1 The Atmosphere u Calculating atmospheric properties Governing
- Slides: 14

1 The Atmosphere u Calculating atmospheric properties – Governing gas equations – Solutions for troposphere and stratosphere u The US standard atmosphere – Reading atmosphere tables – Non-standard atmosphere – Altitude definitions

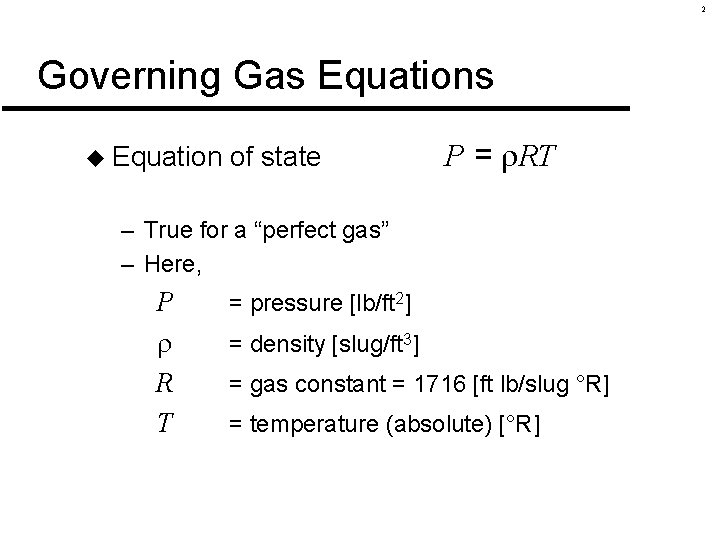
2 Governing Gas Equations u Equation of state P = RT – True for a “perfect gas” – Here, P R T = pressure [lb/ft 2] = density [slug/ft 3] = gas constant = 1716 [ft lb/slug °R] = temperature (absolute) [°R]

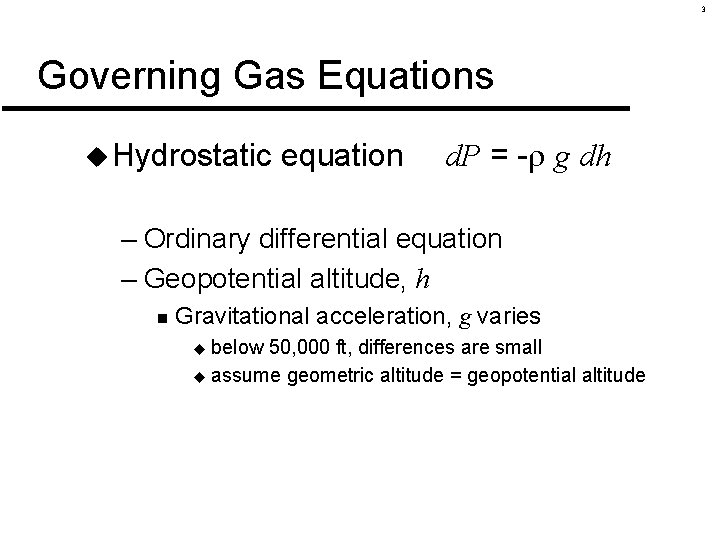
3 Governing Gas Equations u Hydrostatic equation d. P = - g dh – Ordinary differential equation – Geopotential altitude, h n Gravitational acceleration, g varies below 50, 000 ft, differences are small u assume geometric altitude = geopotential altitude u

Calculating Atmospheric Properties u Mathematical relationships for properties in the atmosphere – Based on governing equations and temperature model – Needed for predicting aircraft / rocket performance at different altitudes u Solve the hydrostatic equation (an ordinary differential equation) – Temperature used as boundary conditions – Two regions of interest require two solutions 4

