10 1 Reactions and equations Objectives Recognize evidence

10. 1 Reactions and equations Objectives Recognize evidence of chemical Change Represent chemical reactions with equations

�Evidence of Chemical reactions ◦ Chemical reaction – the process by which atoms of one or more substances are rearranged to form different substances �Also called a chemical change �Affect your life by: breaking down your food; producing the energy you need to live; produce natural fibers like cotton and wool; power the vehicles we drive

How can you tell if a chemical change has taken place Some are hard to detect, while others show a temperature change or release energy in the form of heat and light. Other reactions absorb heat. Another indication is a color change, like nails outside changing from silver to orange-brown. Odor, gas bubbles, and/or the appearance of solids are another indication of chemical change
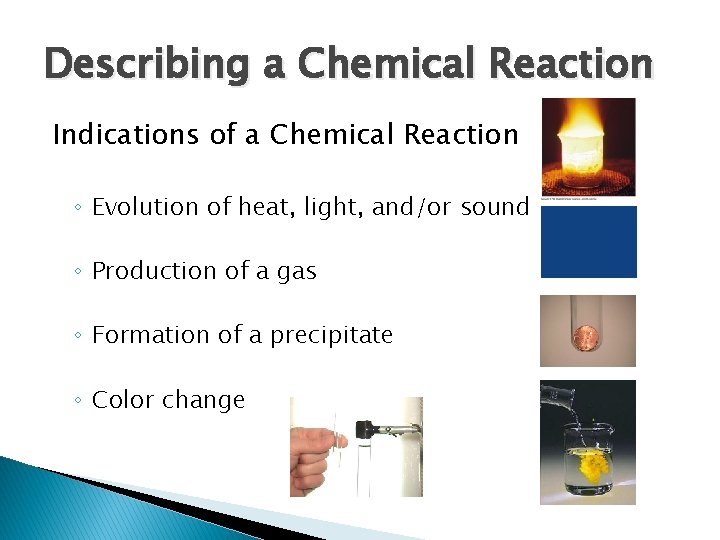
Describing a Chemical Reaction Indications of a Chemical Reaction ◦ Evolution of heat, light, and/or sound ◦ Production of a gas ◦ Formation of a precipitate ◦ Color change
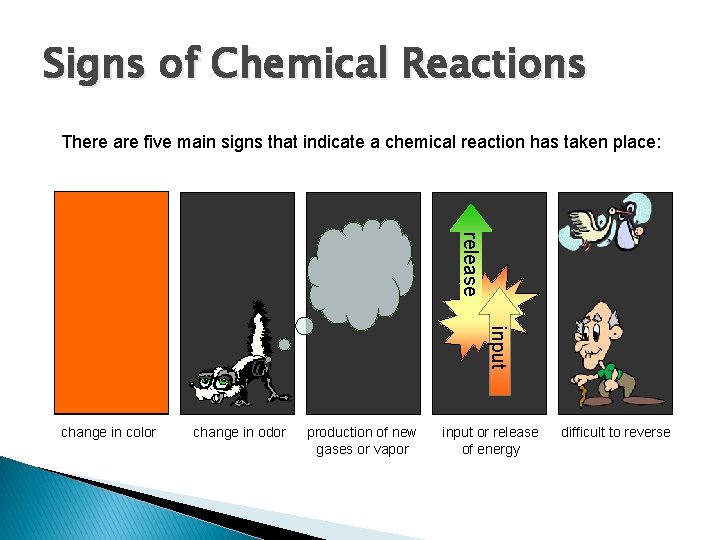
Signs of Chemical Reactions There are five main signs that indicate a chemical reaction has taken place: release input change in color change in odor production of new gases or vapor input or release of energy difficult to reverse

Representing Chemical Reactions Chemists use statements called equations to represent chemical reactions These equations show a reactions reactants which are the starting substances These equations also show the products which are the substances formed during the reaction Chemical equations do not express numerical equalities like mathematical equation because during chemical reactions the reactants are used up as the products are formed Instead the equations show direction in which the reaction progresses

Therefore an arrow not an equal sign is used to separate the reactants from the products Your read the arrow as “react to produce” or “yield” The reactants are written to the arrow’s left and the product are on the arrow’s right When there are two or more reactants or two or more products a plus sign is used to separate each reactant. Example: Reactant 1 + Reactant 2 →Product 1 + Product 2
Chemical Equations Reactants – the substances that exist before a Products – the new substance(s) that are formed chemical change (or reaction) takes place. during the chemical changes. CHEMICAL EQUATION indicates the reactants and products of a reaction. REACTANTS PRODUCTS

�In equations symbols are used to show the physical states of the reactants and products. Reactants and products can be solids, liquids, or gases. When they dissolve in water they are said to be aqueous. �It is important to show the physical states of a reaction’s reactants and products in an equation because the physical state proved clues about how the reaction occurs �

In equations symbols are used to show the physical states of the reactants and products. Reactants and products can be solids, liquids, or gases. When they dissolve in water they are said to be aqueous. It is important to show the physical states of a reaction’s reactants and products in an equation because the physical state proved clues about how the reaction occurs

Additional Symbols Used in Chemical Equations “Yields”; indicates result of reaction Used to indicate a reversible reaction (s) A reactant or product in the solid state; also used to indicate a precipitate Alternative to (s), but used only to indicate a precipitate A reactant or product in the liquid state (l) A reactant or product in an aqueous solution (dissolved in water) A reactant or product in the gaseous state (aq) (g)
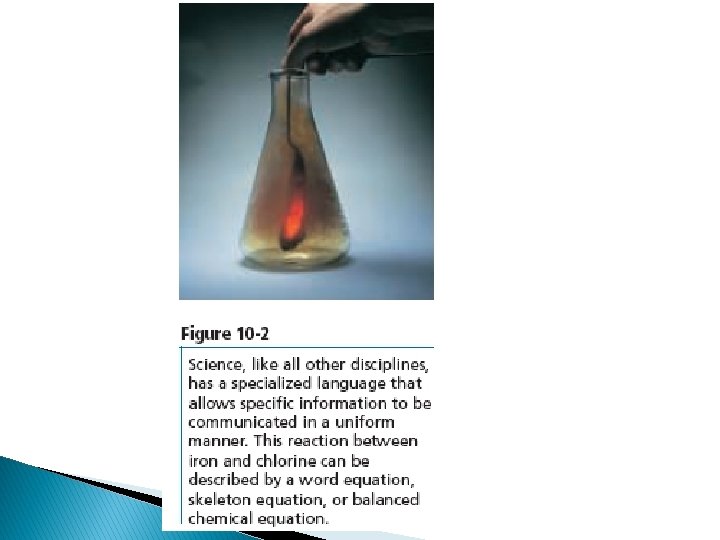

Word Equations: You can use statements called word equations to indicate the reactants and products of chemical reactions. The word equation is read, “Iron and chlorine react to produce iron (III) chloride”
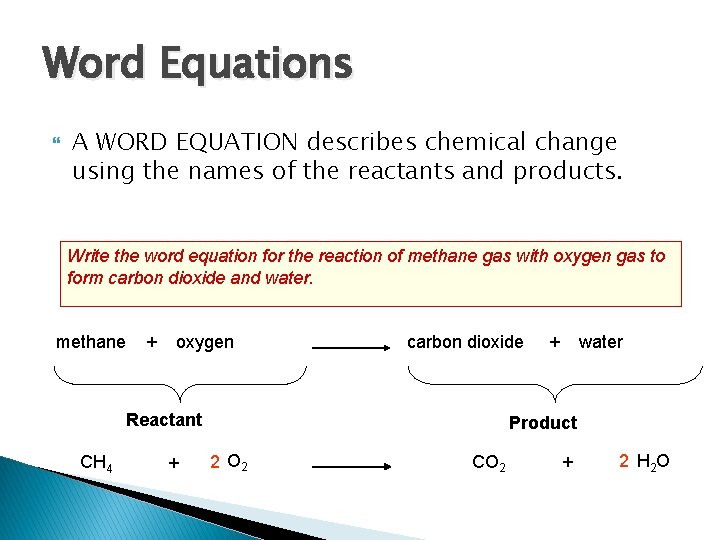
Word Equations A WORD EQUATION describes chemical change using the names of the reactants and products. Write the word equation for the reaction of methane gas with oxygen gas to form carbon dioxide and water. methane + oxygen carbon dioxide Reactant CH 4 + + water Product 2 O 2 CO 2 + 2 H 2 O
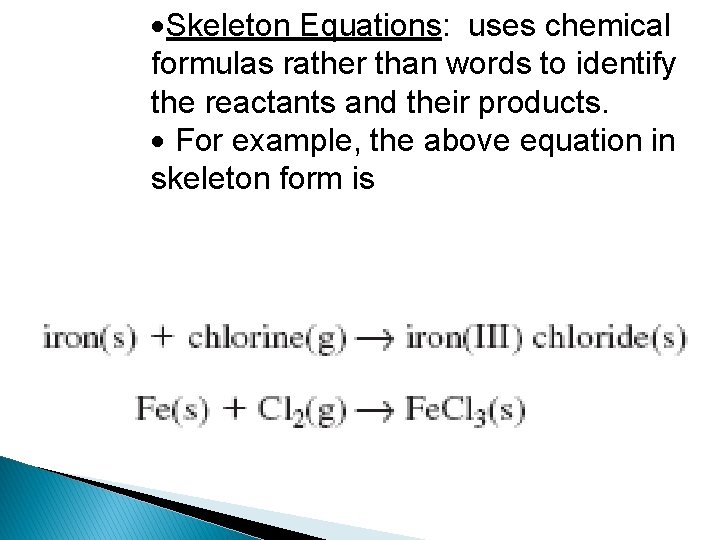
Skeleton Equations: uses chemical formulas rather than words to identify the reactants and their products. For example, the above equation in skeleton form is

