1 States of Matter A brief overview 1

1 States of Matter A brief overview
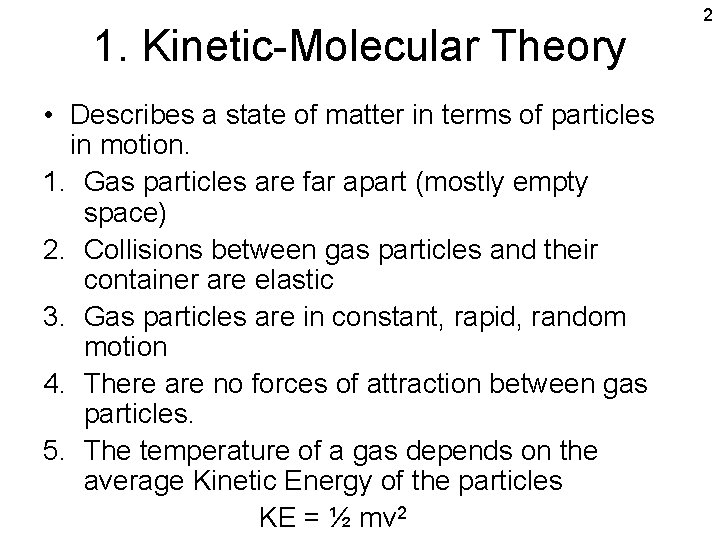
1. Kinetic-Molecular Theory • Describes a state of matter in terms of particles in motion. 1. Gas particles are far apart (mostly empty space) 2. Collisions between gas particles and their container are elastic 3. Gas particles are in constant, rapid, random motion 4. There are no forces of attraction between gas particles. 5. The temperature of a gas depends on the average Kinetic Energy of the particles KE = ½ mv 2 2

Gas Properties • Expansion = expand to fill container • Fluidity = gas particles glide easily past each other • Low Density = because particles are so far apart, they are less dense than other states • Compressibility = gases can be compressed into a small volume • Diffusion = spontaneous mixing of gases due to random motion • Effusion = process by which gas particles pass through a tiny opening

Liquid Properties • Definite volume and takes shape of its container • In constant motion, but particles are closer together (attractive forces are therefore more effective) • Fluid = particle mobility, usually flows downhill naturally due to gravity • Relatively high density = 100 x more dense than in gas state • Relative incompressibility = due to packed particles • Ability to diffuse = mix with other liquids (slower than gases, increases with temp)

Liquid Properties Cont. • Surface Tension = a force that tends to pull adjacent parts of a liquid’s surface together (creates spherical droplets) • Capillary Action = attraction of the surface of a liquid to the surface of a solid • Evaporation = particles escape the surface of a non-boiling liquid to enter the gas state • Boiling = change of liquid to bubbles of vapor that appear throughout the liquid • Formation of Solids = when a liquid is cooled, energy of particles decrease

Solid Properties • Hold relatively fixed positions • Particles are closely packed, highest intermolecular forces • Only vibrational movement, more ordered • 2 Types of Solids 1. Crystalline Solids (consist of crystals) 2. Amorphous solids (particles arranged randomly • Definite Shape & Volume = some are geometrically regular

Solid Properties Cont. • Definite melting point = the kinetic energy of the particles overcome the attractive forces holding them together (amorphous solids don’t have definite melting point, and are often called supercooled liquids) • high density and incompressibility = substances are most dense when a solid • Low rate of Diffusion = extremely slow • Crystalline Solids arranged in crystal structures

3 2. Intermolecular Forces • Inter- vs. Intra • Inter means between two separate particles • Intra refers to a force (or bond) within a particle • Example: CO 2

• Dispersion Forces: – Weakest – Between nonpolar molecules – Example: oxygen • Dipole-Dipole Forces: – Stronger – Between polar molecules – Example: HCl • Hydrogen bonds: – Strongest – Between hydrogens and F, O or N – Example: water 4
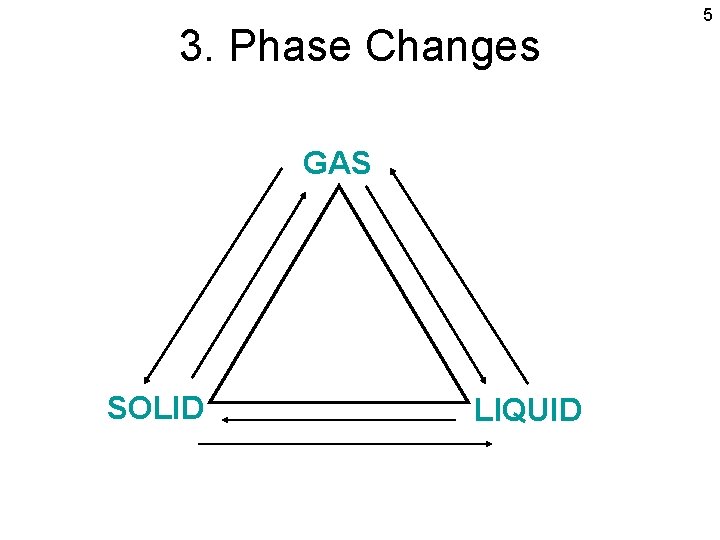
3. Phase Changes GAS SOLID LIQUID 5
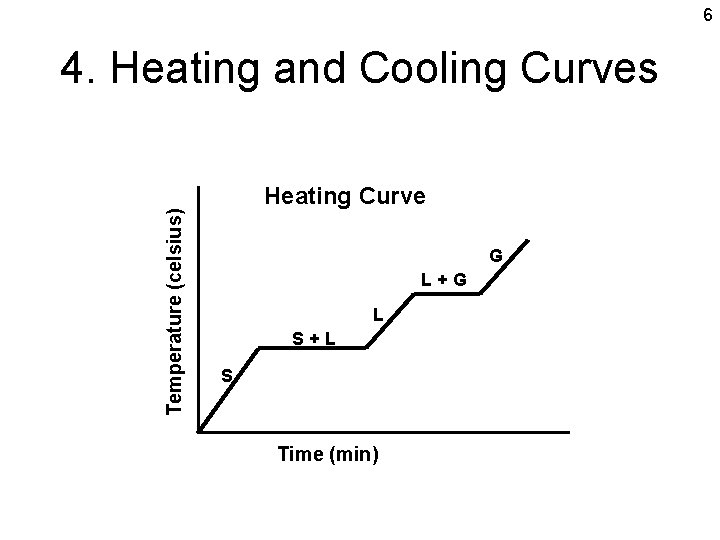
6 Temperature (celsius) 4. Heating and Cooling Curves Heating Curve G L+G L S+L S Time (min)
- Slides: 11