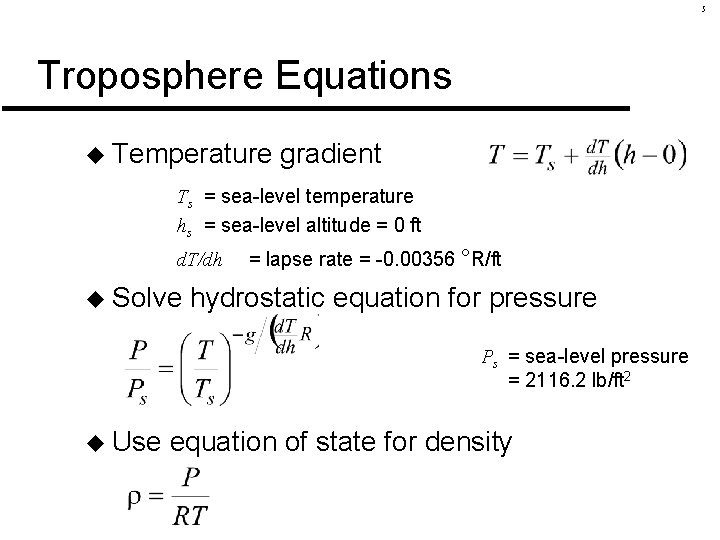
5 Troposphere Equations u Temperature gradient Ts = sea-level temperature hs = sea-level altitude = 0 ft d. T/dh u Solve = lapse rate = -0. 00356 °R/ft hydrostatic equation for pressure Ps = sea-level pressure = 2116. 2 lb/ft 2 u Use equation of state for density

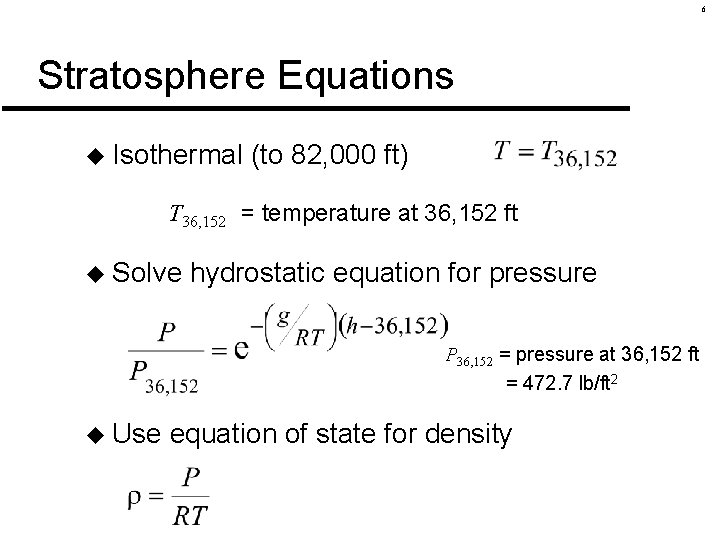
6 Stratosphere Equations u Isothermal (to 82, 000 ft) T 36, 152 = temperature at 36, 152 ft u Solve hydrostatic equation for pressure P 36, 152 = pressure at 36, 152 ft = 472. 7 lb/ft 2 u Use equation of state for density

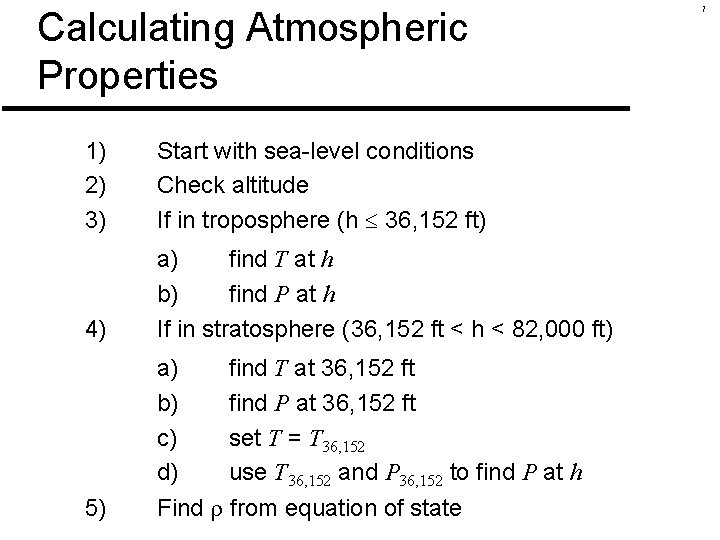
Calculating Atmospheric Properties 1) 2) 3) Start with sea-level conditions Check altitude If in troposphere (h 36, 152 ft) 4) a) find T at h b) find P at h If in stratosphere (36, 152 ft < h < 82, 000 ft) 5) a) find T at 36, 152 ft b) find P at 36, 152 ft c) set T = T 36, 152 d) use T 36, 152 and P 36, 152 to find P at h Find from equation of state 7