Chemical Equations: Chemical equations must show that matter is conserved during a reaction, and a skeleton equation lacks this information. It does not show the exact number of atoms that actually interact
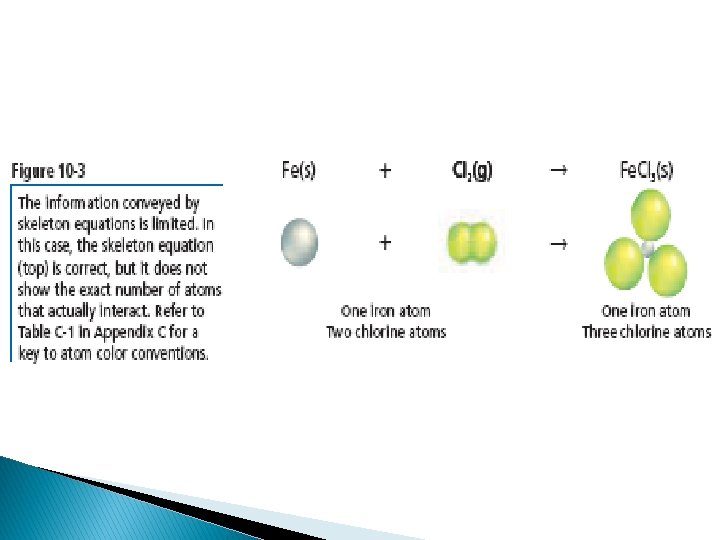

This equation shows that one iron atom and two chlorine atoms react to produce a substance containing one iron atom and 3 chlorine atoms. To accurately represent a chemical reaction the equation must show the law of conservation of mass is obeyed. The equation must show that the number of atoms of each reactant and each product is equal on both sides of the arrow. Such an equation is called a balanced chemical equation
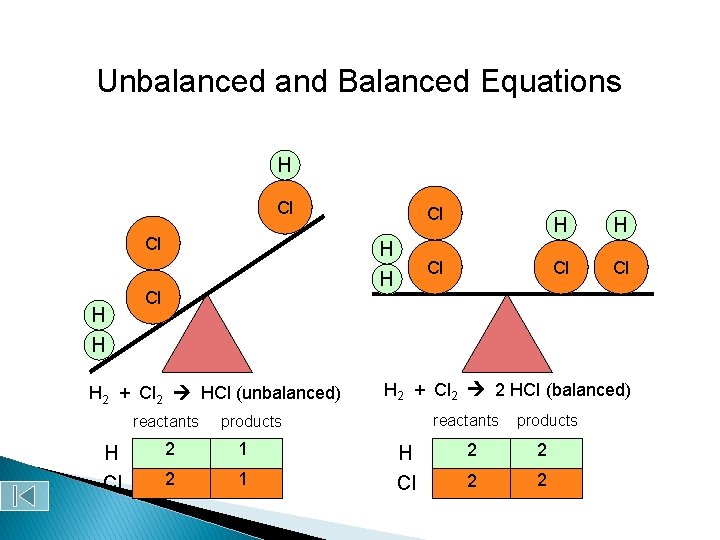
Unbalanced and Balanced Equations H Cl Cl H H Cl H 2 + Cl 2 HCl (unbalanced) reactants H Cl Cl Cl 1 2 1 H Cl Cl H 2 + Cl 2 2 HCl (balanced) reactants products 2 H H Cl products 2 2
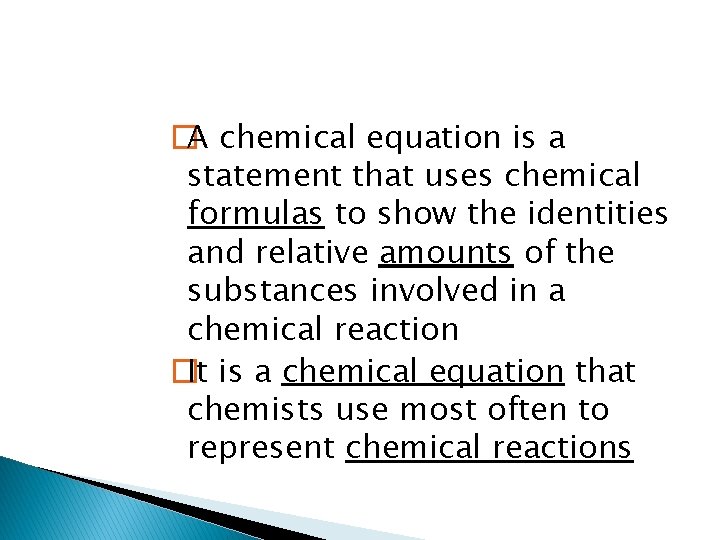
�A chemical equation is a statement that uses chemical formulas to show the identities and relative amounts of the substances involved in a chemical reaction �It is a chemical equation that chemists use most often to represent chemical reactions
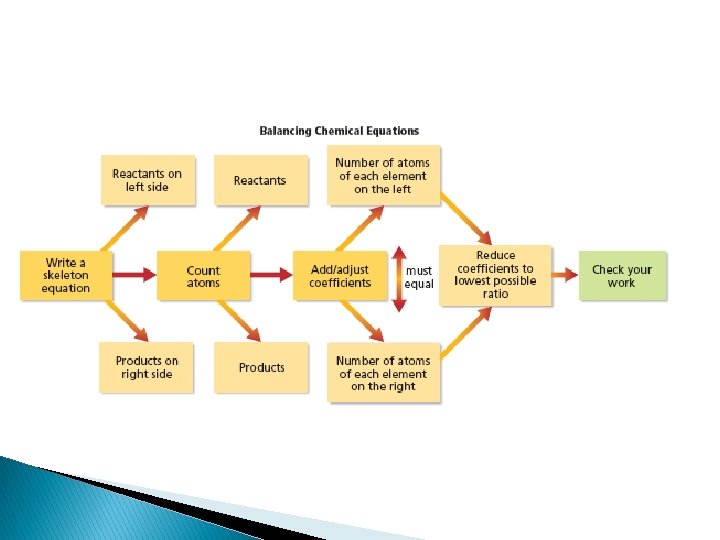
Balancing Chemical Equations The following equation reflects the law of conservation of mass
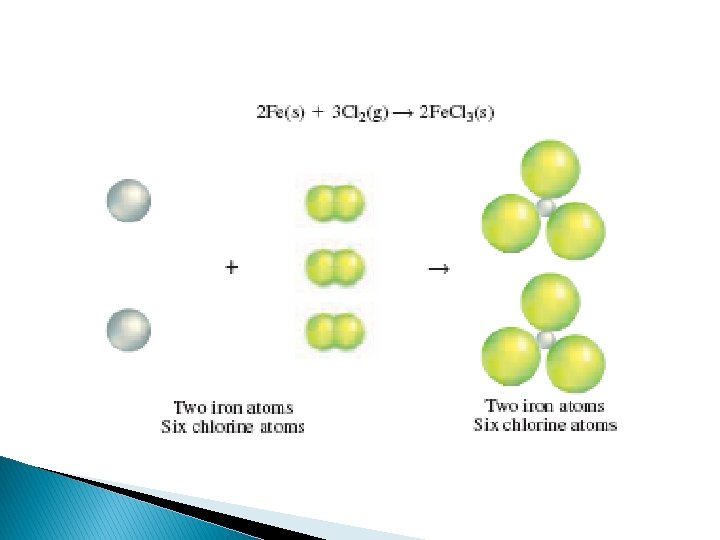

