1 Why are gold and platinum found in




















- Slides: 27

1) Why are gold and platinum found in the ground as uncombined elements? Answers Unreactive-don’t react with oxygen. C 1 TOPIC 4 M. Rahman

2) A rock which contains enough metal to be economically viable to extract is called… Answers Ore C 1 TOPIC 4 M. Rahman

3) The process by which iron extracted from iron oxide on an industrial scale is? a) Electrolysis b) Reduction by carbon Answers Reduction by carbon Bonus: Why Expert answer; Below carbon in reactivity series so does not require electrolysis. Avoid electrolysis when possible because requires a lot of energy and is expensive C 1 TOPIC 4 M. Rahman

4) Reactive metals in rocks are mainly found with … a) Chlorine b) Oxygen c) Carbon dioxide Answers b) Oxygen C 1 TOPIC 4 M. Rahman

5) Name two metals found uncombined in the earth crust Answer Any two from • Gold, silver, platinum C 1 TOPIC 4 M. Rahman

6) Why are some metals found uncombined? Answer Unreactive C 1 TOPIC 4 M. Rahman

7) Reduction is…. Answer Loss of oxygen C 1 TOPIC 4 M. Rahman

8) Oxidation is …. . Answer gain of oxygen C 1 TOPIC 4 M. Rahman

9) What are the two methods of extracting metals from their ore… Answer • Electrolysis • Heating with carbon (reduction) C 1 TOPIC 4 M. Rahman

10) What determines the method used for extraction of metals. a) Reactivity b) Conductivity c) How strong it is Answer a) Reactivity. C 1 TOPIC 4 M. Rahman

11) Which of the following metals are extracted using Electrolysis. a) Aluminium b) Copper c) Iron Answer a) Aluminium C 1 TOPIC 4 M. Rahman

12) Why is aluminium not extracted by heating with carbon? Answer • Aluminium above carbon in reactivity series. • So carbon cannot take an oxygen away from a metal (aluminium) which is more reactive C 1 TOPIC 4 M. Rahman

13) Why would you not use electrolysis to extract iron. Answer • Electrolysis uses a lot of energy and more expensive. • No need to use it when it is not required C 1 TOPIC 4 M. Rahman

14) Which of the following metals are the most reactive. a) Platinum b) Potassium c) Zinc Answer a) Potassium C 1 TOPIC 4 M. Rahman

15) Give three properties of metals (3). Answer a) Strong b) Malleable c) Good conductor of heat and electricity C 1 TOPIC 4 M. Rahman

16) Why is aluminium used for aeroplanes (2) Answer Low density Corrosion resistance. C 1 TOPIC 4 M. Rahman

17) Why is copper used for electrical cables (2). Answer Good conductor of electricity. Has high melting point. C 1 TOPIC 4 M. Rahman

18) Why is copper used for pipes in plumbing (2). Answer Does not react with water-so no corrosion. C 1 TOPIC 4 M. Rahman

19) Why are gold and silver used for Jewellery Answer a) Soft-so can be easily shaped b) Shiny-looks good. C 1 TOPIC 4 M. Rahman

20) Why are gold and silver used for Jewellery Answer a) Soft-so can be easily shaped b) Shiny-looks good. C 1 TOPIC 4 M. Rahman

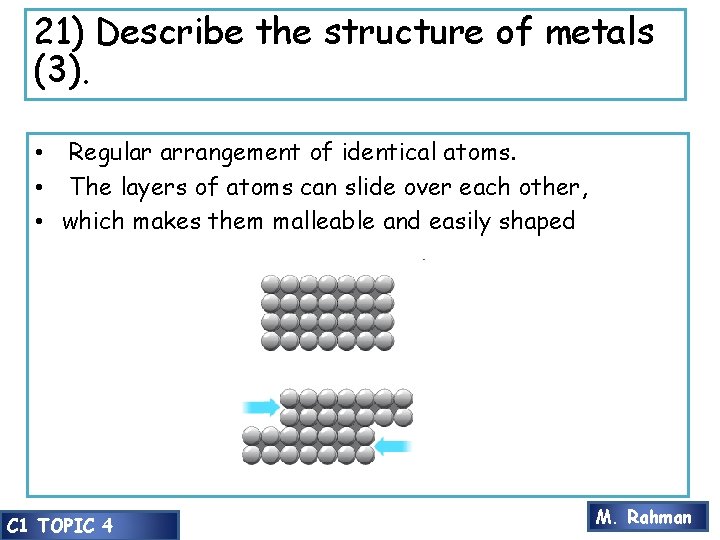
21) Describe the structure of metals (3). • Regular arrangement of identical atoms. • The layers of atoms can slide over each other, • which makes them malleable and easily shaped C 1 TOPIC 4 M. Rahman
22) How can metals like iron be made stronger? Answer Convert into an alloy C 1 TOPIC 4 M. Rahman

23) Define an alloy. Answer A mixture of two or more metals or a metal and nonmetal. C 1 TOPIC 4 M. Rahman

24) Explain why alloys are strong? Answer • Alloys are made up of atoms of different sizes. • This upsets the layers. • The layers cannot slide past each other, making alloys harder. C 1 TOPIC 4 M. Rahman

25) Give an example of smart alloy, describing its properties. Answer • Nitinol-shape memory alloy. • It is an alloy of nickel and titanium. • If you bend it, it will go back to its original shape when heated. C 1 TOPIC 4 M. Rahman

26) Give at least three advantages of recycling metals. Answer 1) There is a limited amount of metals in the earth-recycling conserves that. 2) Saves energy, since mining and extraction require huge amounts of energy 3) Costs less because energy costs money. 4) Reduces the amount of rubbish that goes into landfills. 5) Less pollution because less fossil fuels burnt to provide energy. C 1 TOPIC 4 M. Rahman

27) Give one disadvantages of recycling metals. Answer Recycling is not free- cost involved in collecting, transporting sorting and processing metals C 1 TOPIC 4 M. Rahman