1 of 35 Boardworks Ltd 2008 Lesson Objectives
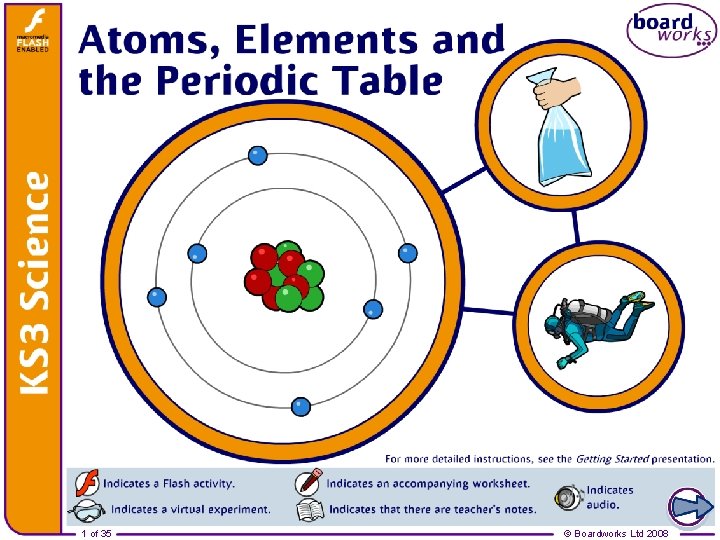
1 of 35 © Boardworks Ltd 2008
Lesson Objectives • Be able to explain the difference between atoms, elements and molecules. • Understand the history of the atom. • Start to become familiar with the symbols for different elements. 2 of 35 © Boardworks Ltd 2008
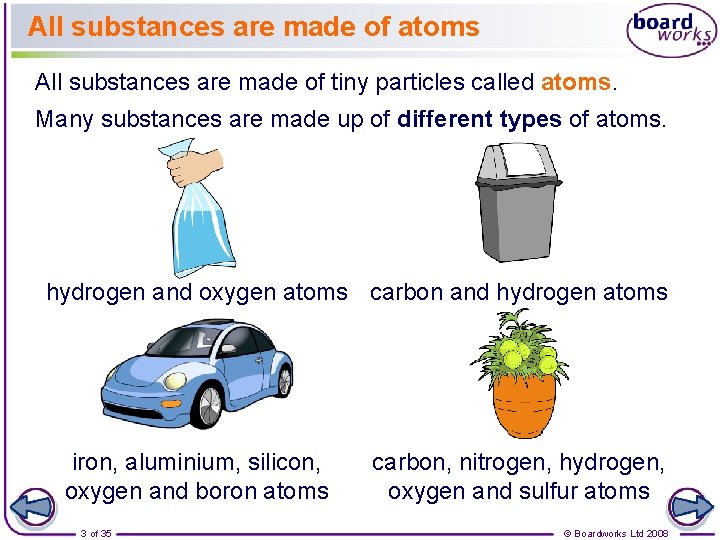
All substances are made of atoms All substances are made of tiny particles called atoms. Many substances are made up of different types of atoms. hydrogen and oxygen atoms carbon and hydrogen atoms iron, aluminium, silicon, oxygen and boron atoms 3 of 35 carbon, nitrogen, hydrogen, oxygen and sulfur atoms © Boardworks Ltd 2008
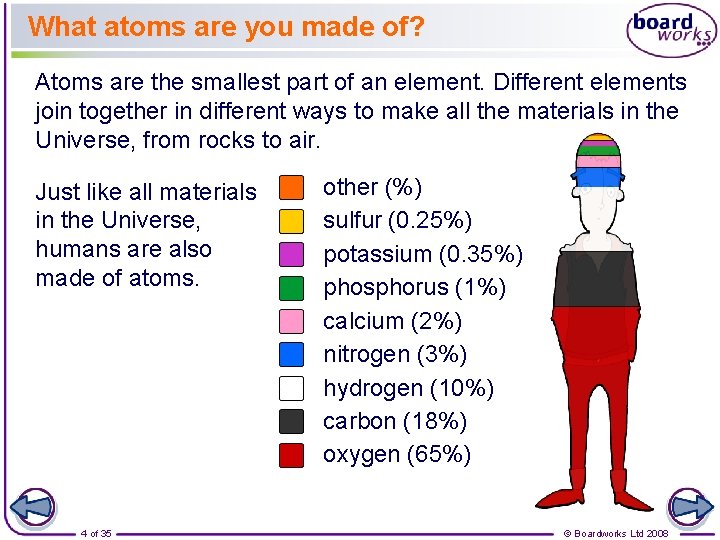
What atoms are you made of? Atoms are the smallest part of an element. Different elements join together in different ways to make all the materials in the Universe, from rocks to air. Just like all materials in the Universe, humans are also made of atoms. 4 of 35 other (%) sulfur (0. 25%) potassium (0. 35%) phosphorus (1%) calcium (2%) nitrogen (3%) hydrogen (10%) carbon (18%) oxygen (65%) © Boardworks Ltd 2008