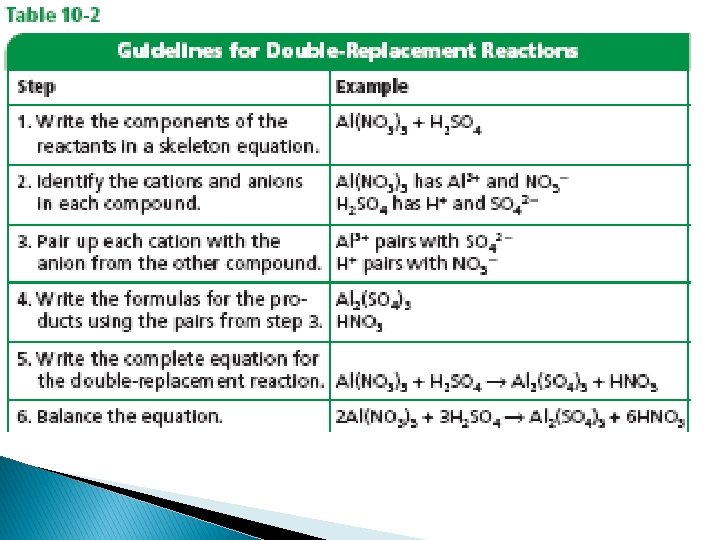
� To balance an equation you must find the correct coefficients for the chemical formulas in the skeleton equation �A coefficient in a chemical equation is the number written in front of each reactant or product. �Coefficients are usually whole numbers, and are not written if the value is 1 �A coefficient tells you the smallest number of particles of the substance involved in the reaction. �The coefficients in a balanced equation describe the lowest whole number ratio of the amounts of all the reactants and products

Steps for balancing equations Step 1: Write the skeleton equation for the reaction
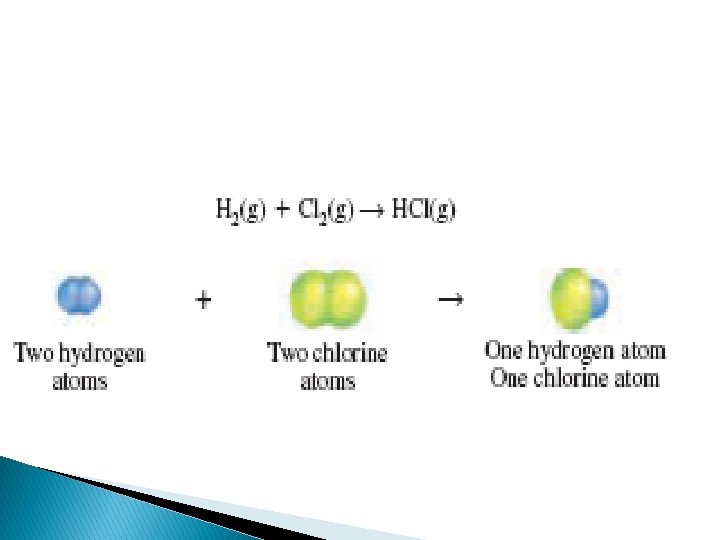
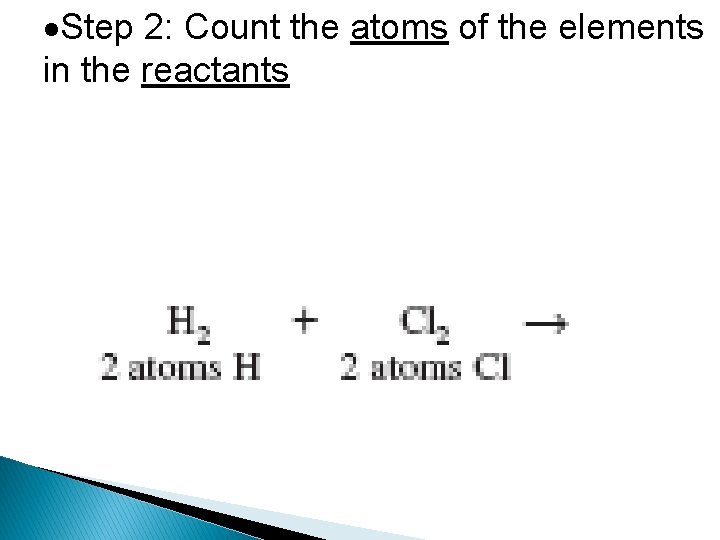
Step 2: Count the atoms of the elements in the reactants

Step 3: Count the atoms of the elements in the products
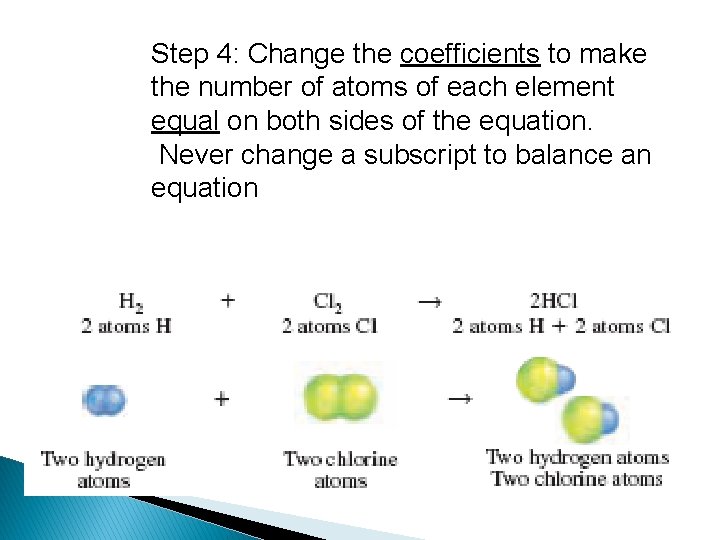
Step 4: Change the coefficients to make the number of atoms of each element equal on both sides of the equation. Never change a subscript to balance an equation

Step 5: Write the coefficients at their lowest possible ratio. Step 6: Check your work.

