1 What is the p H and p













- Slides: 14

#1 • What is the p. H and p. OH of a 1. 2 x 10 -3 M HBr solution?

#2 • Write the equation for nitric acid in water. • Write the equation for the acetate ion in water.

#3 • What is the p. H and p. OH of a 2. 34 x 10 -5 M Na. OH solution?

#4 • What is the p. H and p. OH of a solution made by adding water to 15 grams of hydroiodic acid until the volume of the solution is 2500 m. L?

#5 • What is the p. H and p. OH of a solution that was made by adding 400 m. L of water to 350 m. L of -3 5. 0 x 10 M Na. OH solution?

#6 • What is the p. H and p. OH of a solution with a volume of 5. 40 L that contains 15. 0 grams of hydrochloric acid and 25. 0 grams of nitric acid?

#7 • A swimming pool has a volume of one million liters. How many grams of HCl would need to be added to that swimming pool to bring the p. H down from 7 to 4? (Assume the volume of the HCl is negligible)

#8 • In a titration of H 2 SO 4 with Na. OH, 60. 0 m. L of 0. 020 M Na. OH was needed to neutralize 15. 0 m. L of H 2 SO 4. What is the molarity of the acid?

#9 • A few small drops of water are left in a buret that is then used to titrate a base into an acid solution to determine the concentration of the acid. Will this small amount of water have any effect on the determined value for the concentration of the acid? If so, how is it affected?

#10 • Lulu Labwrecker carefully pipets 25. 0 m. L of 0. 525 M Na. OH into a test tube. She places the test tube into a small beaker to keep it from spilling and then pipets 75. 0 m. L of 0. 355 M HCl into another test tube. When Lulu reaches to put this test tube of acid into the beaker along with test tube of base she accidentally knocks the test tubes together hard enough to break them and their respective contents combine in the bottom of the beaker. Is the solution formed from the contents of the two test tubes acidic or basic? What is the p. H of the resulting solution?

#11 • What is the p. H of a solution that contains 25 grams of hydroiodic acid dissolved in 1. 50 liters of water?

#12 • If a solution has a hydronium concentration of 4. 5 x 10 -7 M, is this an acidic or basic solution?

#13 • List the properties of acids and bases. • Describe how phenolphthalein works.

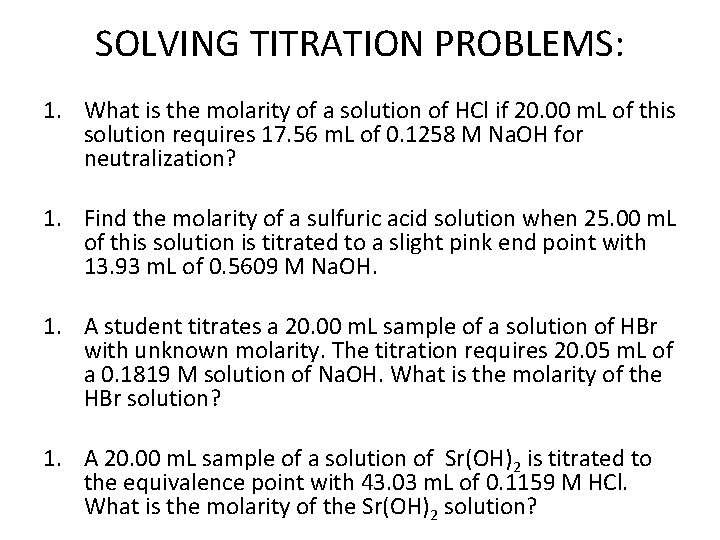
SOLVING TITRATION PROBLEMS: 1. What is the molarity of a solution of HCl if 20. 00 m. L of this solution requires 17. 56 m. L of 0. 1258 M Na. OH for neutralization? 1. Find the molarity of a sulfuric acid solution when 25. 00 m. L of this solution is titrated to a slight pink end point with 13. 93 m. L of 0. 5609 M Na. OH. 1. A student titrates a 20. 00 m. L sample of a solution of HBr with unknown molarity. The titration requires 20. 05 m. L of a 0. 1819 M solution of Na. OH. What is the molarity of the HBr solution? 1. A 20. 00 m. L sample of a solution of Sr(OH)2 is titrated to the equivalence point with 43. 03 m. L of 0. 1159 M HCl. What is the molarity of the Sr(OH)2 solution?