How big are atoms? Atoms have a diameter of about 0. 00000001 cm, which is far too small to be seen with your eyes. However, microscopes called Scanning Tunnelling Microscopes allow scientists to see the outlines of atoms. In one glass of water there around: l 12, 000, 000, 000 oxygen atoms l 24, 000, 000, 000 hydrogen atoms. 5 of 35 © Boardworks Ltd 2008

Activity • Complete worksheet on „history of the atom“ while we review the history and the differents models used by scientists to describe the atom. 6 of 35 © Boardworks Ltd 2008

Democtritus • Ancient Greek philosopher (460 – 370 BC) • Suggested that matter cannot be cut into smaller pieces forever • Gave a name to the smallest particle of matter: the ATOM ατομοσ 7 of 35 © Boardworks Ltd 2008

The ancient Greeks theorised that everything was made of fire, earth, water and air. Only after over 1600 years did scientists realise matter was composed of different elements. In 1805 John Dalton refined a vague idea that matter was indivisible. 8 of 35 © Boardworks Ltd 2008

John Dalton (1766 – 1844) • Dalton was an English schoolteacher • Began teaching mathematics and chemistry at the age of 12 • Revived the idea of Democritus’ “smallest piece” of matter 9 of 35 © Boardworks Ltd 2008

In 1805, John Dalton proposed explanations for some observations he had made during experiments. He developed an atomic theory. 1. Elements are made of extremely small particles called atoms. 2. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. 3. Atoms cannot be subdivided, created, or destroyed. 4. Atoms of different elements combine in simple whole-number ratios to form chemical compounds. 5. In chemical reactions, atoms are combined, separated, or rearranged. 10 of 35 © Boardworks Ltd 2008
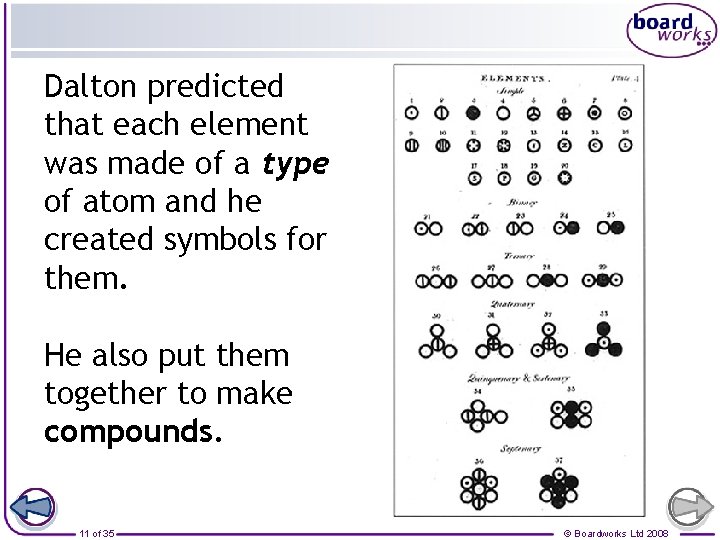
Dalton predicted that each element was made of a type of atom and he created symbols for them. He also put them together to make compounds. 11 of 35 © Boardworks Ltd 2008

