1 REACTIVE CHEMICAL HAZARDS This module provides a



















































- Slides: 58

1 REACTIVE CHEMICAL HAZARDS This module provides a systematic and simplified way to to understand identify reactive chemical hazards (RCH in short).

2 About the module • This module is developed for undergraduate Chemical Engineering students prior to plant design project. • The goal of this module is to: 1. 2. 3. 4. 5. Raise awareness of RCH study Motivate students to study RCH by showcase the consequences from RCH Help students to understand the nature of RCH Provide tools for students to identify RCH Provide examples for students to estimate the extent of RCH • Control and Prevention of RCH will not be discussed in details in this module as it involves substantial technical and theoretical learning.

3 What can happen?

4 Table of Contents • Introduction • Definition of Hazard and Risk • Definition of Reactive Chemical Hazards (RCH) • Desired and Undesired Reactions • Type of RCH • Process of RCH Studies • Understanding • Qualify RCH • Quantify RCH • Control and Prevention • References

5 Hazard and Risk Definition • “ An intrinsic chemical, physical, societal, economic or political condition that has the potential for causing damage to a risk receptor (people, property or the environment)” • • “A measure of the human injury, environmental damage or economic loss in terms of both the frequency and the magnitude of the loss of injury” • • • -- Definition from the Centre for Chemical Process Safety Introduction Type of RCH Process of RCH Studies References

6 Reactive Chemical Hazard (RCH) Definition • Reactivity: • Tendency of substances to undergo chemical change • A reactive chemical hazard (RCH) is a situation with the potential for an UNCONTROLLED chemical reaction – with significant increases in Temperature, Pressure, and/or gas evolution – that can result directly or indirectly in serious harm to people, property or the environment Introduction Type of RCH Process of RCH Studies References

7 Nature of RCH… • Chemical reactions involve energy changes • Most reactions liberate energy as heat – exothermic • Some energy is absorbed into products – endothermic • RCH involves high rates of energy release • Too high to be absorbed by the immediate environment of the reacting system • Result in damages • Safeguarding information is provided later in this presentation Introduction Type of RCH Process of RCH Studies References

8 Desired and Undesired Reactions • Desired reactions can be controlled • Process Hazards Analysis – assess effect of deviations on process conditions • E. g. temperature, feed rate, pressure, etc… • Undesired reactions must be prevented • Types of undesired reactions: • Side reactions • Mixing of incompatible chemicals • Formation of self-reacting chemicals • Unintended decomposition Introduction Type of RCH Process of RCH Studies References

9 Significant Disasters in History Involving Desired Reactions Involving Undesired Reactions Seveso, Italy 1976 Negaunee, Michigan 1878 (alkaline hydrolysis runaway reaction) Nitro-Glycerine Tragedy (self-reacting impact sensitive) Jacksonville, Florida 2007 (T 2 laboratory explosion due to runaway reactions) Introduction Type of RCH Bhopal, India 1984 (mixing of incompatible chemicals: MIC+water) Process of RCH Studies References

10 Recap • After reading the case studies, which of the following is NOT a cause from chemical reactivity disasters? A. Untrained labours B. False alarm C. Uncontrolled reaction D. Lack of responsibility E. None of the above ALL contributes to a potential disaster!!! Introduction Type of RCH Process of RCH Studies References

11 Types of RCH? 1. Self-Reacting impact-sensitive or thermally sensitive materials 2. Runaway reactions 3. Chemical incompatibility Introduction Type of RCH Process of RCH Studies References

12 Self-Reacting impact-sensitive or thermally sensitive materials • When subjected to heat or impact, these chemicals may rapidly decompose, resulting in a potentially explosive release of energy. • These are undesired or unintentional reactions • Examples: • organic peroxides • copper acetylide Introduction Type of RCH Process of RCH Studies References

