1 State symbols a subscript letter in brackets
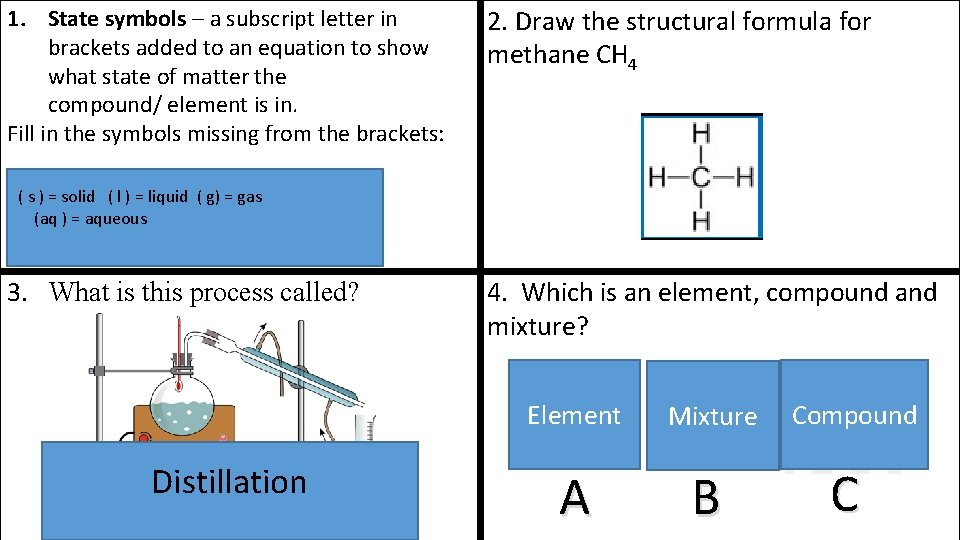
1. State symbols – a subscript letter in brackets added to an equation to show what state of matter the compound/ element is in. Fill in the symbols missing from the brackets: = solid ) = liquid ( s() =) solid ( l ) =(liquid ( g) = gas( ((aq)) == aqueous ) = gas 3. What is this process called? Distillation 04 September 2021 2. Draw the structural formula for methane CH 4 4. Which is an element, compound and mixture? Element Mixture A B Compound C

Good progress: • Identify substances as pure or formulations Outstanding Progress: • Use melting point and boiling point data to distinguish pure from impure substances. • Describe the tests for hydrogen, oxygen, carbon dioxide and chlorine

Formulations How is paint made? BBC Teach Watch this video and answer the questions below: 1. Give two uses of paint. 2. What is paint made of? White p_____, t_____, b_______, d______ 3. Paint is a formulation - So is a formulation a pure substance or a mixture? A formulation is a mixture that has been designed as a useful product.
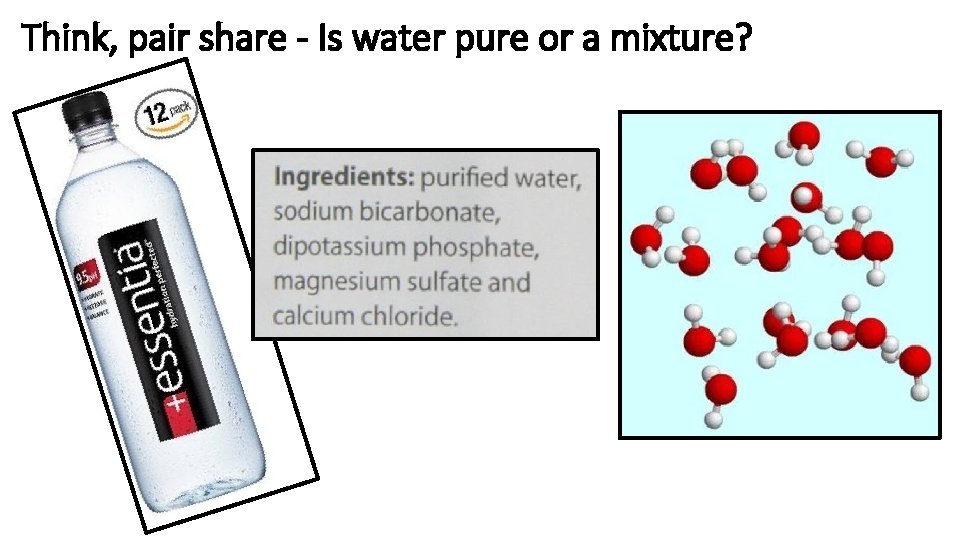
Think, pair share - Is water pure or a mixture?

