1 Stoichiometry Unit 7 Lesson 1 2 Stoichiometry






- Slides: 14

1 Stoichiometry Unit 7 Lesson 1

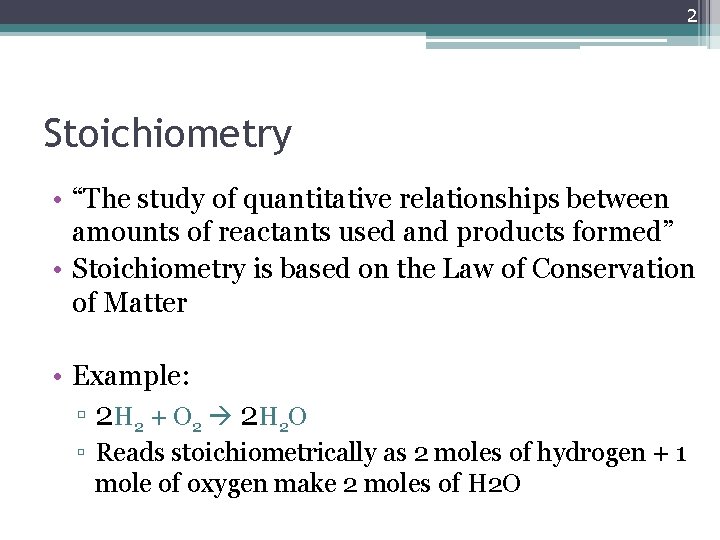
2 Stoichiometry • “The study of quantitative relationships between amounts of reactants used and products formed” • Stoichiometry is based on the Law of Conservation of Matter • Example: ▫ 2 H 2 + O 2 2 H 2 O ▫ Reads stoichiometrically as 2 moles of hydrogen + 1 mole of oxygen make 2 moles of H 2 O

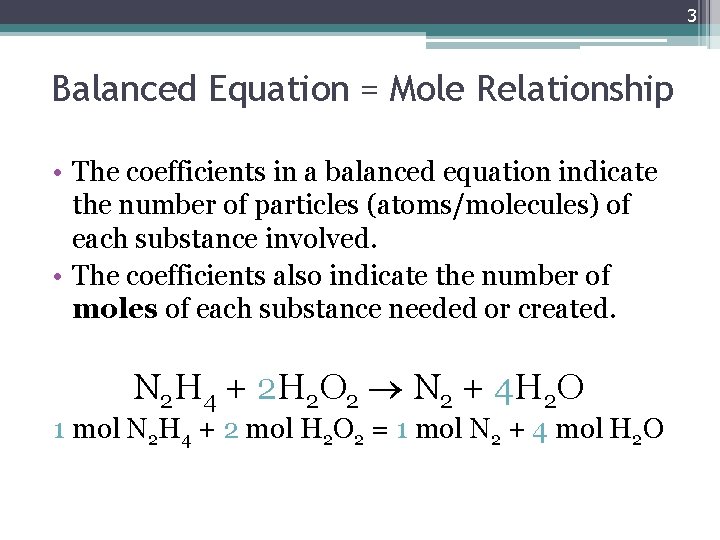
3 Balanced Equation = Mole Relationship • The coefficients in a balanced equation indicate the number of particles (atoms/molecules) of each substance involved. • The coefficients also indicate the number of moles of each substance needed or created. N 2 H 4 + 2 H 2 O 2 N 2 + 4 H 2 O 1 mol N 2 H 4 + 2 mol H 2 O 2 = 1 mol N 2 + 4 mol H 2 O

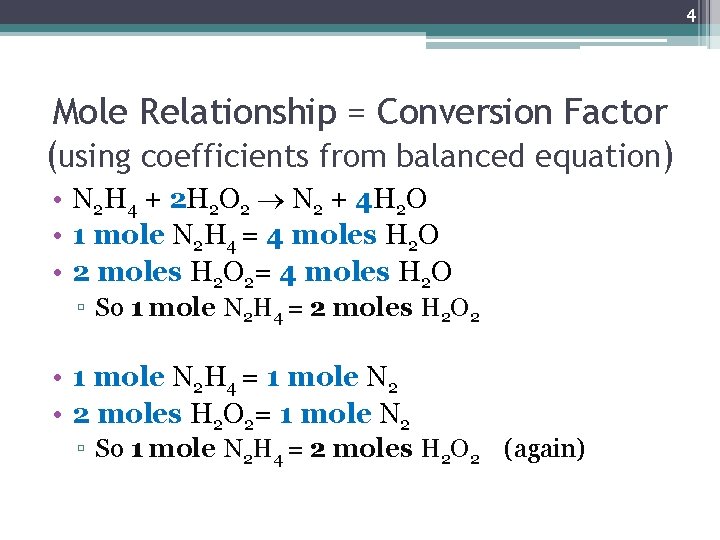
4 Mole Relationship = Conversion Factor (using coefficients from balanced equation) • N 2 H 4 + 2 H 2 O 2 N 2 + 4 H 2 O • 1 mole N 2 H 4 = 4 moles H 2 O • 2 moles H 2 O 2= 4 moles H 2 O ▫ So 1 mole N 2 H 4 = 2 moles H 2 O 2 • 1 mole N 2 H 4 = 1 mole N 2 • 2 moles H 2 O 2= 1 mole N 2 ▫ So 1 mole N 2 H 4 = 2 moles H 2 O 2 (again)