13 Chemical Incompatibility • Between two or more substances • These hazards occur when a chemical is suddenly mixed or comes into contact with another chemical, resulting in a violent reaction. • These are undesired and unintentional reactions • Examples: • Strong acids and strong bases • Water reactive materials (sodium metal and water) • Pyrophoric materials (iron sulfide and oxygen) Introduction Type of RCH Process of RCH Studies References

14 Runaway Reactions • Predominantly involves desired/intentional reactions • Self-reactive chemicals or mixtures • In an out-of-control reaction involving a chemical or chemical mixture, the rate at which heat is generated exceeds the rate at which it is removed through cooling media and surroundings. For example: • Polystyrene batch reaction and loss of jacketed cooling control • Acetylene hydrogenation reaction and inadequate heat removal per gas flow through reactor • Usually occur during scale-up as the system becomes more adiabatic as it increases in size Introduction Type of RCH Process of RCH Studies References

15 Recap • “In order to prevent RCH for undesired chemical reactions, we need to establish sufficient control via safety mechanisms” True or False? • Answer: False • “In order to prevent Reactive Chemical Hazards for undesired chemical reactions, the easiest way is to PREVENT incompatible materials from contacting” Introduction Type of RCH Process of RCH Studies References

16 BREAK TIME Bhopal disaster made into a movie (watch the trailer here) Introduction Type of RCH Process of RCH Studies References

17 Process for RCH Studies Step 1: What reactivity hazard question is being studied? Undesired Single chemical: instability More than one chemical: incompatibility Desired Concern for runaway Step 2: Conduct Literature Research (qualitative review) Step 3: Is information researched sufficient to make a definitive decision on reactivity hazard for specific situation under consideration? Conclude investigation, document findings and necessary recommendations Introduction Type of RCH YES NO Process of RCH Studies Use qualified labs to conduct controlled/safe lab testing. Quantification can involve calorimetry test for heat release and kinetics References

18 Step 1: Understanding • After defining the system boundary, ask this question: Which of the two scenario is involved? 1. Chemical reacting by design/Desired reaction • e. g. desired production 2. Chemical reacting by accident/Undesired reaction • e. g. inadvertent mixing of chemicals Introduction Type of RCH Process of RCH Studies References

19 Step 2: Qualify RCH – a screening tool Sources to Identify RCH 1. Reactive Functional Groups 2. MSDS (Material Safety Data Sheet) 3. International Chemical Safety Data Card 4. NOAA reactivity worksheet 5. S 2 S RCH online assessment 6. Oxygen Balance Introduction Type of RCH Process of RCH Studies References

20 Reactive Functional Groups • The presence of certain functional groups is considered an indicator of reactivity. • Some examples of chemicals containing functional groups can be considered potentially reactive: • -NO 2 organic nitro compounds • N=N=N organic/inorganic azides, a linear anion • -O-O-, -O-OH organic/inorganic peroxide and hydroperoxide compounds • -C≡C- triple bonded carbon atoms as in acetylene and acetylenic compounds Introduction Type of RCH Process of RCH Studies References

21 Reactive Functional Groups • Simplest reactivity screening method possible and serves as a guideline for further analysis. • Cornell University’s EHS website • List of Functional Groups Properties and Hazards, including Reactivity Hazards Introduction Type of RCH Process of RCH Studies References

22 Recap • Which of the following functional group reacts vigorously with concentrated mineral acids? (Hint: available in Cornell’s EHS website) • A. aldehydes • B. aliphatic amines • C. alicyclic hydrocarbons • D. alcohols • Answer: • B. aliphatic amines Introduction Type of RCH Process of RCH Studies References

23 Reactive Functional Groups • Useful source: EPA’s Chemical Compatibility Chart • To determining the compatibility of chemicals and the result of mixing Introduction Type of RCH Process of RCH Studies References

24 EPA Chemical Compatibility Chart Available Here: Introduction Type of RCH Process of RCH Studies References

25 Recap Q: Using the EPA compatibility chart, what will happen by mixing amides and oxidizing mineral acids? Introduction Type of RCH Process of RCH Studies References

