1 OxidationReduction Reactions and Electron Carriers many metabolic

1
Oxidation-Reduction Reactions and Electron Carriers • many metabolic processes involve oxidation-reduction reactions (electron transfers) • electron carriers are often used to transfer electrons from an electron donor to an electron acceptor 2

Oxidation-reduction (redox) reactions • transfer of electrons from a donor to an acceptor can result in energy release, which can be conserved and used to form ATP 3

Redox reactions • Oxidation – Removal of electrons • Fe 2+ Fe 3+ + 1 e- – Releases energy • Reduction – Accepts electrons • O 2 + 4 e- (+ 4 H+) 2 H 2 O – Requires energy 4

Redox reactions • Each oxidation requires a simultaneous reduction – Therefore, an oxidation half reaction requires a reduction half reaction • 4 Fe 2+ 4 Fe 3+ + 4 e • O 2 + 4 e- (+ 4 H+) 2 H 2 O 4 Fe 2+ + O 2 + 4 H+ 4 Fe 3+ + 2 H 2 O 5

Convention for depicting redox couples, and definitions Acceptor + ne- donor 2 H+ + 2 e. H 2 or 2 H+/H 2 Electron donor: reductant, reducing agent, is energy rich Electron acceptor: oxidant, oxidizing agent, is energy poor 6