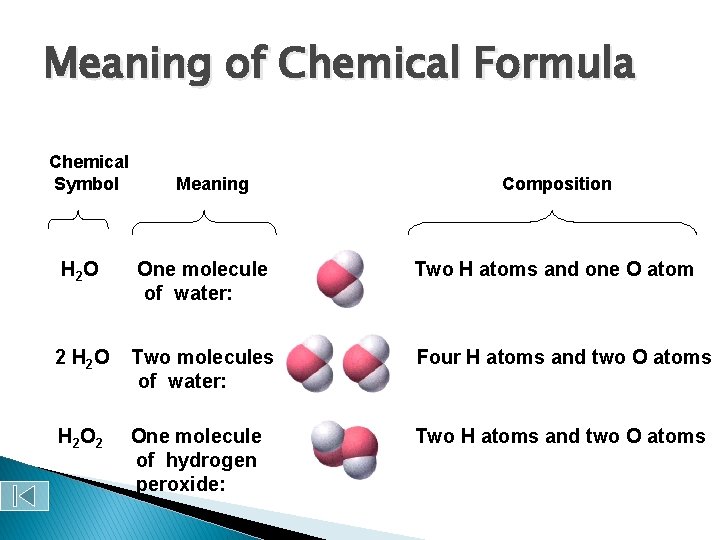
Meaning of Chemical Formula Chemical Symbol Meaning Composition H 2 O One molecule of water: Two H atoms and one O atom 2 H 2 O Two molecules of water: Four H atoms and two O atoms H 2 One molecule of hydrogen peroxide: Two H atoms and two O atoms
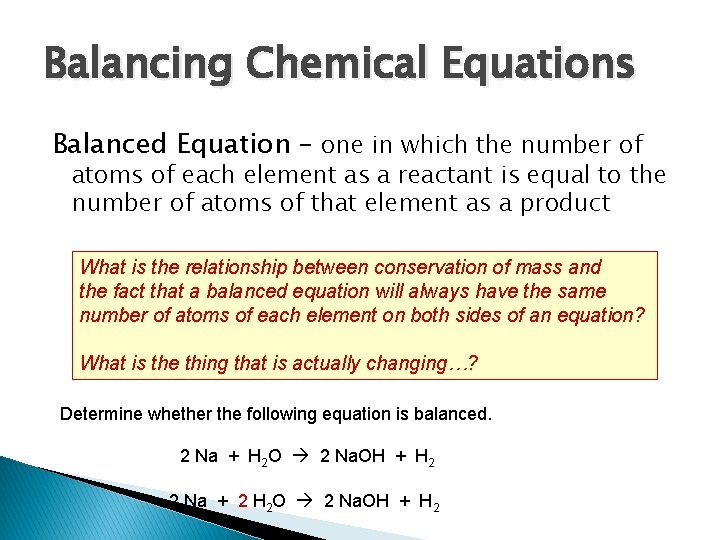
Balancing Chemical Equations Balanced Equation – one in which the number of atoms of each element as a reactant is equal to the number of atoms of that element as a product What is the relationship between conservation of mass and the fact that a balanced equation will always have the same number of atoms of each element on both sides of an equation? What is the thing that is actually changing…? Determine whether the following equation is balanced. 2 Na + H 2 O 2 Na. OH + H 2 2 Na + 2 H 2 O 2 Na. OH + H 2
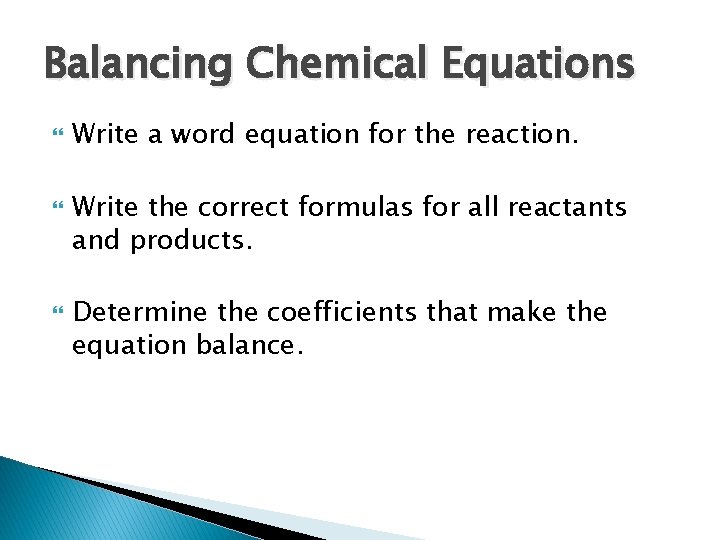
Balancing Chemical Equations Write a word equation for the reaction. Write the correct formulas for all reactants and products. Determine the coefficients that make the equation balance.
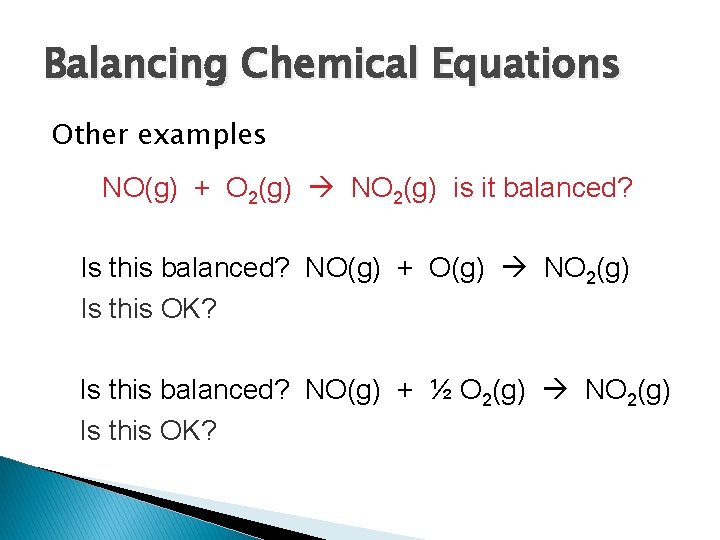
Balancing Chemical Equations Other examples NO(g) + O 2(g) NO 2(g) is it balanced? Is this balanced? NO(g) + O(g) NO 2(g) Is this OK? Is this balanced? NO(g) + ½ O 2(g) NO 2(g) Is this OK?
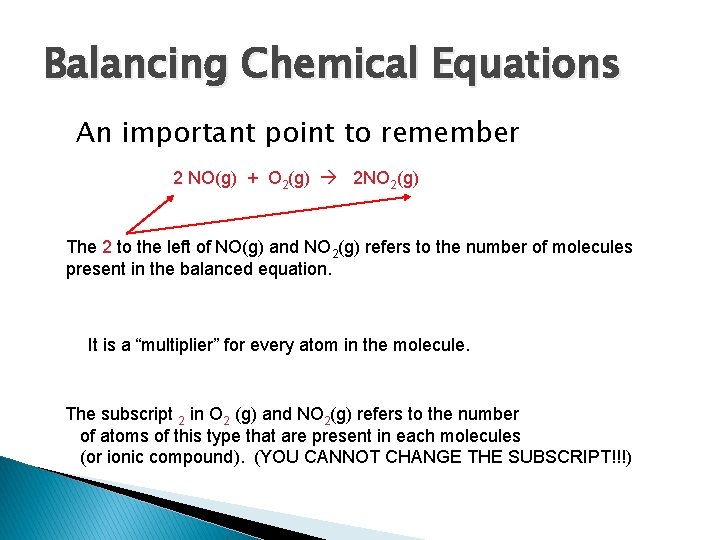
Balancing Chemical Equations An important point to remember 2 NO(g) + O 2(g) 2 NO 2(g) The 2 to the left of NO(g) and NO 2(g) refers to the number of molecules present in the balanced equation. It is a “multiplier” for every atom in the molecule. The subscript 2 in O 2 (g) and NO 2(g) refers to the number of atoms of this type that are present in each molecules (or ionic compound). (YOU CANNOT CHANGE THE SUBSCRIPT!!!)
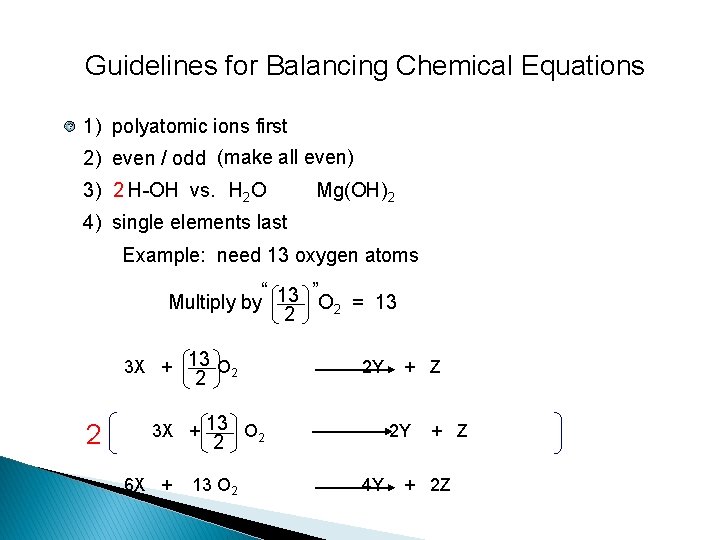
Guidelines for Balancing Chemical Equations ? 1) polyatomic ions first 2) even / odd (make all even) 3) 2 H-OH vs. H 2 O Mg(OH)2 4) single elements last Example: need 13 oxygen atoms “ ” Multiply by 13 O 2 = 13 2 3 X + 13 O 2 2 Y 2 6 X + 13 O 2 + Z 4 Y + Z + 2 Z
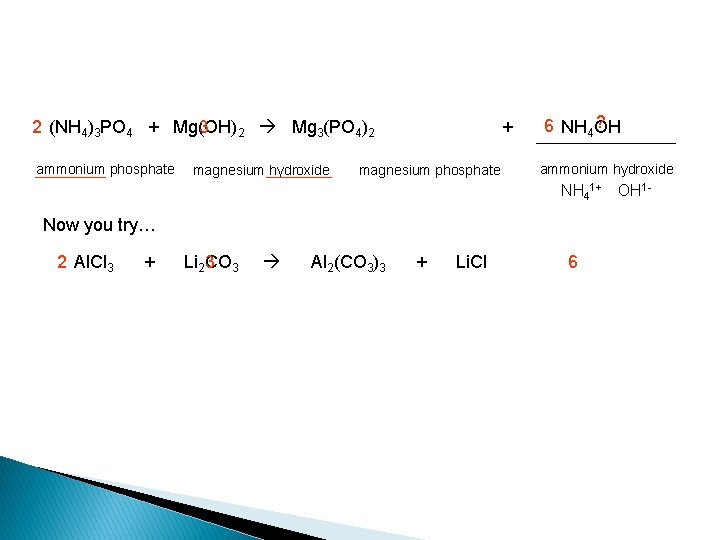
2 (NH 4)3 PO 4 + Mg(OH) 3 2 Mg 3(PO 4)2 ammonium phosphate magnesium hydroxide + magnesium phosphate ? 6 NH 4 OH ammonium hydroxide NH 41+ OH 1 - Now you try… 2 Al. Cl 3 + Li 23 CO 3 Al 2(CO 3)3 + Li. Cl 6
10. 2 Classifying Chemical Reactions Objectives: Classify Chemical reactions Identify the characteristics of different classes of chemical reactions