26 Recap Solution Answer: Toxic gas formation and Heat Introduction Type of RCH Process of RCH Studies References

27 MSDS (Material Safety Data Sheet) • Contact your supplier for MSDS first • Under Section ‘Stability and Reactivity’ • Limited details Introduction Type of RCH Process of RCH Studies References

28 International Chemical Safety Card • If MSDS is not available, this is the secondary source • Available on ILO (International Labour Organization) Website • OR Google international chemical safety data card • CDC (Centers for Disease Control and Prevention) website • Start search: Introduction Type of RCH Process of RCH Studies References

29 Recap • Using ICSD cards, find out at least one key reactive hazard for lead chromate. • Solution: • Decomposes on heating. This produces toxic fumes including lead oxides. • Reacts violently with many substances such as combustible substances, amines, bases and metals. This generates fire and explosion hazard. Introduction Type of RCH Process of RCH Studies References

30 NOAA Reactivity Worksheet (CRW) • A software to find out hazards of: • Chemicals: a database of reactivity information for more than 5, 000 common hazardous chemicals • Reactive Groups: chemicals are assigned to 64 reactive groups to generate reactivity predictions • Mixtures of chemicals: rule-based algorithm allowing you to virtually “mix” chemicals to determine compatibility of two or more chemicals • Available online Introduction Type of RCH Process of RCH Studies References

31 NOAA: Use functional groups instead of chemicals when… • You know the chemical class of a chemical, but not its exact name or CAS (Chemical Abstracts Service) registry number. • For instance, you may be able to tell that it's a powdered metal • A new compound that hasn't yet been included in major chemical databases. • You work with (or store) proprietary chemicals that are not included in the CRW's chemical database. In this case, you can either use a reactive group to approximate the chemical or you could create a custom chemical datasheet in the CRW. Introduction Type of RCH Process of RCH Studies References

32 NOAA Example - Single chemical Search Results When working with mixture, see next slide Introduction Type of RCH Process of RCH Studies References

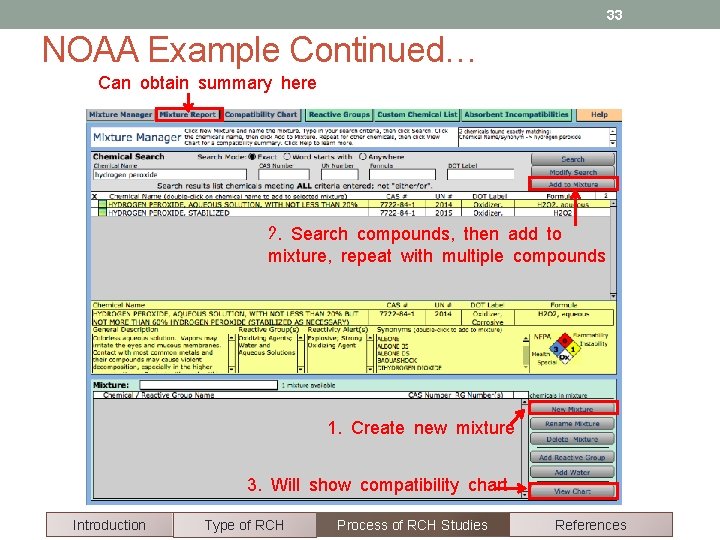
33 NOAA Example Continued… Can obtain summary here 2. Search compounds, then add to mixture, repeat with multiple compounds 1. Create new mixture 3. Will show compatibility chart Introduction Type of RCH Process of RCH Studies References

34 Recap: Try this on NOAA… • Mix sodium hypochlorite and hydrogen peroxide together • What are the predicted hazards? Introduction Type of RCH Process of RCH Studies References

35 S 2 S RCH Online Assessment • To assess the hazardous properties of your substances or the hazardous properties of your process. • Select “Self assessment Reactivity Hazards” Introduction Type of RCH Process of RCH Studies References