Exam practice: Self assess: Pure substance in chemistry = A single element or a single compound Pure substance in everyday life = A substance that has had nothing added to it ? 2

Pure or a formulation? Use the statements to complete the table. Pure substance Milk Formulation Water Fuel Cotton Milk Paint Cleaning agent Medicine r e t a r W e s i l i t r Some foods Fe Some foods Gold Medicine Formulation Cotton Fuel Gold

Excellent Progress: Use melting point and boiling point data to distinguish pure from impure substances. Describe the graph. What can you say about the temperature at the melting point or boiling point? EXTENSION! This is a graph for a pure substance. Can you predict what a graph would look like for an impure substance?
Carry out practical on cooling of stearic acid
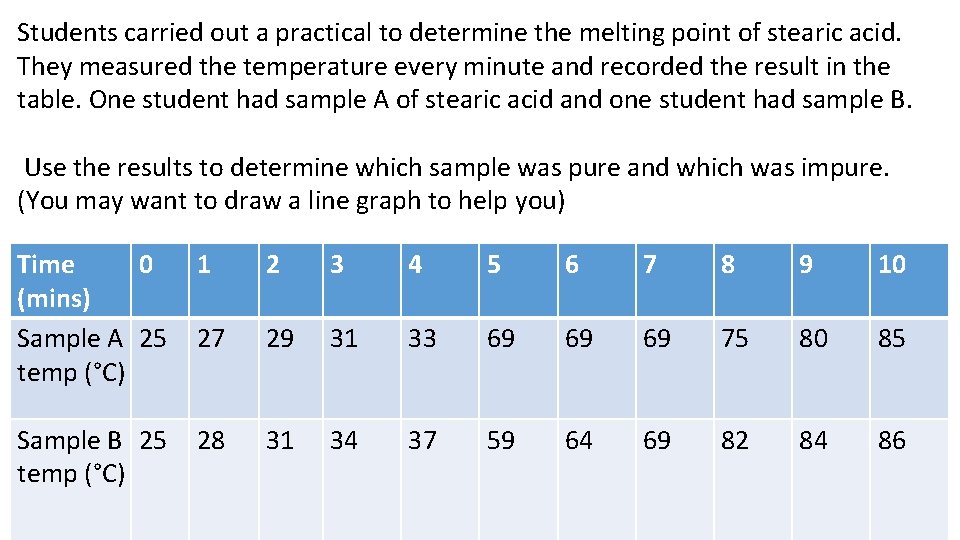
Students carried out a practical to determine the melting point of stearic acid. They measured the temperature every minute and recorded the result in the table. One student had sample A of stearic acid and one student had sample B. Use the results to determine which sample was pure and which was impure. (You may want to draw a line graph to help you) Time 0 (mins) Sample A 25 temp (°C) 1 2 3 4 5 6 7 8 9 10 27 29 31 33 69 69 69 75 80 85 Sample B 25 temp (°C) 28 31 34 37 59 64 69 82 84 86