�Chemists classify chemical reactions in order to organize the many reactions that occur daily in living things, laboratories and industries. �Knowing the categories of chemical reactions can help you remember and understand them �Chemists classify reactions in different ways

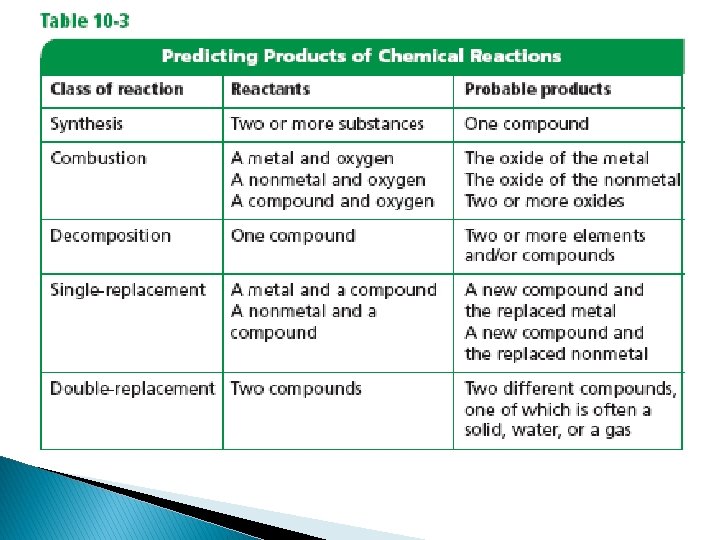
�One ways is to distinguish among the five types of chemical reactions: �synthesis, �combustion, � decomposition, �single-replacement, �and double-replacement reactions. � Some reactions fit well into more than one of these classes
Synthesis Reactions – a chemical reaction in which two or more substances react to produce a single product When two elements react, the reaction is always a synthesis reaction.
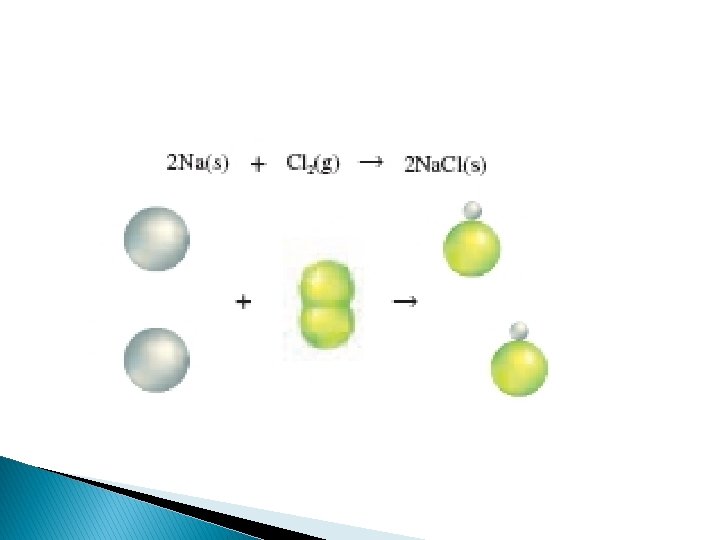
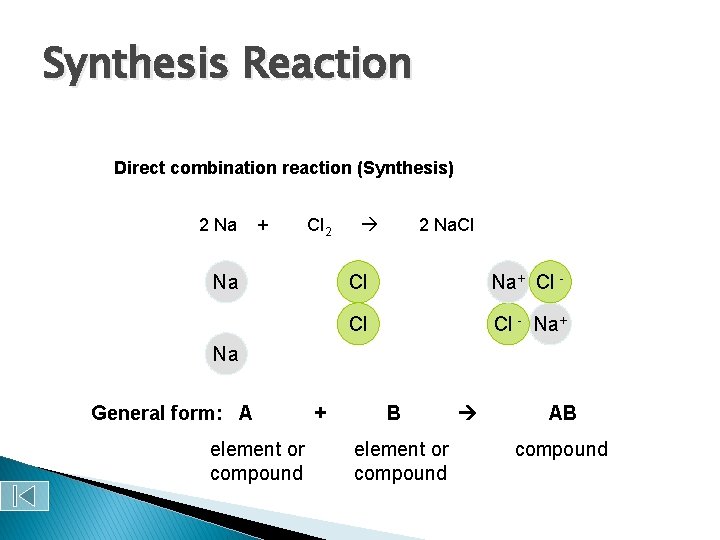
Synthesis Reaction Direct combination reaction (Synthesis) 2 Na + Cl 2 Na. Cl Cl Na+ Cl - Cl Cl - Na+ Na General form: A element or compound + B element or compound AB compound
� Combustion reactions – oxygen combines with a substance and releases energy in the form of heat and light. � Oxygen can combine with many different substances making combustion reactions common
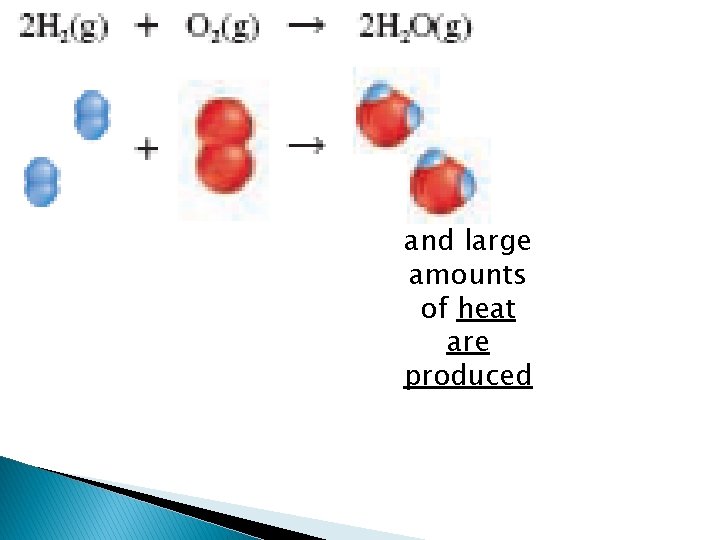
and large amounts of heat are produced

�Another example is when coal is burned to produce energy. �Coal is called a fossil fuel because it contains the remains of plants that lived long ago. It is composed primarily of the element carbon. �Coal burning power plants generate electric power in many parts of the US
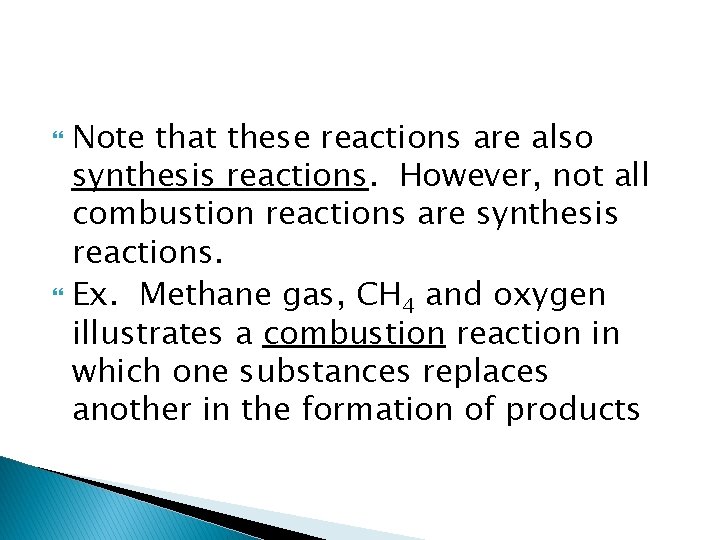
Note that these reactions are also synthesis reactions. However, not all combustion reactions are synthesis reactions. Ex. Methane gas, CH 4 and oxygen illustrates a combustion reaction in which one substances replaces another in the formation of products