36 S 2 S RCH Online Assessment • Follow the prompt from S 2 S online assessment, practice with sodium azide: Na. N 3 • You can use facts regarding Na. N 3 in MSDS or ICSC • Results in a summary report indicating: • Deficiencies in Good Practice • Adequate fulfillment of needs • Insufficient Knowledge and Practices • Instruction: • Take a screenshot of your assessment and submit HERE. Introduction Type of RCH Process of RCH Studies References

37 Oxygen Balance (OB, or OB%) • Used to indicate degree to which an explosive can be oxidized • If an explosive molecule contains just enough chemically-bonded oxygen to convert all of its carbon to carbon dioxide, all of its hydrogen to water, and all of its metal to metal oxide with NO EXCESS, the molecule is said to have a zero oxygen balance • Positive OB: molecule contains more chemically-bonded oxygen than is needed. • Negative OB: molecule contains less chemically-bonded oxygen than is needed. • For OB>-200, it is considered potential high risk Important clarification - Use of the oxygen-balance tool implies the presence of oxidizing groups (functional groups) like nitro, nitrate, chlorate, peroxy in the molecule. *Using the oxygen balance without this additional information often will lead in wrong (nonsensical) results. Introduction Type of RCH Process of RCH Studies References

38 Oxygen Balance (OB, or OB%) Lothrop and Handrick (1949) defined OB and will be calculated as: For an organic compound: Cx. Hy. Oz + (x+y/4 - z/2) O 2 ⇒ x CO 2 + y/2 H 2 O and M is the molecular weight. Can also be calculated on S 2 S website Introduction Type of RCH Process of RCH Studies References

39 BREAK TIME T 2 Laboratory Runaway Watch Online: https: //www. youtube. com/watch? v=C 561 PCq 5 E 1 g Introduction Type of RCH Process of RCH Studies References

40 Step 3: Quantify RCH – an estimating tool Tools to Quantify RCH 1. Calorimetry 2. Adopting TCPA (Toxic Catastrophe Prevention Act) 3. Calculated Adiabatic Reaction Temperature (CART) 4. ASTM CHETAH program Introduction Type of RCH Process of RCH Studies References

41 Calorimetry • To measure the heat effect of: • Physical changes (melting, evaporation, dehydration) • Chemical changes (acid-base reaction, dissolving, solid-state reaction, crystal phase transition) • It can be used to determine: • Enthalpy formation trends • Phase stability • Heat capacity • Surface effect • According to relationship between time and heat release per mole, we can, for intended reactions, design the safety response accordingly (e. g. cooling rate, set pressure alarm, etc. ) Introduction Type of RCH Process of RCH Studies References

42 Calorimeter • An instrument determines heat effect in it by measuring temperature. • Based on state of system, classified into two types: 1. Adiabatic • Directly measures the temperature change in insulated system 2. Non-adiabatic • Measures heat flow of the system, with heat transfer to surrounding • Based on working conditions, classified into two types: 1. Constant pressure (e. g. coffee cup calorimeter) 2. Constant volume (e. g. bomb calorimeter) • Other types: solution calorimetry, scanning calorimetry

43 Schematic of a simplified calorimeter 1. 2. 3. 4. 5. Reactant Stirrer Thermometer Calorimeter liquid Heater Picture reference: Calorimetry: Fundamentals, Instrumentation and Applications, 1 st ed. (Stefan M. Sarge, Gunther W. H. Hohne, and Wolfgang Hemminger.

44 Calorimetry Example When 0. 7022 g of oxalic acid (C 2 O 4 H 2) is burnt in the calorimeter under the same conditions as Example 6, the temperature increased by 1. 602°C. The heat capacity of the calorimeter is 1. 238 k. J/K. Calculate d. H°combustion. Solution: The balanced equation and various quantities calculated are given in a logical order below: C 2 O 4 H 2(s) + 0. 5 O 2(g) 2 CO 2 (g) + H 2 O(l) dn = 1. 5 q = C d. T = 1. 238*1. 602 = 1. 984 k. J n of oxalic acid = 0. 7022/90 = 0. 00780 mol d. E = -1. 984 / 0. 00780 = -354. 4 k. J/mol d. H = d. E + dn. RT = -254. 4 k. J + 1. 5 mol * 0. 008314 k. J/(K mol)* 298 K = -250. 6 k. J/mol Similarly, temperature increase can be calculated knowing the reaction, amount of reactants, heat capacity, and energy release. Introduction Type of RCH Process of RCH Studies References