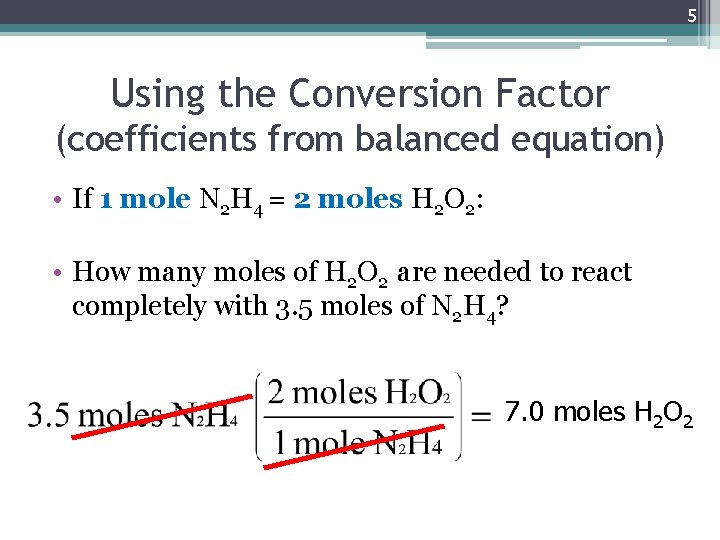
5 Using the Conversion Factor (coefficients from balanced equation) • If 1 mole N 2 H 4 = 2 moles H 2 O 2: • How many moles of H 2 O 2 are needed to react completely with 3. 5 moles of N 2 H 4? 7. 0 moles H 2 O 2

6 Using the Conversion Factor (coefficients from balanced equation) • If 1 mole N 2 H 4 = 4 moles H 2 O: • How many moles of water can be made if we react 3. 5 moles of N 2 H 4? 14 moles H 2 O

7 Steps for Solving Stoichiometry 1. Write & balance chemical equation 2. Plan conversions using coefficients from balanced equation 3. Write your conversion factors and check units 4. Calculate 5. Adjust for significant figures and include units

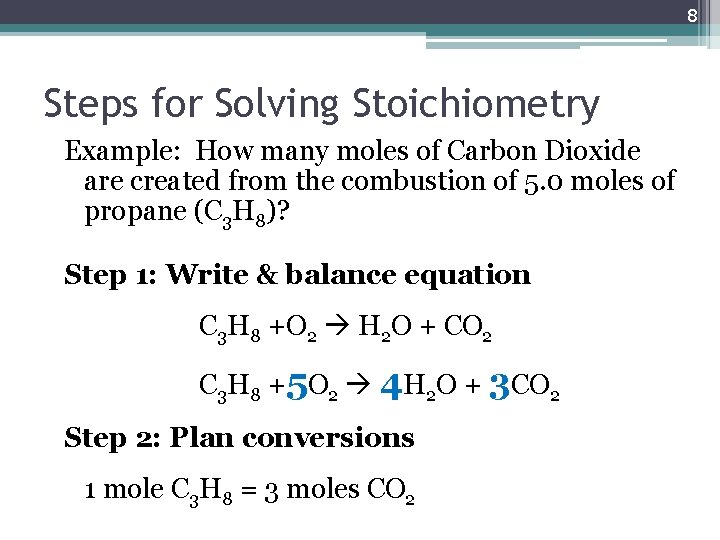
8 Steps for Solving Stoichiometry Example: How many moles of Carbon Dioxide are created from the combustion of 5. 0 moles of propane (C 3 H 8)? Step 1: Write & balance equation C 3 H 8 +O 2 H 2 O + CO 2 C 3 H 8 +5 O 2 4 H 2 O + 3 CO 2 Step 2: Plan conversions 1 mole C 3 H 8 = 3 moles CO 2