Methane which belongs to a group of substances called hydrocarbons is the major component of natural gas. All hydrocarbons contain carbon and hydrogen and burn in oxygen to yield the same products as methane does… carbon dioxide and water In engines gasoline is combined with oxygen, producing carbon dioxide, water and energy that powers vehicles

Decomposition reactions – when a single compound breaks down into two or more elements or new compounds AB→A+B Decomposition reactions often require an energy source such as heat, light or electricity
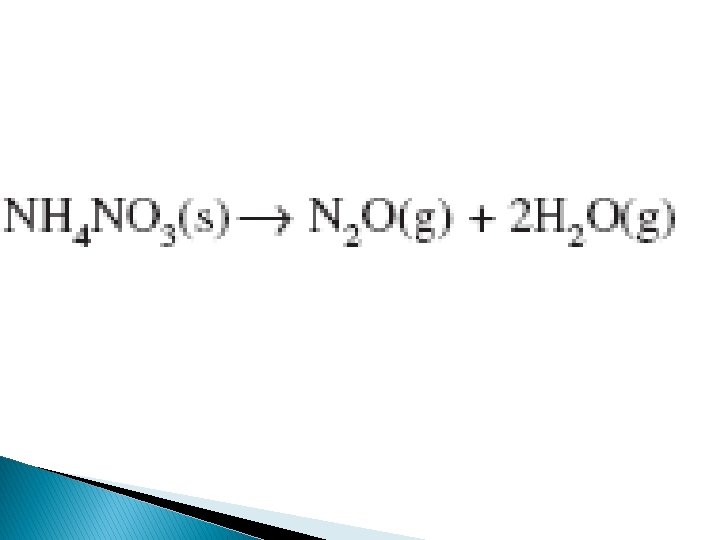

Another example is when air bags inflate rapidly as sodium azide pellets decompose after an electric charge is applies and decomposes into nitrogen that inflates the bag
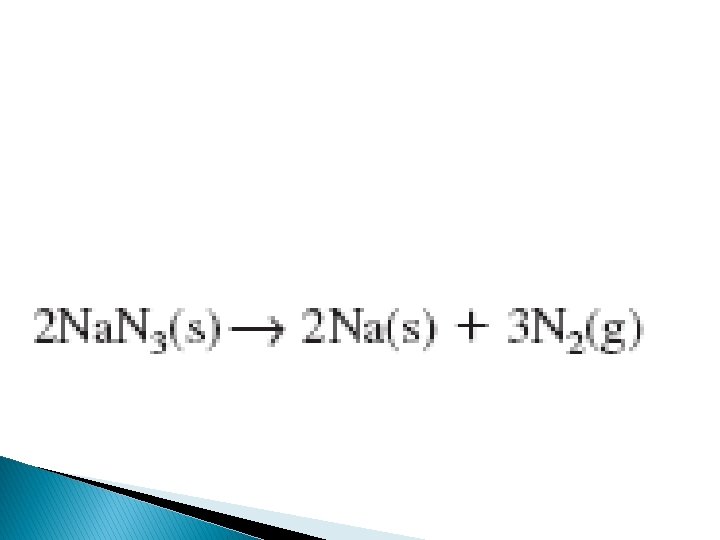
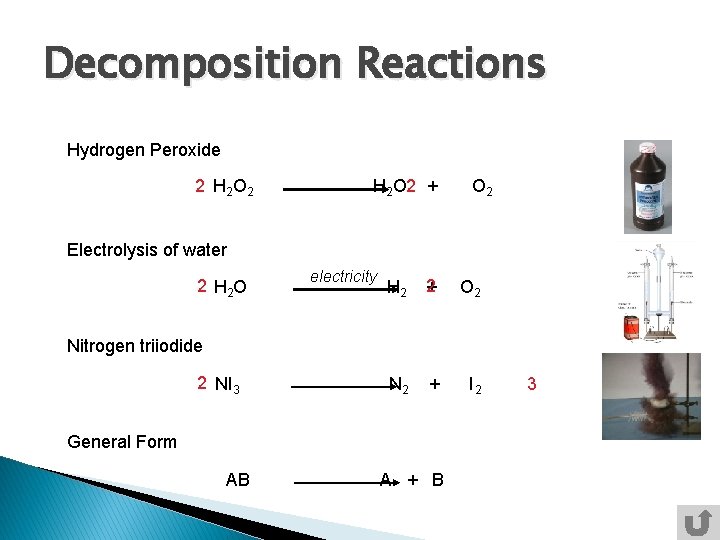
Decomposition Reactions Hydrogen Peroxide 2 H 2 O 2 H 2 O 2 + O 2 Electrolysis of water 2 H 2 O electricity H 2 2 + O 2 N 2 + I 2 Nitrogen triiodide 2 NI 3 General Form AB A + B 3
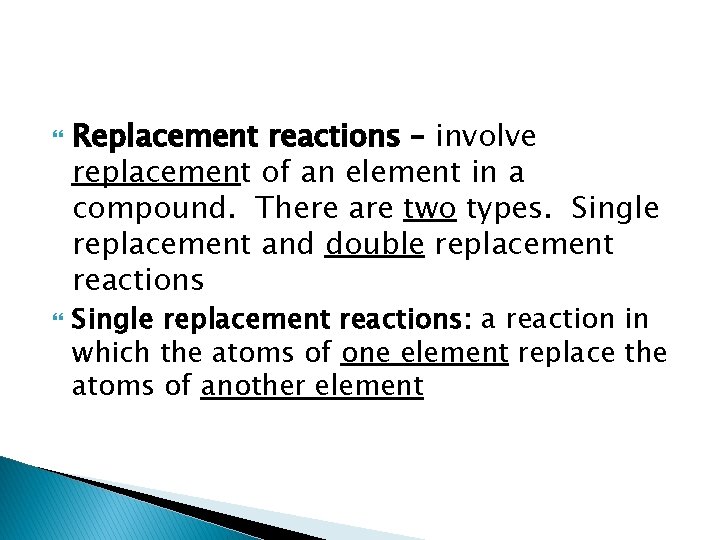
Replacement reactions – involve replacement of an element in a compound. There are two types. Single replacement and double replacement reactions Single replacement reactions: a reaction in which the atoms of one element replace the atoms of another element
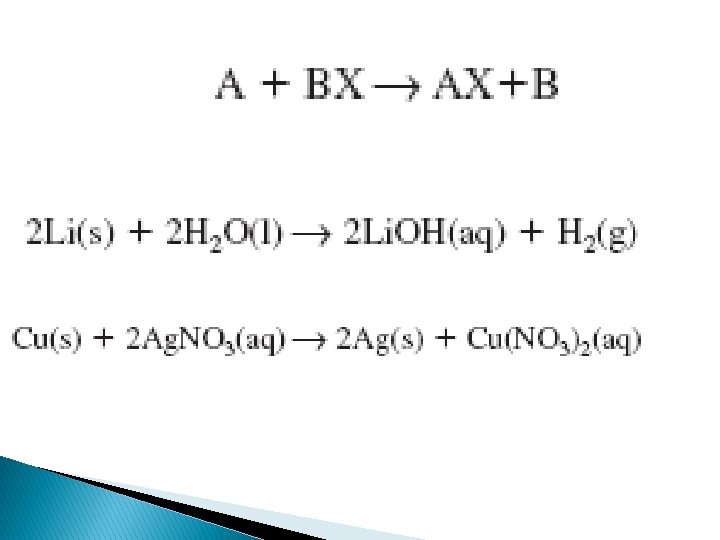

A metal will not always replace another metal in a compound dissolved in water This is because metals differ in their relativities A metal’s reactivity is its ability to react with another substance the most reactive metals, which are those that do replace the metal in a compound are at the top of the list, the least are at the bottom of the list
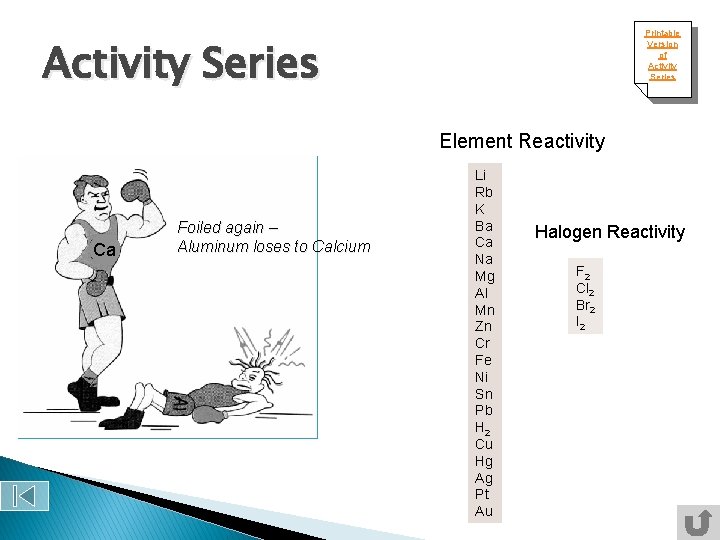
Printable Version of Activity Series Element Reactivity Ca Foiled again – Aluminum loses to Calcium Li Rb K Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H 2 Cu Hg Ag Pt Au Halogen Reactivity F 2 Cl 2 Br 2 I 2