Standard reduction potential (E 0) • equilibrium constant for an oxidation-reduction reaction • a measure of the tendency of the reducing agent to lose electrons more negative E 0 better electron donor more positive E 0 better electron acceptor 7
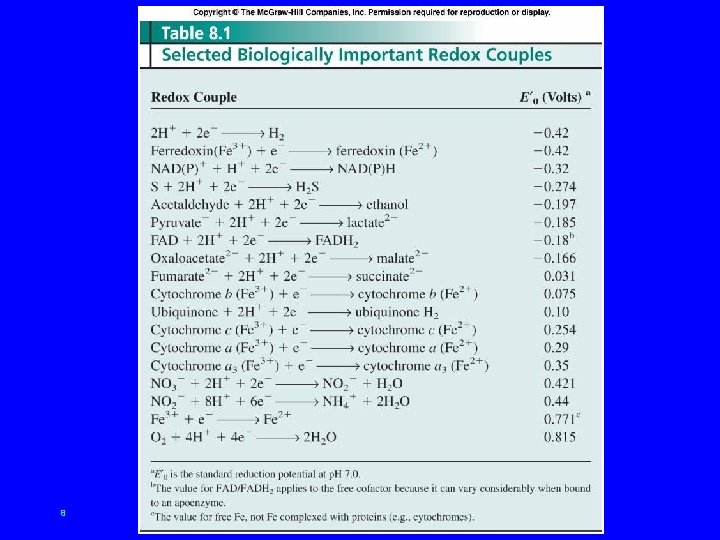
8
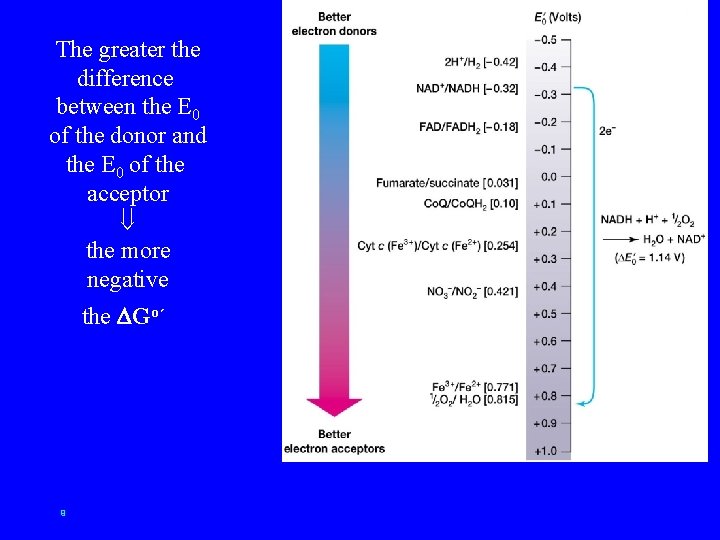
The greater the difference between the E 0 of the donor and the E 0 of the acceptor the more negative the Go´ 9
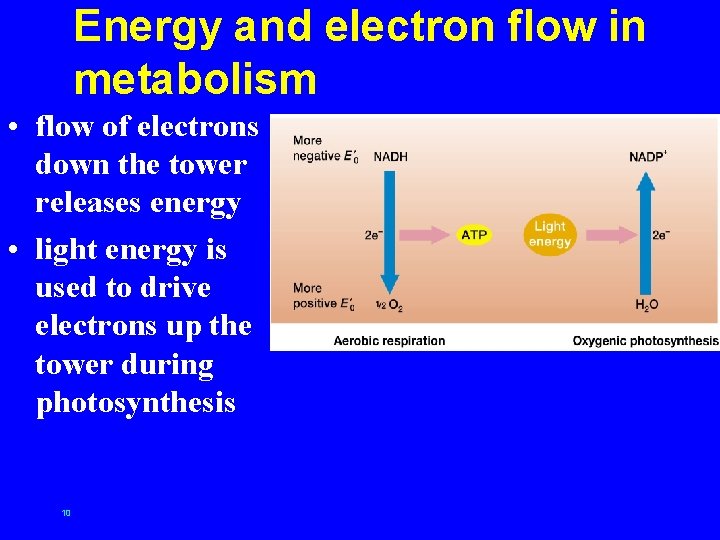
Energy and electron flow in metabolism • flow of electrons down the tower releases energy • light energy is used to drive electrons up the tower during photosynthesis 10
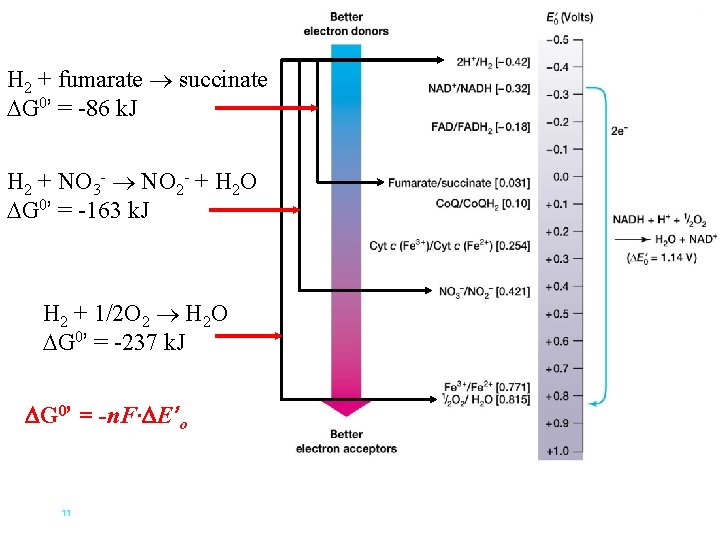
H 2 + fumarate succinate G 0’ = -86 k. J H 2 + NO 3 - NO 2 - + H 2 O G 0’ = -163 k. J H 2 + 1/2 O 2 H 2 O G 0’ = -237 k. J G 0’ = -n. F· E’o 11
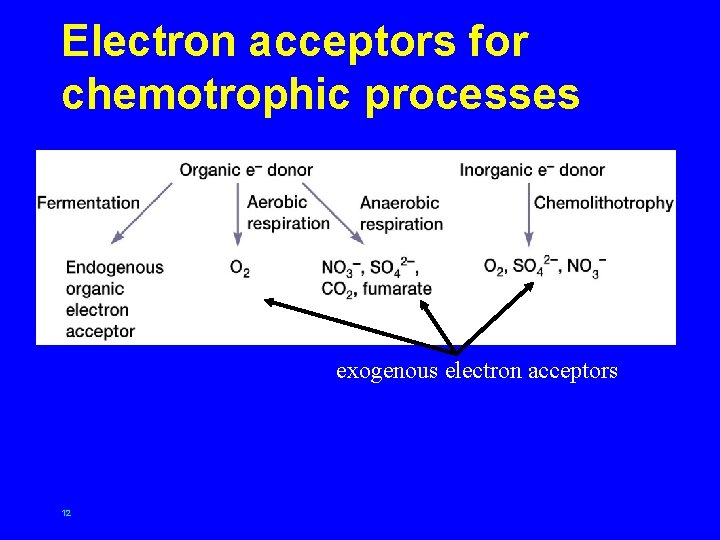
Electron acceptors for chemotrophic processes exogenous electron acceptors 12

Chemoorganotrophic metabolism • aerobic respiration – energy source degraded using oxygen as exogenous electron acceptor – yields large amount of energy, primarily by electron transport activity 13

