1 Universidade Federal de Minas Gerais INORGANIC FIBERS

1

Universidade Federal de Minas Gerais INORGANIC FIBERS AND MATERIALS OF CARBON 2
Contents (Inorganic Fibres) 1. What are fibres? 2. Classification of Fibres 3. Preparation of Inorganic Fibres 4. 5. 6. 7. Properties of Inorganic Fibres Examples of Inorganic Fibres Applications of Inorganic Fibres Future Perspectives of Inorganic Fibres 3

1. What are fibres? Material that has a length-to-diameter ratio of at least 10: 1, with a cross-sectional area of less than 0. 005 mm 2 and a thickness of less than 0. 25 mm The man made fibres, derived from Inorganic Substance is called Inorganic Fibres. Glass, Carbon, Ceramics, and Metal are the examples of Inorganic Fibres. A 6 μm diameter carbon filament (running from bottom left to top right) compared to a human hair. Fibers, 11. Inorganic Fibers, Survey BERND CLAUB, ITCF Denkendorf, Germany ERICH FITZER, 4
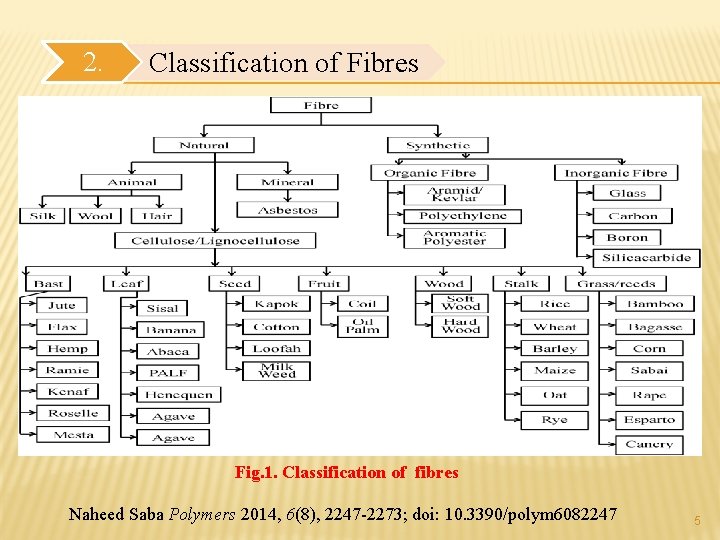
2. Classification of Fibres Fig. 1. Classification of fibres Naheed Saba Polymers 2014, 6(8), 2247 -2273; doi: 10. 3390/polym 6082247 5
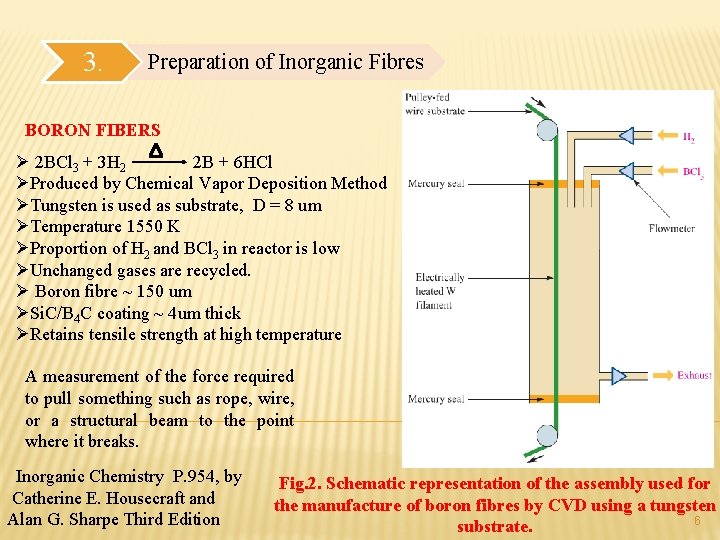
3. Preparation of Inorganic Fibres BORON FIBERS Ø 2 BCl 3 + 3 H 2 2 B + 6 HCl ØProduced by Chemical Vapor Deposition Method ØTungsten is used as substrate, D = 8 սm ØTemperature 1550 K ØProportion of H 2 and BCl 3 in reactor is low ØUnchanged gases are recycled. Ø Boron fibre ~ 150 սm ØSi. C/B 4 C coating ~ 4սm thick ØRetains tensile strength at high temperature A measurement of the force required to pull something such as rope, wire, or a structural beam to the point where it breaks. Inorganic Chemistry P. 954, by Catherine E. Housecraft and Alan G. Sharpe Third Edition Fig. 2. Schematic representation of the assembly used for the manufacture of boron fibres by CVD using a tungsten 6 substrate.