Joseph Thomson (1856 -1940) • English physicist Call me “JJ” • Worked with Cathode-Ray Tubes (CRTs) • Credited with the discovery of the electron 12 of 35 © Boardworks Ltd 2008
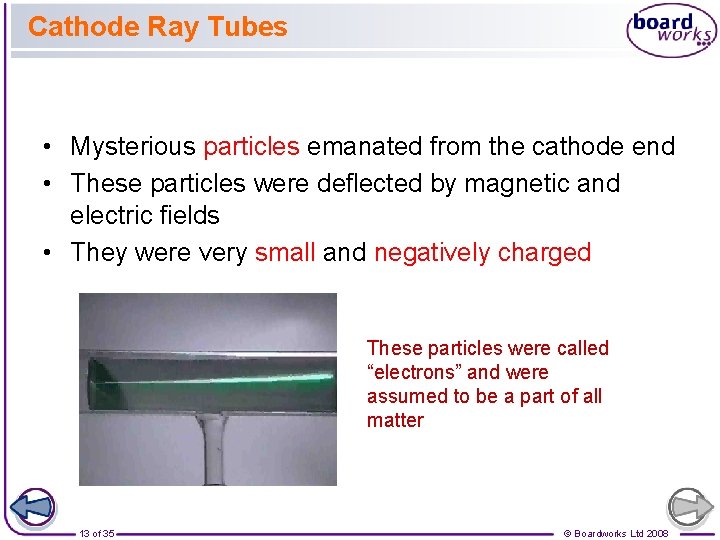
Cathode Ray Tubes • Mysterious particles emanated from the cathode end • These particles were deflected by magnetic and electric fields • They were very small and negatively charged These particles were called “electrons” and were assumed to be a part of all matter 13 of 35 © Boardworks Ltd 2008
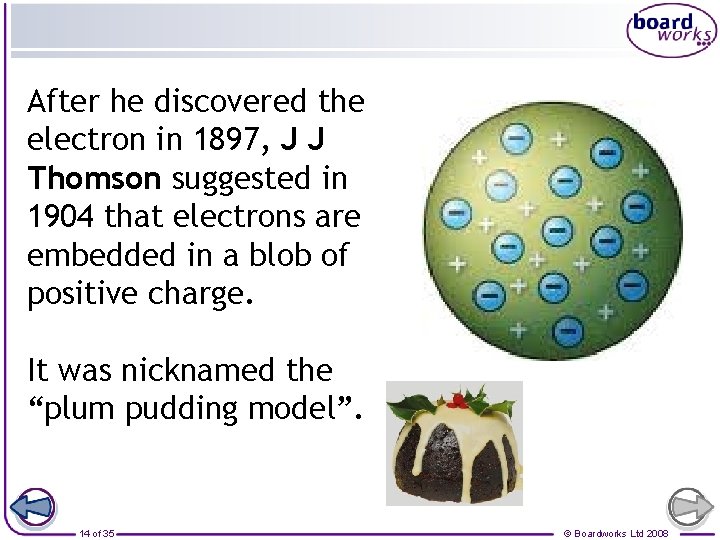
After he discovered the electron in 1897, J J Thomson suggested in 1904 that electrons are embedded in a blob of positive charge. It was nicknamed the “plum pudding model”. 14 of 35 © Boardworks Ltd 2008
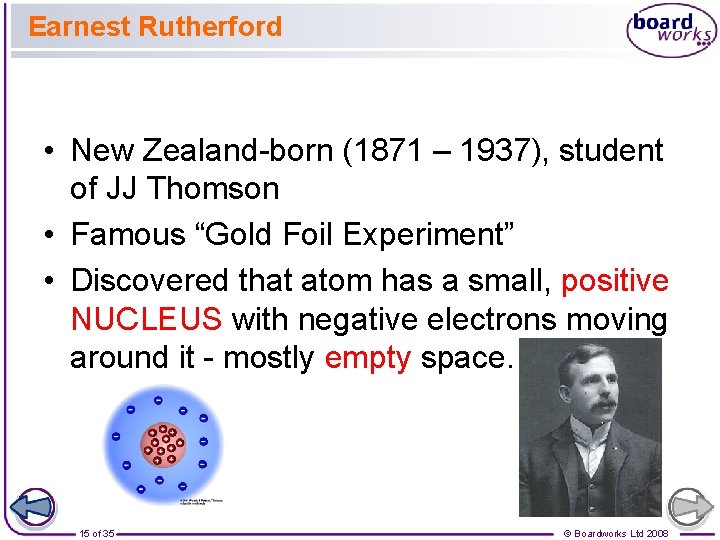
Earnest Rutherford • New Zealand-born (1871 – 1937), student of JJ Thomson • Famous “Gold Foil Experiment” • Discovered that atom has a small, positive NUCLEUS with negative electrons moving around it - mostly empty space. 15 of 35 © Boardworks Ltd 2008
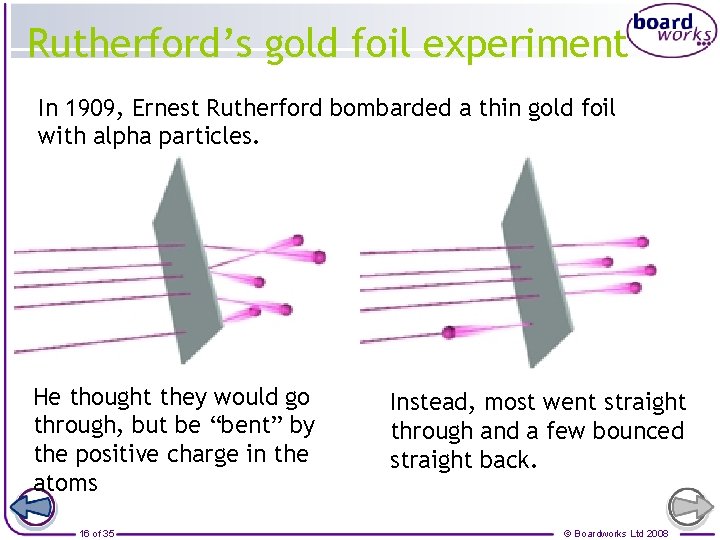
Rutherford’s gold foil experiment In 1909, Ernest Rutherford bombarded a thin gold foil with alpha particles. He thought they would go through, but be “bent” by the positive charge in the atoms 16 of 35 Instead, most went straight through and a few bounced straight back. © Boardworks Ltd 2008
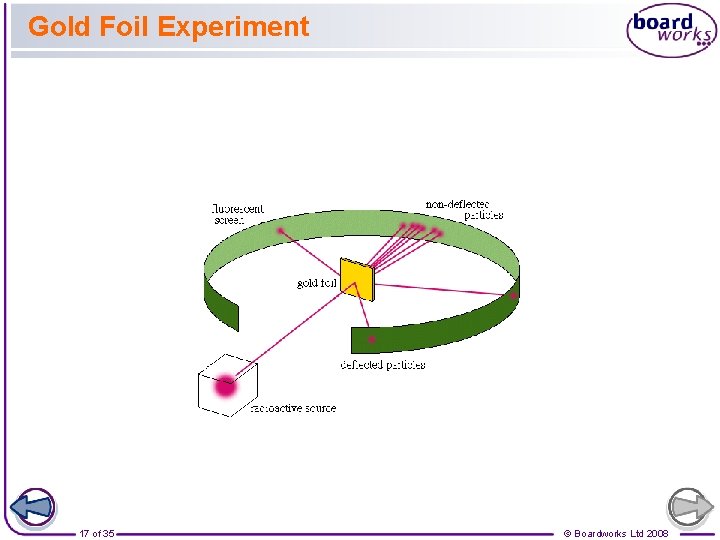
Gold Foil Experiment 17 of 35 © Boardworks Ltd 2008