45 Adopting TCPA (New Jersey) (Toxic Catastrophe Prevention Act) • TCPA’s goal: Protect the public from catastrophic releases of extraordinarily hazardous substances (EHS). • TCPA includes two categories of reactive chemicals: • Reactive Hazard Substances (RHS), list of chemicals • Reactive Hazard Substance Mixtures (RHSM) determined by functional groups Threshold quantity is calculated as: TQ = threshold quantity of the RHS, lb; D = distance to property line, ft; E = energy of explosion of the RHS; 24 = scaled distance for the mass of TNT that results in a blast pressure of 2. 3 psi; 1024 = energy of explosion of TNT, cal/g. Introduction Type of RCH Process of RCH Studies References

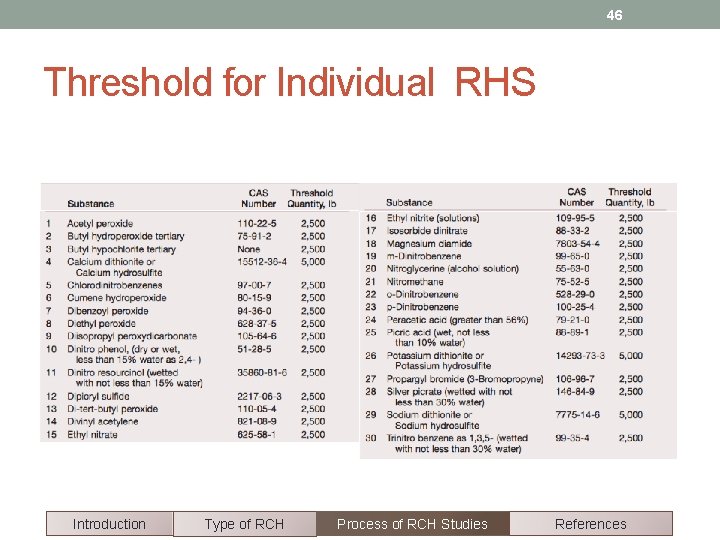
46 Threshold for Individual RHS Introduction Type of RCH Process of RCH Studies References

47 Calculation adopting from TCPA • Reaction threshold can be calculated with heat of reaction ΔH, and obtain using the table below: • Distance from the reactor to the property line can also be calculated adopting this method. NOTE: TCPA is not our center of attention, it is the calculation that can be adopted to help with RCH evaluation. Introduction Type of RCH Process of RCH Studies References

48 Recap: TCPA Example Now, practice with the following copolymerization example: Styrene and acrylonitrile forms SAN (styrene-acrylonitrile) Literature value: - Heat of reaction is -261 kcal/mol with 70: 30 feed weight ratio Find out: - For a reactor filled with 8500 lb of SAN, what would be the minimum distance for us to keep the reactor from? Solution: Introduction Type of RCH Process of RCH Studies References

49 Calculated Adiabatic Reaction Temperature (CART) • Also known as adiabatic flame temperature • “For a combustion process that takes place adiabatically with no shaft work, the temperature of the products is referred to as the adiabatic flame temperature. ” Δh 1+Δh 2=Δhadiabatic=0 for no work done and no heat exchanged (overall enthalpy from initial to final state is zero). Introduction Type of RCH Process of RCH Studies References