CARBON FIBRES ØDifferent grades of carbon fibre are manufactured by thermal degradation of a polymeric organic three carbon-containing precursors: 1. Rayon 2. Pitch 3. Polyacrylonitrile (PAN) Preparation from Rayon fibres Air 500– 700 K H 2 O, CO 2 and CH 4 Product No Air/N 2 1300 K Graphite-like structure (Carbon Fibres) ØLow density 1. 7 g cm-3 ØLow tensile ØSuch fibres have limited uses and are not suitable for structural applications. norganic Chemistry P. 954, 955 by Catherine E. Housecraft and Alan G. Sharpe Third Editio 7
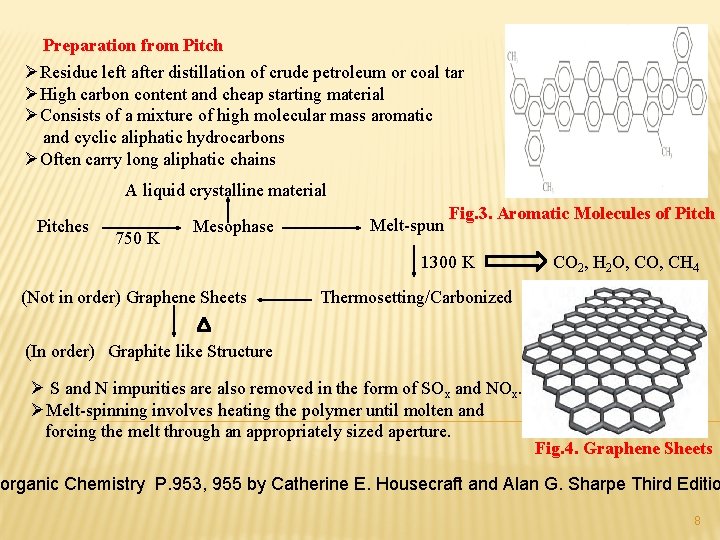
Preparation from Pitch ØResidue left after distillation of crude petroleum or coal tar ØHigh carbon content and cheap starting material ØConsists of a mixture of high molecular mass aromatic and cyclic aliphatic hydrocarbons ØOften carry long aliphatic chains A liquid crystalline material Pitches 750 K Mesophase Melt-spun Fig. 3. Aromatic Molecules of Pitch 1300 K (Not in order) Graphene Sheets CO 2, H 2 O, CH 4 Thermosetting/Carbonized (In order) Graphite like Structure Ø S and N impurities are also removed in the form of SOx and NOx. ØMelt-spinning involves heating the polymer until molten and forcing the melt through an appropriately sized aperture. Fig. 4. Graphene Sheets norganic Chemistry P. 953, 955 by Catherine E. Housecraft and Alan G. Sharpe Third Editio 8
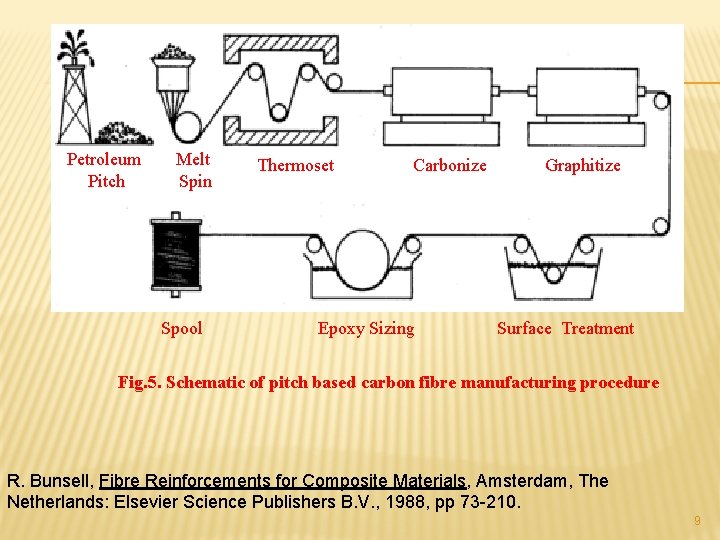
Petroleum Pitch Melt Spin Spool Thermoset Carbonize Epoxy Sizing Graphitize Surface Treatment Fig. 5. Schematic of pitch based carbon fibre manufacturing procedure R. Bunsell, Fibre Reinforcements for Composite Materials, Amsterdam, The Netherlands: Elsevier Science Publishers B. V. , 1988, pp 73 -210. 9