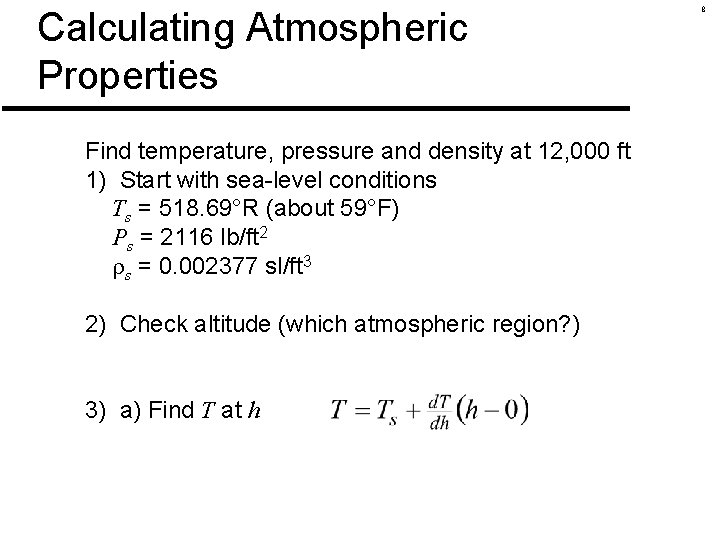
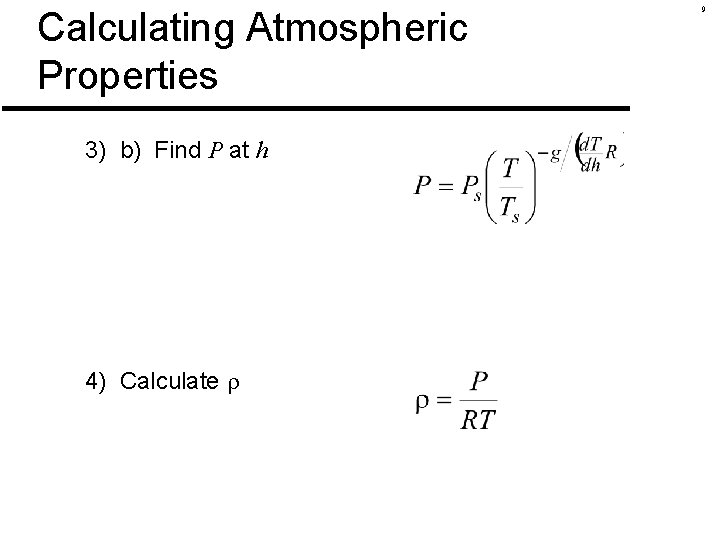
Calculating Atmospheric Properties Find temperature, pressure and density at 12, 000 ft 1) Start with sea-level conditions Ts = 518. 69°R (about 59°F) Ps = 2116 lb/ft 2 s = 0. 002377 sl/ft 3 2) Check altitude (which atmospheric region? ) 3) a) Find T at h 8

Calculating Atmospheric Properties 3) b) Find P at h 4) Calculate 9

10 The US Standard Atmosphere u Based on mean annual US temperature – Uses empirical temperature data – Reference of atmospheric conditions u Appendix B, Introduction to Aeronautics – Table B. 1 English (USCS) units, Table B. 2 SI units – Includes speed of sound, a and viscosity, m