You can use this chart to see if certain reaction will occur. A specific metal can replace any metal listed below it that is in a compound, it cannot replace any metal listed above it

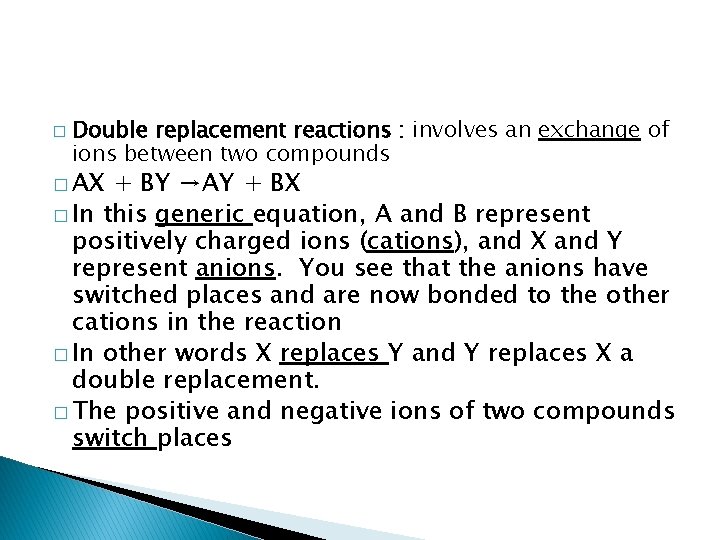
� Double replacement reactions : involves an exchange of ions between two compounds � AX + BY →AY + BX � In this generic equation, A and B represent positively charged ions (cations), and X and Y represent anions. You see that the anions have switched places and are now bonded to the other cations in the reaction � In other words X replaces Y and Y replaces X a double replacement. � The positive and negative ions of two compounds switch places
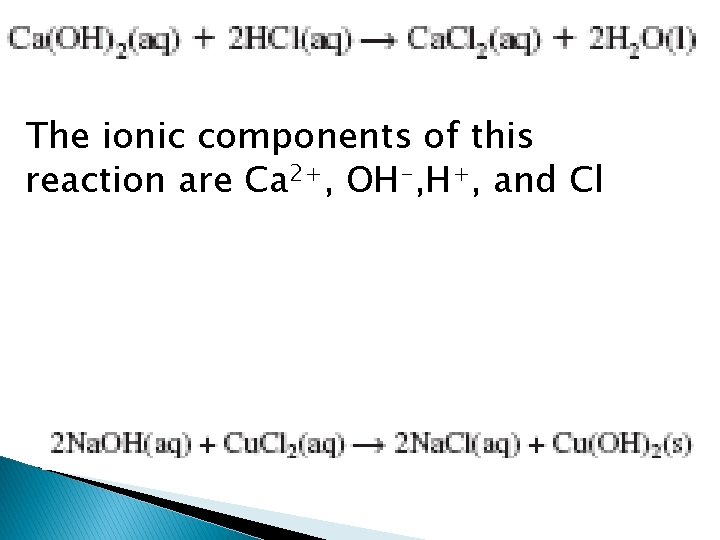
The ionic components of this reaction are Ca 2+, OH-, H+, and Cl