Chemoorganotrophic metabolism • anaerobic respiration – energy source oxidized and degraded using molecules other than oxygen as exogenous electron acceptors – can yield large amount of energy (depending on reduction potential of energy source and electron acceptor), primarily by electron transport activity 14
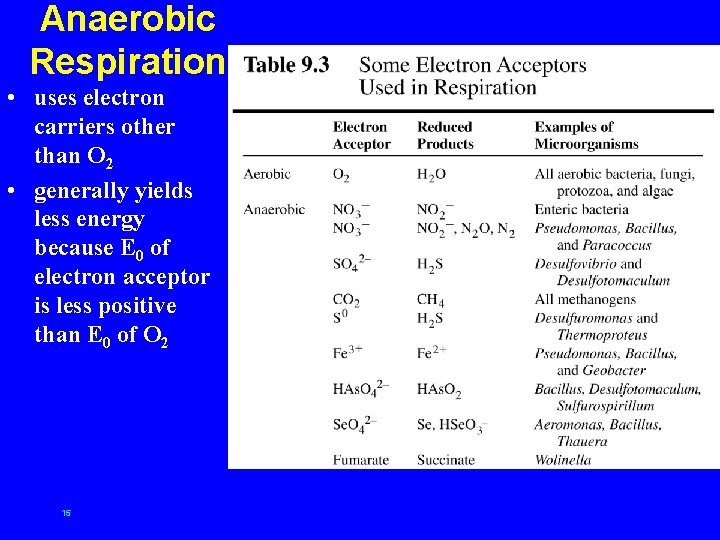
Anaerobic Respiration • uses electron carriers other than O 2 • generally yields less energy because E 0 of electron acceptor is less positive than E 0 of O 2 15
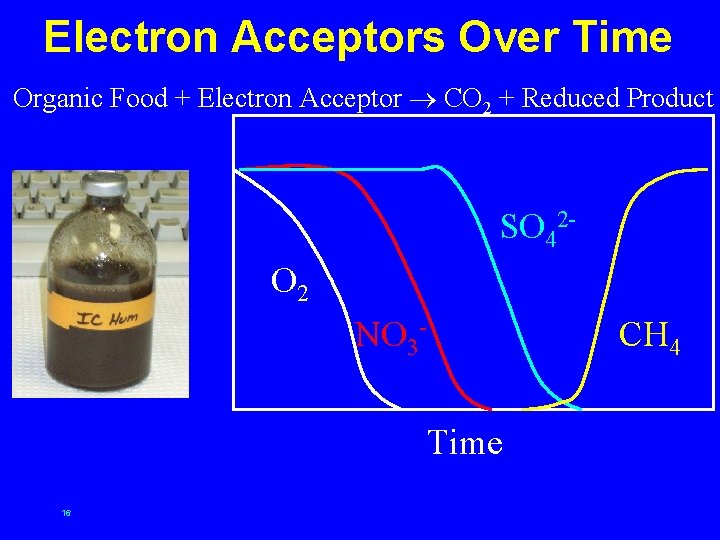
Electron Acceptors Over Time Organic Food + Electron Acceptor CO 2 + Reduced Product SO 42 O 2 NO 3 - CH 4 Time 16
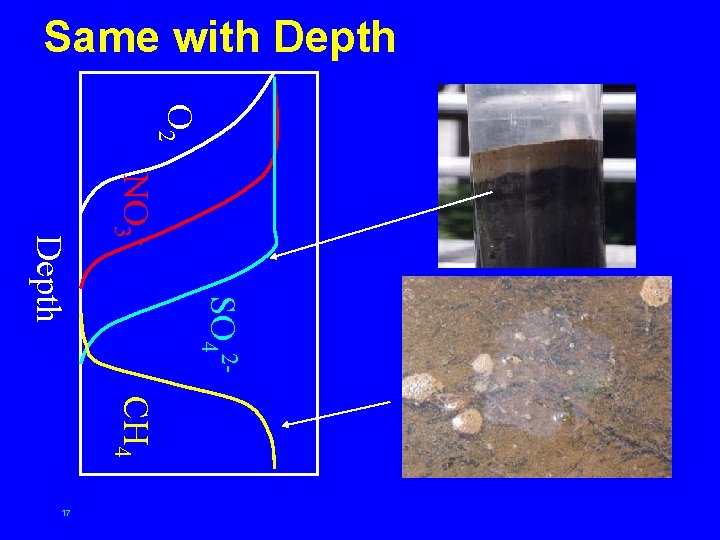
Same with Depth O 2 NO 3 - SO 42 - Depth CH 4 17