50 Calculated Adiabatic Reaction Temperature (CART) • CART relates to reaction mechanism and KNOWN stoichiometry • Energy release, HRXN • The io. Mosaic Reactivity Hazard Index can then be used to compare/rank reactivity hazards • “Neglible Reactivity Hazard” • HRXN no more negative than – 100 cal/g, and • CART 700 K • “Low Reactivity Hazard” (energetic reaction but not likely to be explosive) • HRXN between – 100 and – 287 cal/g, and • CART 700 K • “Intermediate Reactivity Hazard” (energetic reaction but not likely to be explosive) • HRXN between – 287 and – 717 cal/g, or • 700 < CART 1600 K • “High Reactivity Hazard” (strong potential for being explosive reaction) • HRXN more negative than – 717 cal/g, or • CART 1600 K Introduction Type of RCH Process of RCH Studies References

51 CART Calculation Continued For process 1: h 2 – hi = -q 1 = (hof )unit mass where q 1 is the “heat of reaction” For process 2: we put this amount back into the products to raise their temperature to the final level. hf – h 2 = -q 1 or, if we can approximate the specific heat as constant cp, avg (Tf – T 2) = q 1 Temperature change during this second process is approximately , where Tf is the adiabatic flame temperature Introduction Type of RCH Process of RCH Studies References

52 Recap: CART Practice Determine the constant pressure adiabatic flame temperature for the combustion of methane with a stoichiometric air at 1 atm pressure. The reactant temperature at initial condition, Ti=298 K. The reaction is CH 4 + 2 O 2 + 7. 52 N 2 = CO 2 + 2 H 2 O + 7. 524 N 2 SUBMIT ANSWER (in K) Introduction Type of RCH Process of RCH Studies References

53 Recap: CART Solution Introduction Type of RCH Process of RCH Studies References

54 ASTM CHETAH program • A computer program for chemical thermodynamic and energy release evaluation • Predict RCH associated with a pure chemical, a mixture of chemicals, or a chemical reaction. • Used for: • Classifying materials for their ability to decompose with violence • Estimating heats of reaction or combustion • Predicting lower flammable limits • Obtaining flammability parameters • Complementing experimental results to help identify IF further testing is needed. • NOT used: • As a replacement for physical testing of materials

55 Control and Prevention • Undesired reactions need to be prevented • Prevention examples: • Segregate storage tanks/dykes • Use different fittings/flanges to reduce mixups • Desired reactions need to be controlled • By controlling concentration, temperature, pressure, phase, surface area of reactants, amount of catalyst • Kinetically, and • Thermodynamically • This section is simplified due to the scope and goal of this module. Introduction Type of RCH Process of RCH Studies References

56 Control and Prevention • Various methods to manage reactivity hazards: • Inherent • e. g. Use an intended reaction pathway that uses less hazardous chemicals • Passive • e. g. Use separate storage for incompatible chemicals • Active • e. g. Provide properly designed control systems to control intended reactive chemicals in the process • Procedural • e. g. Manage process changes that may involve reactive chemicals For a complete hierarchy of methods, please refer to R. W. Johnson, S. W. Rudy, and S. D. Unwin, Essential Practices for Managing Chemical Reactivity Hazards (NY: AICh. E Center for Chemical Process Safety, 2003) Introduction Type of RCH Process of RCH Studies References

57 Other sources for RCH Information and Tools Source Location “Essential Practices for Managing Chemical Reactivity Hazards” E-book on Knovel The U. S. Coast Guard’s (USCG) Chemical Hazard Response Information System (CHRIS) database Available Online Brethericks Handbook of Reactive Chemical Hazards, P. Urben, ed. (2006) Google books or Elsevier Publishers Sax’s Dangerous Properties of Industrial Materials, R. J. Lewis, ed. (2007) John Wiley and Sons, Inc. Introduction Type of RCH Process of RCH Studies References

58 Other sources for RCH Information and Tools Source Location Sigma Aldrich Library of Chemical Safety Data, R. E. Lenga, ed. (1988) Sigma-Aldrich Fire Protection Guide to Hazardous Materials (2010) National Fire Protection Association (NFPA) Computer Program for Chemical Thermodynamics and Energy Release Evaluation (CHETAH) American Society for Testing and Materials (ASTM) Chemical Risk Analysis, in practical working situations Google book Introduction Type of RCH Process of RCH Studies References