Identify four common gases Test for hydrogen Test for chlorine Go to each activity station and complete the test for the common gas and rotate clockwise Test for carbon dioxide Test for oxygen Test for hydrogen – What happens when you hold a burning splint at the end of a test tube of hydrogen? Test for oxygen – What happens when you hold a glowing splint inside a test tube of oxygen? Test for carbon dioxide – What happens when carbon dioxide is bubbled through limewater? Test for chlorine - What happens when you put damp litmus paper in chlorine?
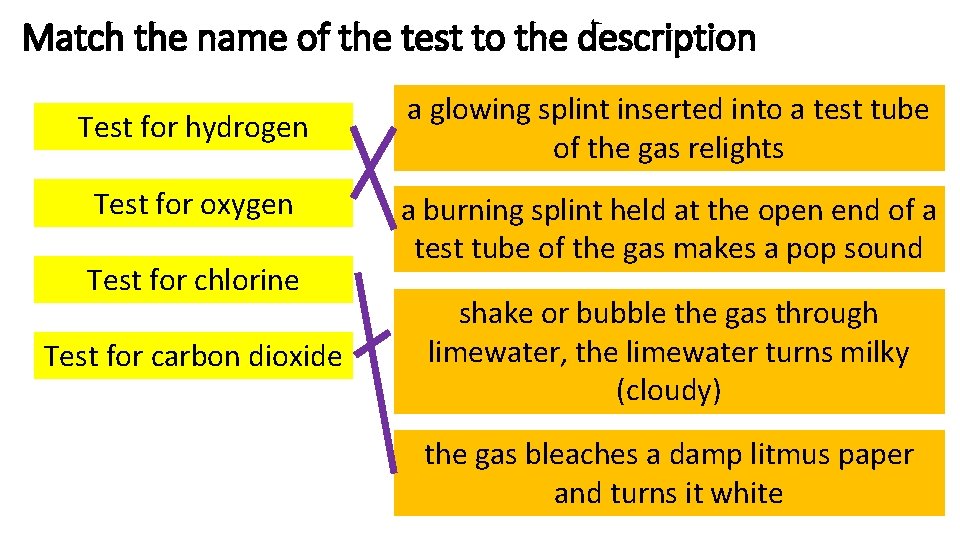
Match the name of the test to the description Test for hydrogen Test for oxygen Test for chlorine Test for carbon dioxide a glowing splint inserted into a test tube of the gas relights a burning splint held at the open end of a test tube of the gas makes a pop sound shake or bubble the gas through limewater, the limewater turns milky (cloudy) the gas bleaches a damp litmus paper and turns it white
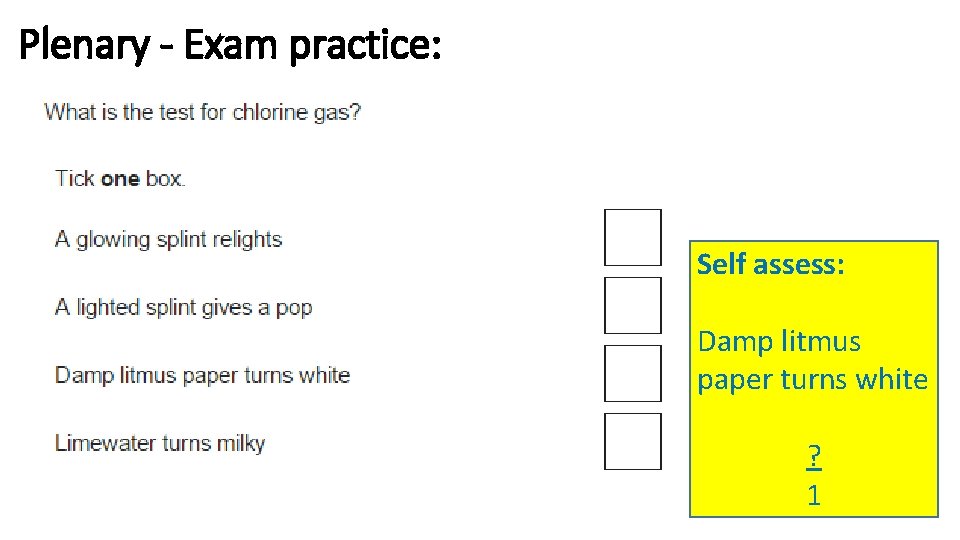
Plenary - Exam practice: Self assess: Damp litmus paper turns white ? 1
- Slides: 12