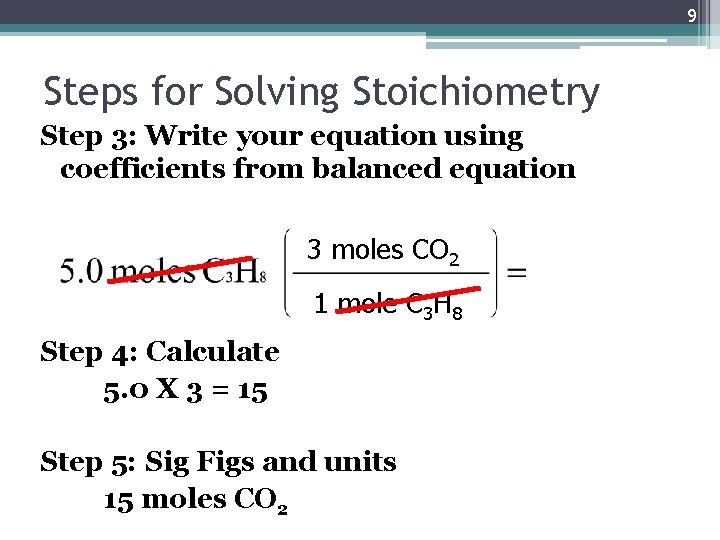
9 Steps for Solving Stoichiometry Step 3: Write your equation using coefficients from balanced equation 3 moles CO 2 1 mole C 3 H 8 Step 4: Calculate 5. 0 X 3 = 15 Step 5: Sig Figs and units 15 moles CO 2

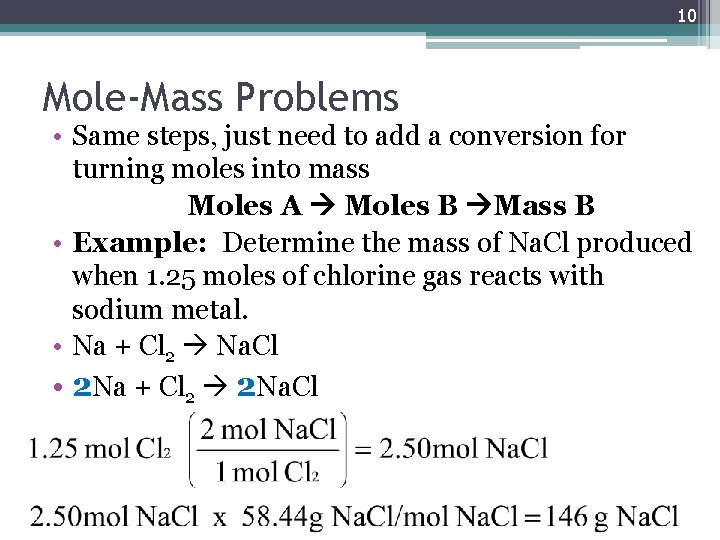
10 Mole-Mass Problems • Same steps, just need to add a conversion for turning moles into mass Moles A Moles B Mass B • Example: Determine the mass of Na. Cl produced when 1. 25 moles of chlorine gas reacts with sodium metal. • Na + Cl 2 Na. Cl • 2 Na + Cl 2 2 Na. Cl

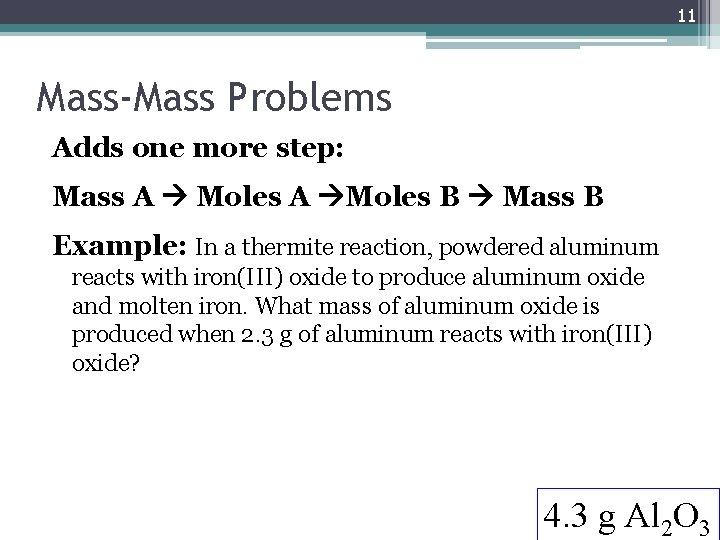
11 Mass-Mass Problems Adds one more step: Mass A Moles A Moles B Mass B Example: In a thermite reaction, powdered aluminum reacts with iron(III) oxide to produce aluminum oxide and molten iron. What mass of aluminum oxide is produced when 2. 3 g of aluminum reacts with iron(III) oxide? 4. 3 g Al 2 O 3

12 Sample Problem What volume of hydrogen gas, along with nitrogen gas, is necessary to produce 16. 0 L of ammonia gas (NH 3) at STP? 24. 0 L H 2

13 Sample Problem How many moles of bromine (Br 2) are produced when fluorine reacts with 347. 5 g of potassium bromide? 1. 460 mol Br 2

14 Sample Problem How many liters of oxygen gas are necessary for the combustion of 340. g of ethanol (C 2 H 5 OH) gas at 0°C and 1 atm? 497 L O 2