Chemoorganotrophic metabolism • fermentation – energy source oxidized and degraded using endogenous electron acceptor – often occurs under anaerobic conditions – limited energy made available 18 Example: CH 3 COOH CH 4 + CO 2 e- donor e- acceptor
Oxidation of Inorganic Molecules • carried out by chemolithotrophs • electrons released from energy source – transferred to terminal electron acceptor • ATP synthesized by oxidative phosphorylation 19
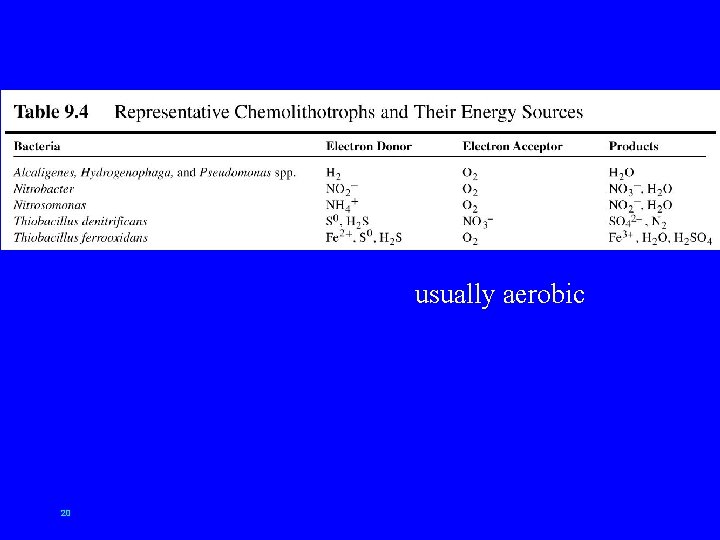
usually aerobic 20
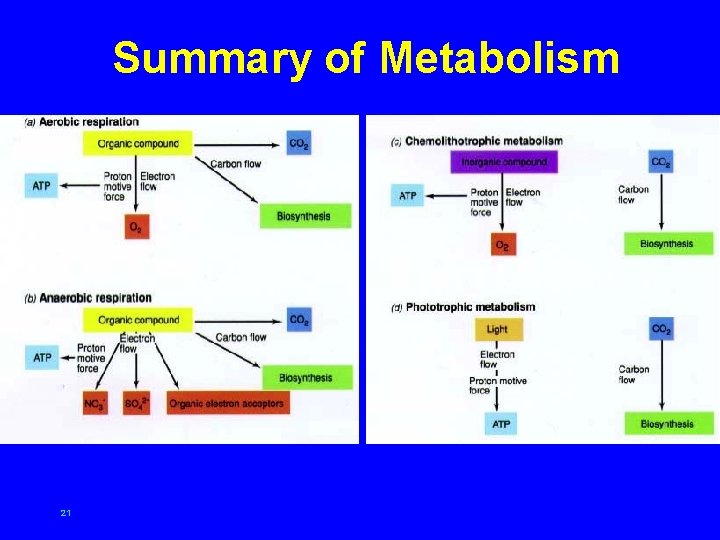
Summary of Metabolism 21
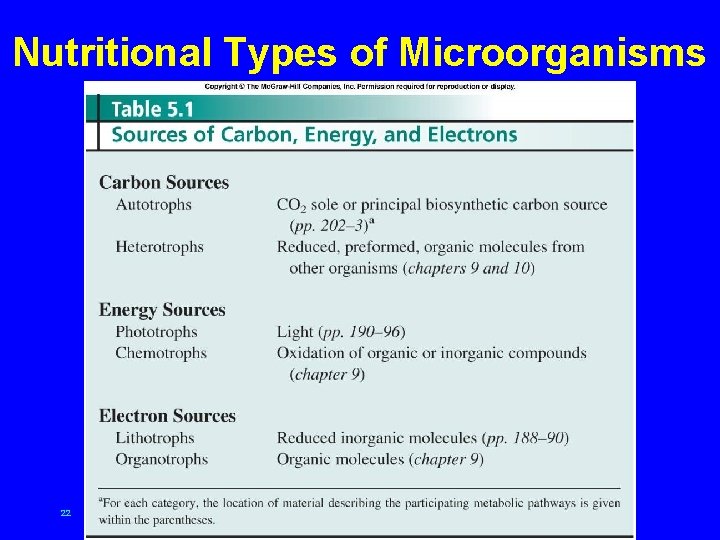
Nutritional Types of Microorganisms 22
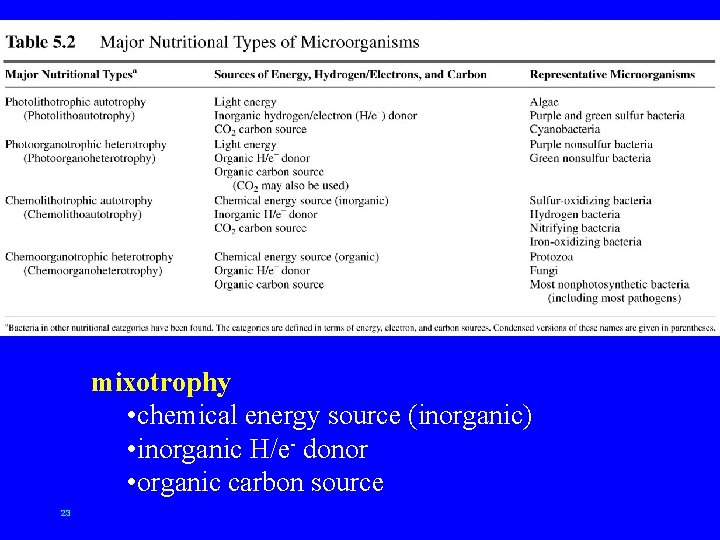
mixotrophy • chemical energy source (inorganic) • inorganic H/e- donor • organic carbon source 23
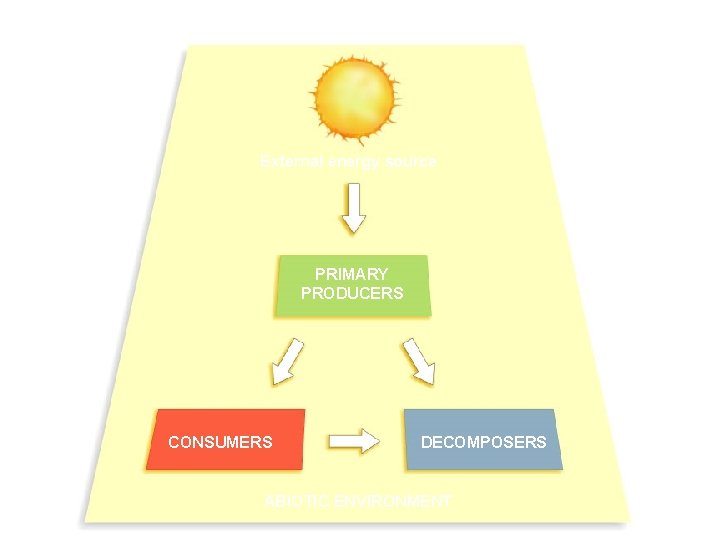
External energy source PRIMARY PRODUCERS CONSUMERS 24 DECOMPOSERS ABIOTIC ENVIRONMENT