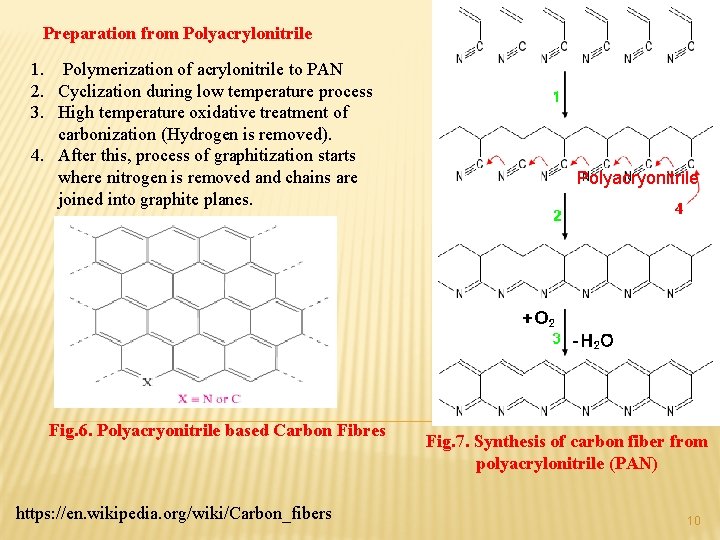
Preparation from Polyacrylonitrile 1. Polymerization of acrylonitrile to PAN 2. Cyclization during low temperature process 3. High temperature oxidative treatment of carbonization (Hydrogen is removed). 4. After this, process of graphitization starts where nitrogen is removed and chains are joined into graphite planes. Fig. 6. Polyacryonitrile based Carbon Fibres https: //en. wikipedia. org/wiki/Carbon_fibers Polyacryonitrile Fig. 7. Synthesis of carbon fiber from polyacrylonitrile (PAN) 10
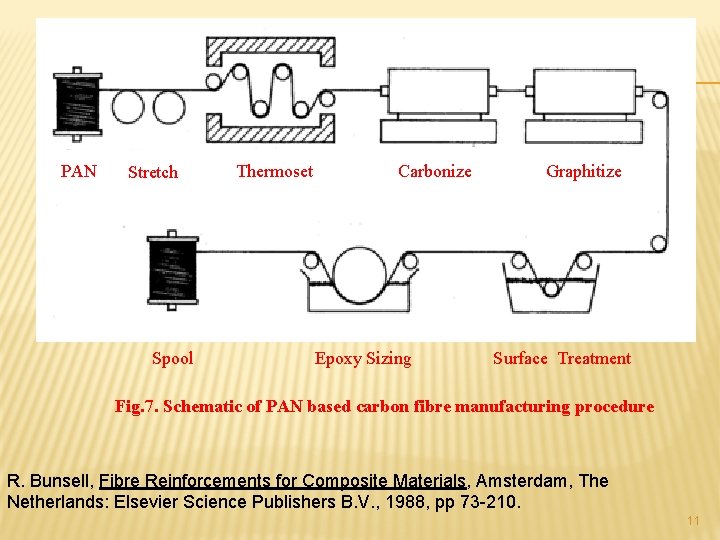
PAN Stretch Spool Thermoset Carbonize Epoxy Sizing Graphitize Surface Treatment Fig. 7. Schematic of PAN based carbon fibre manufacturing procedure R. Bunsell, Fibre Reinforcements for Composite Materials, Amsterdam, The Netherlands: Elsevier Science Publishers B. V. , 1988, pp 73 -210. 11
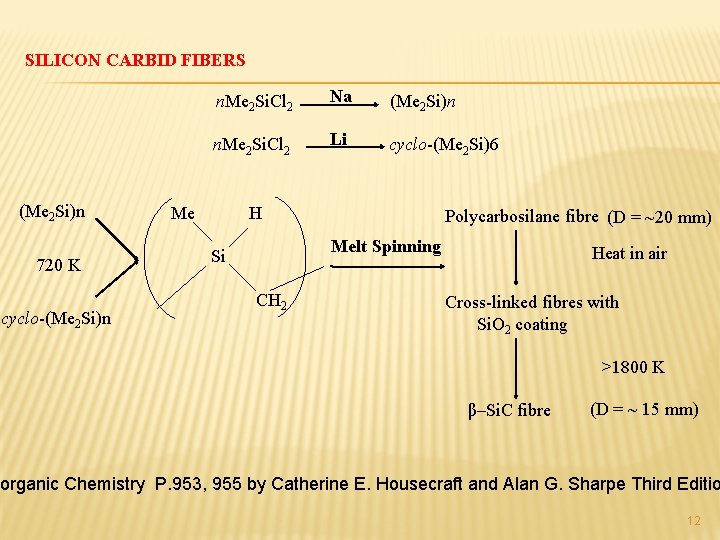
SILICON CARBID FIBERS (Me 2 Si)n 720 K cyclo-(Me 2 Si)n n. Me 2 Si. Cl 2 Na (Me 2 Si)n n. Me 2 Si. Cl 2 Li cyclo-(Me 2 Si)6 Me H Polycarbosilane fibre (D = ~20 mm) Melt Spinning Si CH 2 Heat in air Cross-linked fibres with Si. O 2 coating >1800 K β–Si. C fibre (D = ~ 15 mm) norganic Chemistry P. 953, 955 by Catherine E. Housecraft and Alan G. Sharpe Third Editio 12

Interesting, right? This is just a sneak preview of the full presentation. We hope you like it! To see the rest of it, just click here to view it in full on Power. Show. com. Then, if you’d like, you can also log in to Power. Show. com to download the entire presentation for free.
- Slides: 13