Gold Foil Experiment “It was quite the most incredible event that ever happened to me in my life. It was almost as incredible as if you fired a 15 -inch shell at a piece of tissue paper and it came back and hit you!” 18 of 35 © Boardworks Ltd 2008
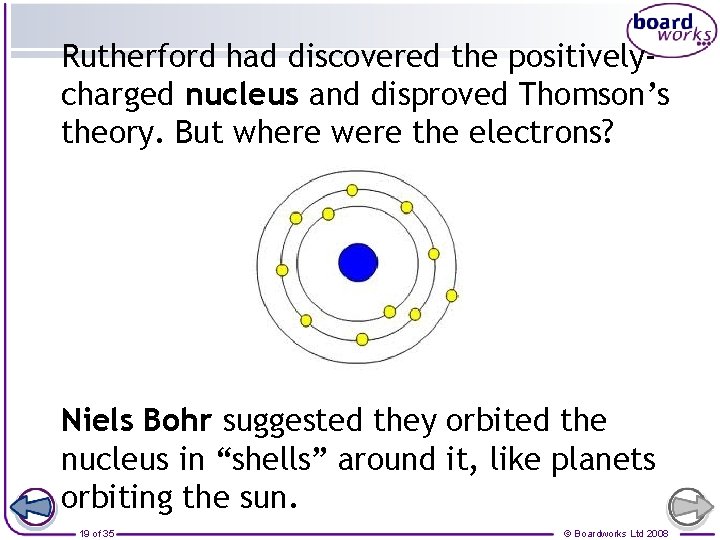
Rutherford had discovered the positivelycharged nucleus and disproved Thomson’s theory. But where were the electrons? Niels Bohr suggested they orbited the nucleus in “shells” around it, like planets orbiting the sun. 19 of 35 © Boardworks Ltd 2008
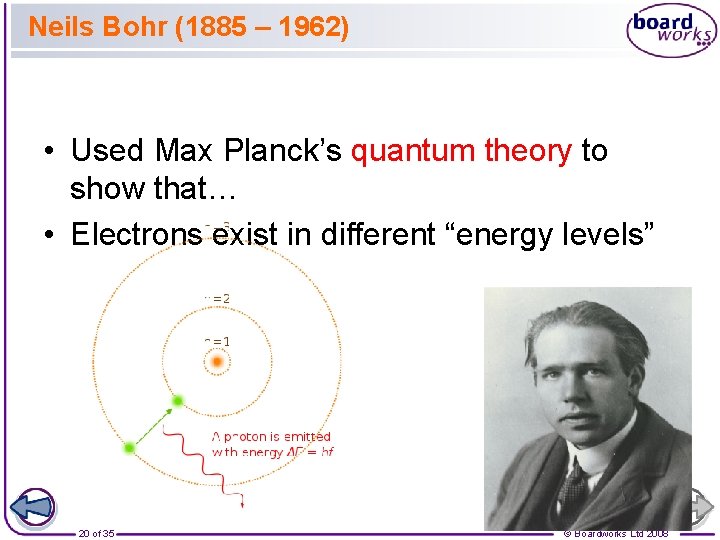
Neils Bohr (1885 – 1962) • Used Max Planck’s quantum theory to show that… • Electrons exist in different “energy levels” 20 of 35 © Boardworks Ltd 2008