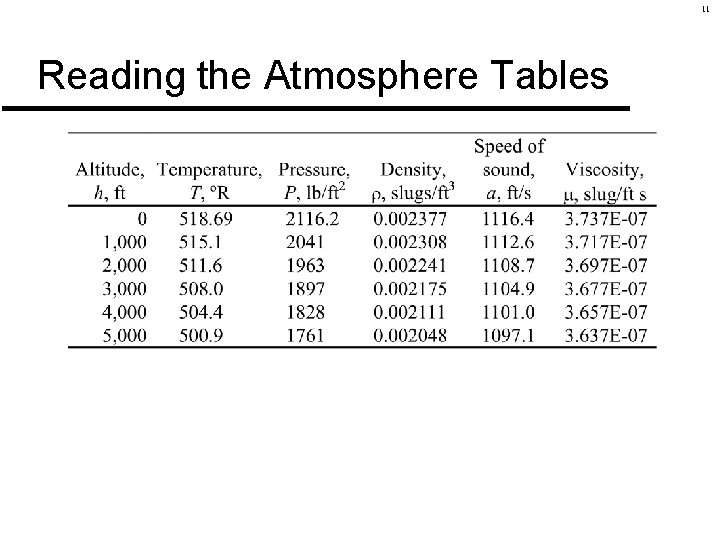
11 Reading the Atmosphere Tables

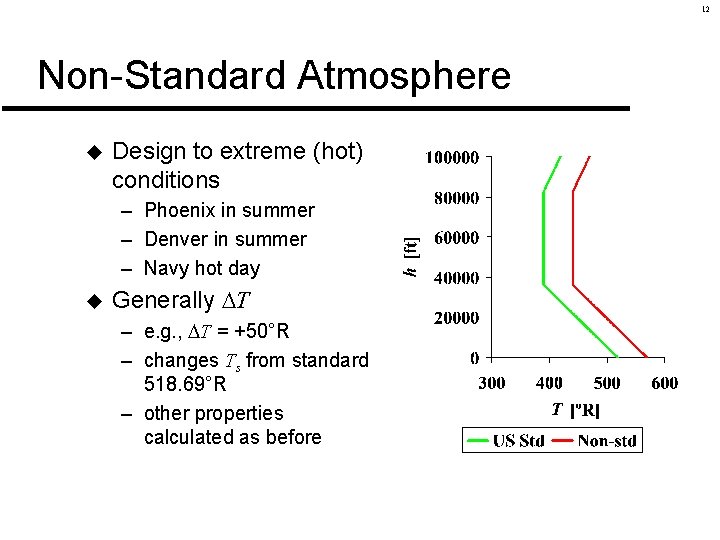
12 Non-Standard Atmosphere u Design to extreme (hot) conditions – Phoenix in summer – Denver in summer – Navy hot day u Generally T – e. g. , T = +50°R – changes Ts from standard 518. 69°R – other properties calculated as before

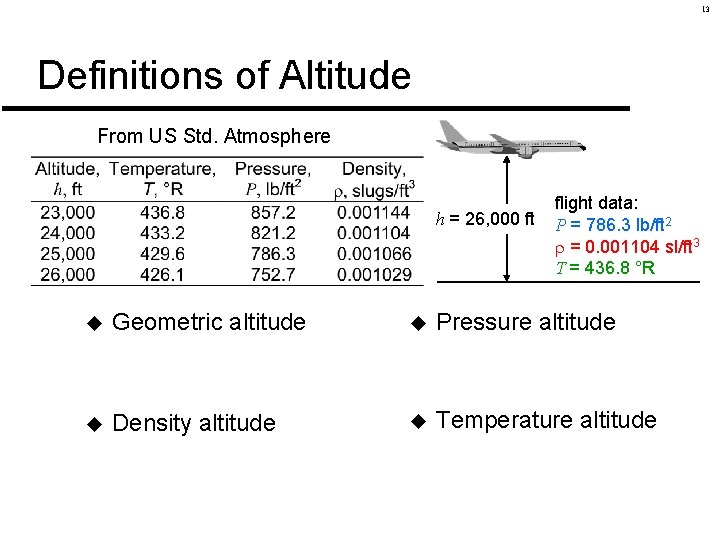
13 Definitions of Altitude From US Std. Atmosphere h = 26, 000 ft flight data: P = 786. 3 lb/ft 2 = 0. 001104 sl/ft 3 T = 436. 8 °R u Geometric altitude u Pressure altitude u Density altitude u Temperature altitude

14 Martian Atmosphere u Mostly carbon dioxide – close to perfect gas u Hydrostatic equation and equation of state still valid u Different constants and “sea-level” conditions R = gas constant = 1148 [ft lb/slug °R] or 192 [J/kg K] g = gravitational constant = 12. 21 [ft/s 2] or 3. 72 [m/s 2] T 0 = “sea-level” temperature = 410. 4 [°R] or 228 [K] P 0 = “sea-level” pressure = 16. 165 [lb/ft 2] or 774 [Pa] 0 = “sea-level” density = 3. 43× 10 -5 [slug/ft 3] or 0. 01768 [kg/m 3] d. T/dh = lapse rate = -0. 0016 [°R/ft] or -0. 0030 [K/m]