In this equation the result is a solid product, Copper (II) hydroxide, The solid produced during a chemical reaction in a solution is called a precipitate All double replacement reaction produce either a precipitate, a gas, or water
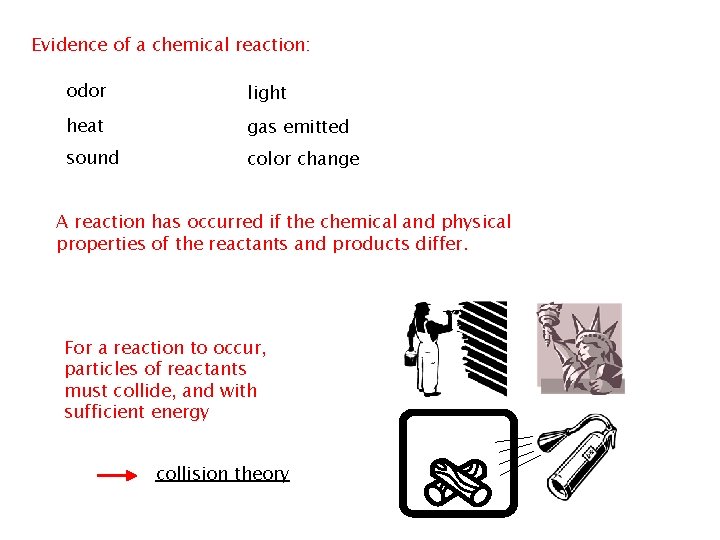
Evidence of a chemical reaction: odor light heat gas emitted sound color change A reaction has occurred if the chemical and physical properties of the reactants and products differ. For a reaction to occur, particles of reactants must collide, and with sufficient energy collision theory
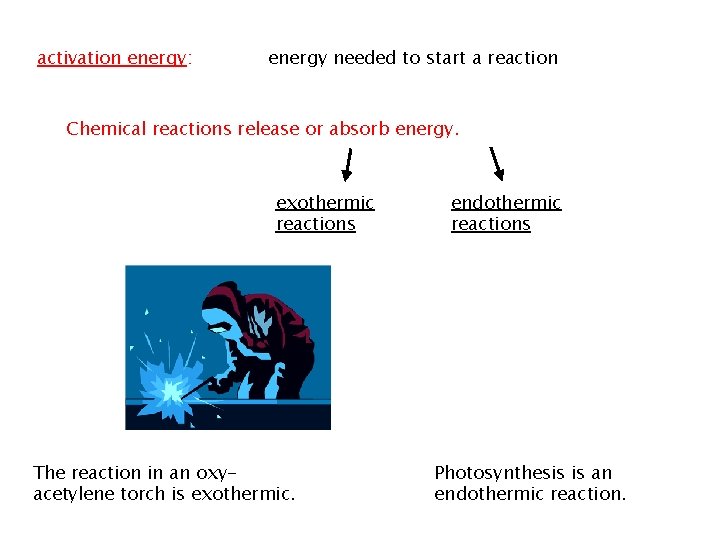
activation energy: energy needed to start a reaction Chemical reactions release or absorb energy. exothermic reactions The reaction in an oxyacetylene torch is exothermic. endothermic reactions Photosynthesis is an endothermic reaction.
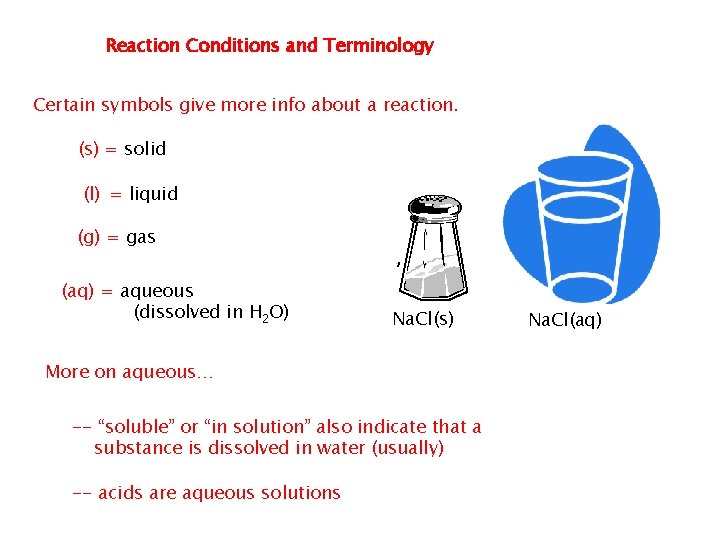
Reaction Conditions and Terminology Certain symbols give more info about a reaction. (s) = solid (l) = liquid (g) = gas (aq) = aqueous (dissolved in H 2 O) Na. Cl(s) More on aqueous… -- “soluble” or “in solution” also indicate that a substance is dissolved in water (usually) -- acids are aqueous solutions Na. Cl(aq)
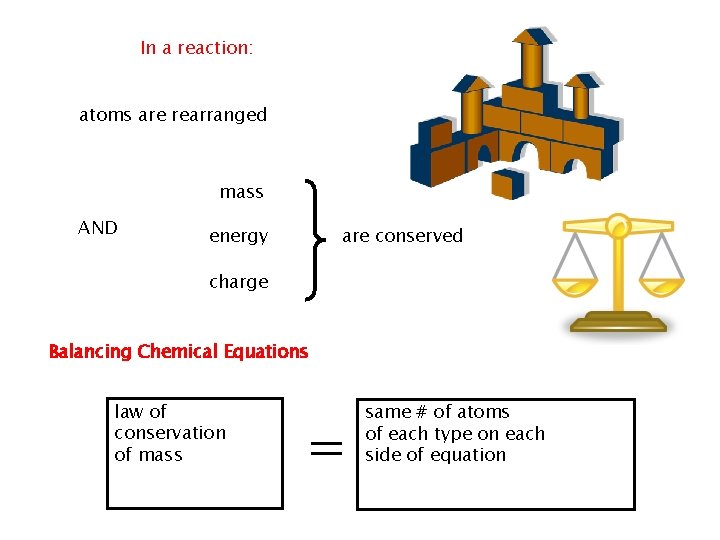
In a reaction: atoms are rearranged mass AND are conserved energy charge Balancing Chemical Equations law of conservation of mass = same # of atoms of each type on each side of equation
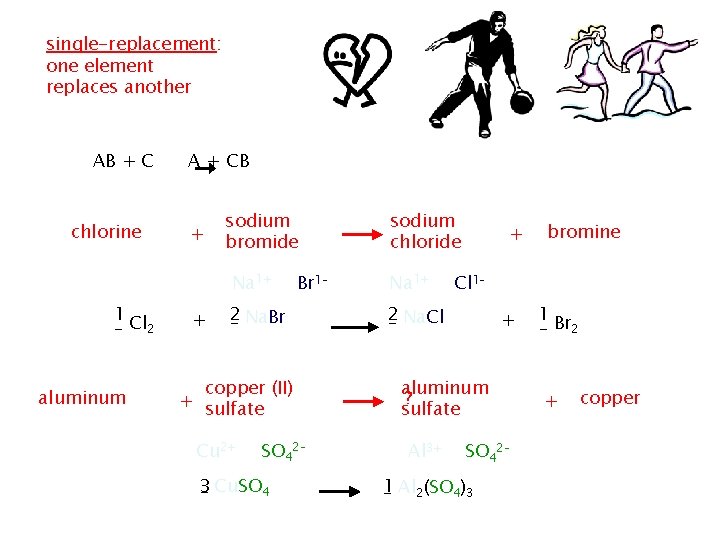
single-replacement: one element replaces another AB + C A + CB chlorine + AB + C sodium bromide Na 1+ 1 _ Cl 2 + 2 _ Na. Br copper (II) + sulfate aluminum Cu 2+ _2 Al Br 1– + SO 42– 3 _ Cu. SO 4 B + AC sodium chloride Na 1+ + Cl 1– 2 _ Na. Cl + aluminum ? sulfate Al 3+ bromine 1 _ Br 2 + copper SO 42– 1 _ Al 2(SO 4)3 _ Cu + 3
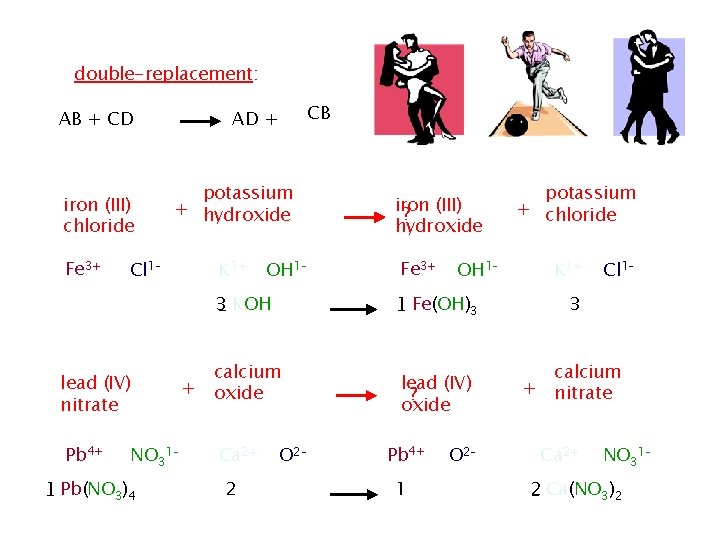
double-replacement: iron (III) chloride Fe 3+ potassium + hydroxide Cl 1– 1 _ Fe. Cl 3 K 1+ 3 _ KOH + calcium oxide NO 31– 1 _ Pb(NO 3)4 OH 1– + lead (IV) nitrate Pb 4+ CB AD + AB + CD Ca 2+ _ Ca. O + 2 iron (III) ? hydroxide Fe 3+ OH 1– 1 _ Fe(OH)3 O 2– lead (IV) ? oxide Pb 4+ potassium + chloride O 2– 1 _ Pb. O 2 K 1+ + + Cl 1– 3 _ KCl calcium nitrate Ca 2+ NO 31– _ Ca(NO 3)2 + 2
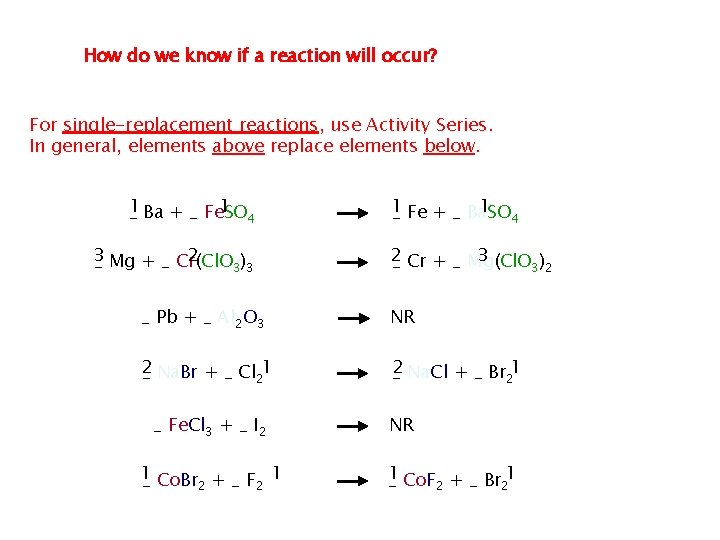
How do we know if a reaction will occur? For single-replacement reactions, use Activity Series. In general, elements above replace elements below. 1 1 _ Ba + _ Fe. SO 4 3 2 _ Mg + _ Cr(Cl. O 3)3 1 1 _ Fe + _ Ba. SO 4 2 3 _ Cr + _ Mg(Cl. O 3)2 _ Pb + _ Al 2 O 3 NR 2 _ Na. Br + _ Cl 21 _2 Na. Cl + _ Br 21 _ Fe. Cl 3 + _ I 2 1 _ Co. Br 2 + _ F 2 1 NR _1 Co. F 2 + _ Br 21
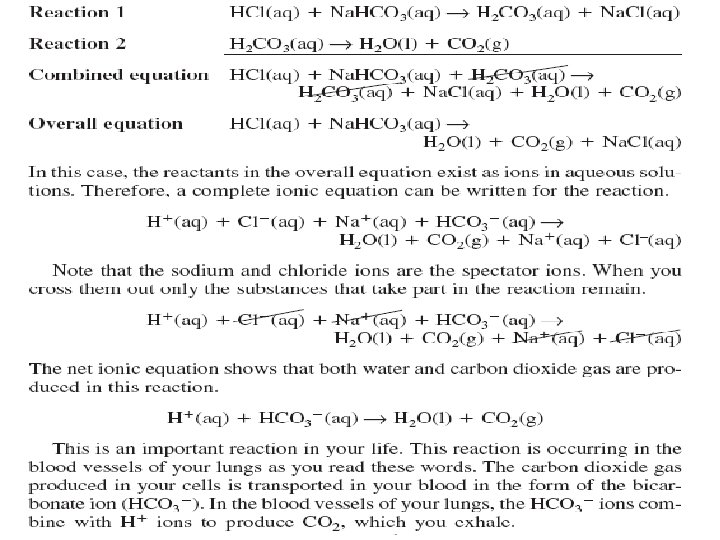
- Slides: 73