1 Solutions 2 Zanichelli editore 2016 Solutions and


- Slides: 14

1

Solutions 2 © Zanichelli editore 2016

Solutions and miscible substances Solutions are made up of two or more chemical species that are mixed to form a homogeneous system. The main characteristic of solutions is that they are formed by miscible substance. Miscibility of substances depends on their physical state as well as on the bonds between solvent and solute. 3 © Zanichelli editore 2016

Miscibility and the physical states Gases are miscible in any ratio, regardless of their chemical nature. Miscibility of a liquid solution’s components depends on the strength of the bonds that break and form. Solid solutions are formed only if two solids have similar crystal structures. 4 © Zanichelli editore 2016

Solid solutions There are some mineral groups of silicates which are solid solutions called isomorphic mixtures. Man-made metal mixtures (alloys) can be homogeneous or heterogeneous. Homogenous alloys are actual solid solutions, while heterogeneous solutions have distinct phases. 5 © Zanichelli editore 2016

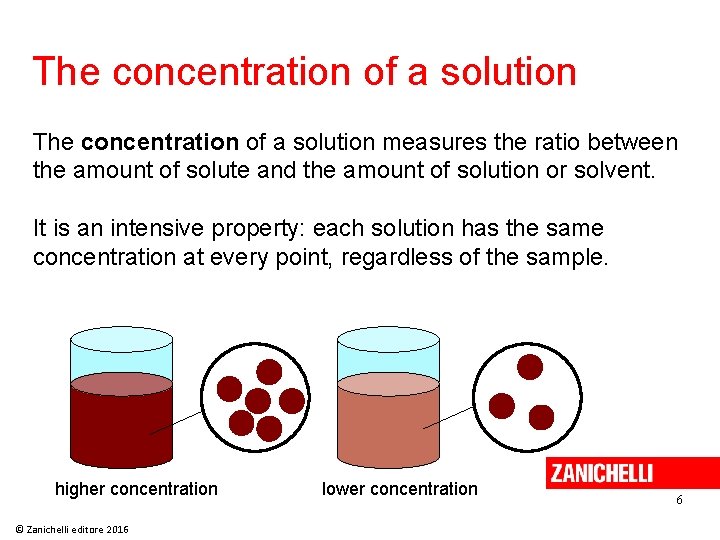
The concentration of a solution measures the ratio between the amount of solute and the amount of solution or solvent. It is an intensive property: each solution has the same concentration at every point, regardless of the sample. higher concentration © Zanichelli editore 2016 lower concentration 6

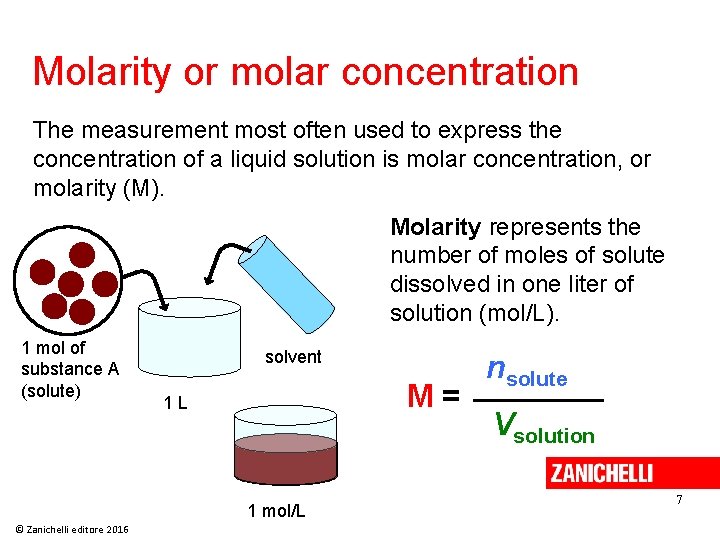
Molarity or molar concentration The measurement most often used to express the concentration of a liquid solution is molar concentration, or molarity (M). Molarity represents the number of moles of solute dissolved in one liter of solution (mol/L). 1 mol of substance A (solute) solvent M= 1 L 1 mol/L © Zanichelli editore 2016 nsolute Vsolution 7

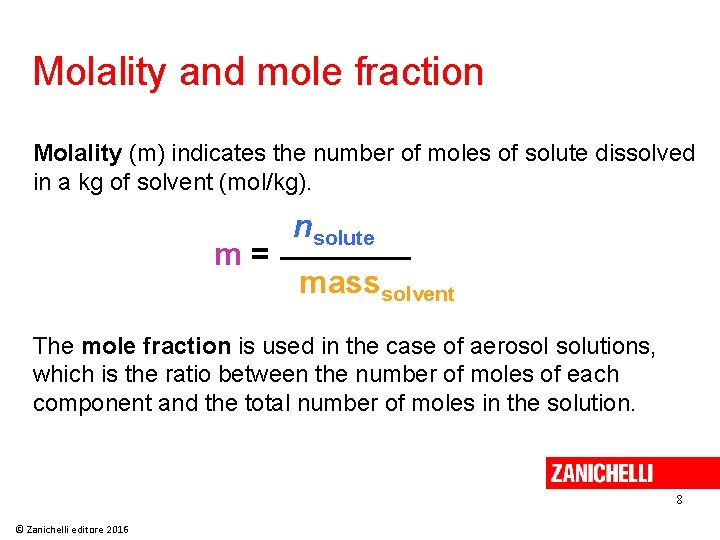
Molality and mole fraction Molality (m) indicates the number of moles of solute dissolved in a kg of solvent (mol/kg). m= nsolute masssolvent The mole fraction is used in the case of aerosol solutions, which is the ratio between the number of moles of each component and the total number of moles in the solution. 8 © Zanichelli editore 2016