Trophic levels Trophic level 4 Feeding strategy Grazing food chain Secondary carnivore Cooper’s hawk 3 Carnivore Robin 25 Decomposer food chain Owl Shrew 2 Herbivore Cricket Earthworm 1 Autotroph Maple tree leaves Dead maple leaves
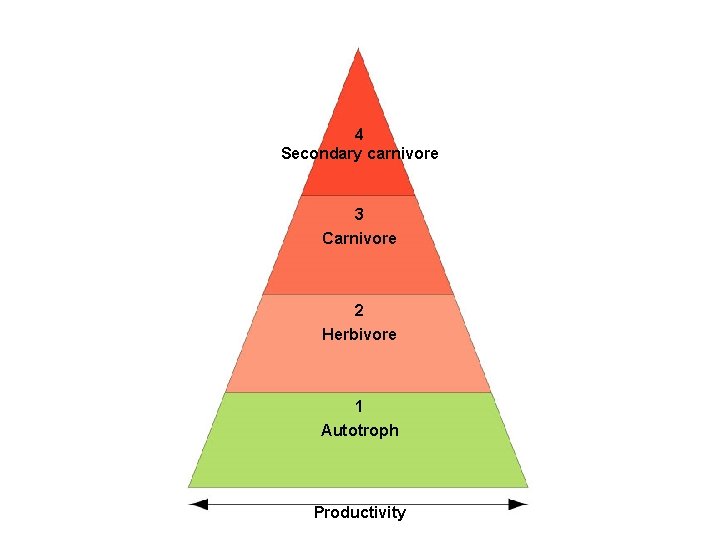
Pyramid of productivity 4 Secondary carnivore 3 Carnivore 2 Herbivore 1 Autotroph 26 Productivity
- Slides: 26