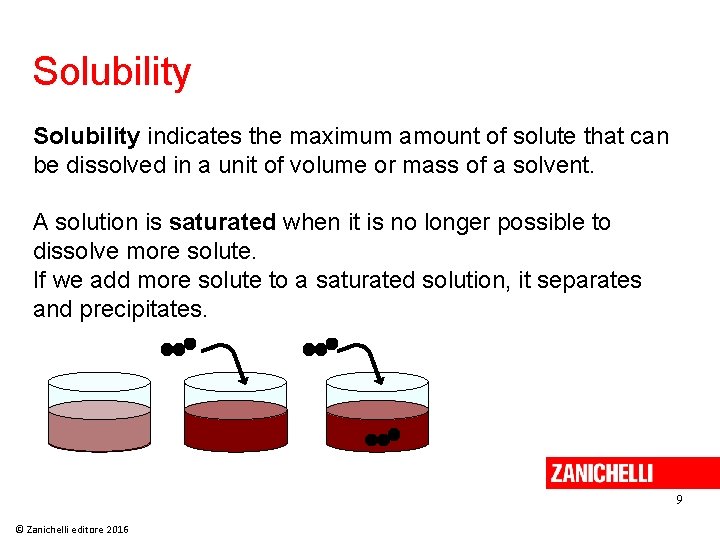
Solubility indicates the maximum amount of solute that can be dissolved in a unit of volume or mass of a solvent. A solution is saturated when it is no longer possible to dissolve more solute. If we add more solute to a saturated solution, it separates and precipitates. 9 © Zanichelli editore 2016

Solubility and temperature For most solid solutes, solubility increases when temperature increases. For gases, solubility in water generally decreases when temperature increases. 10 © Zanichelli editore 2016

Colligative properties Some properties of aqueous solutions - called colligative properties - depend on the concentration of a solute rather than on its chemical nature. Two important properties are: • the colligative freezing point depression (reduction of the melting temperature); • the boiling point elevation (an increase in the boiling temperature). 11 © Zanichelli editore 2016

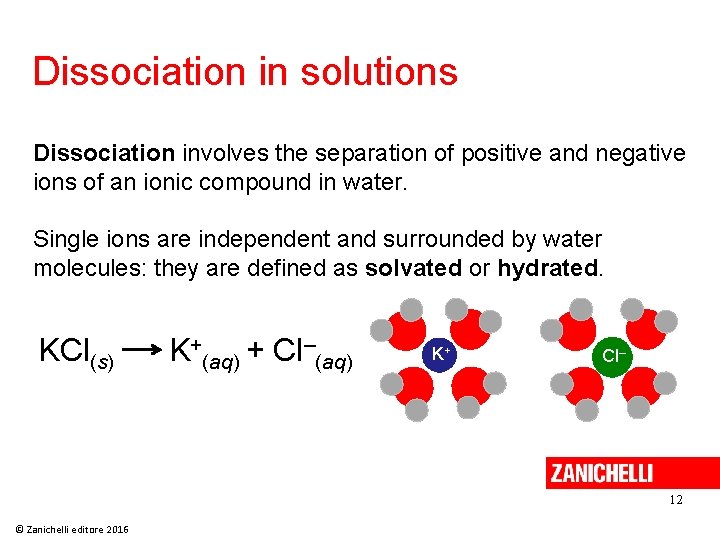
Dissociation in solutions Dissociation involves the separation of positive and negative ions of an ionic compound in water. Single ions are independent and surrounded by water molecules: they are defined as solvated or hydrated. KCl(s) K+(aq) + Cl–(aq) K+ Cl– 12 © Zanichelli editore 2016

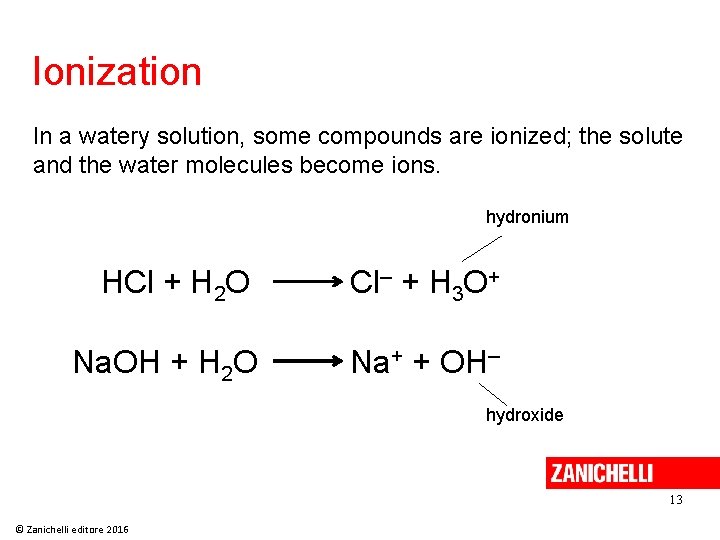
Ionization In a watery solution, some compounds are ionized; the solute and the water molecules become ions. hydronium HCl + H 2 O Cl– + H 3 O+ Na. OH + H 2 O Na+ + OH– hydroxide 13 © Zanichelli editore 2016

Metals and non-metals in water Many metals and oxides of non-metals and metals also react in water: • oxides of non-metals form oxoacids; • metals and oxides of metals form hydroxides. 14 © Zanichelli editore 2016