James Chadwick 20 October 1891 – 24 July 1974 … • A fellow researcher with Rutherford • Discovered years later that the nucleus contained not one, but TWO types of particles • This second particle was called the “neutron” because it had no electrical charge 21 of 35 mmmm, neutrons … © Boardworks Ltd 2008
Atoms, Elements and Molecules 22 of 35 © Boardworks Ltd 2008
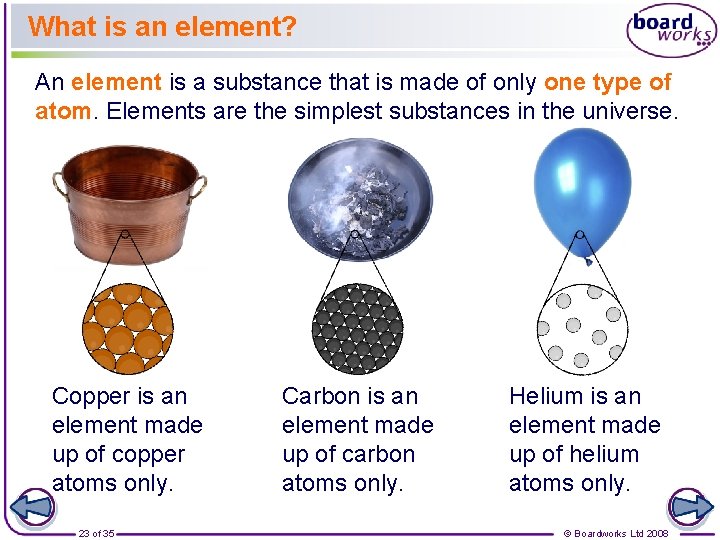
What is an element? An element is a substance that is made of only one type of atom. Elements are the simplest substances in the universe. Copper is an element made up of copper atoms only. 23 of 35 Carbon is an element made up of carbon atoms only. Helium is an element made up of helium atoms only. © Boardworks Ltd 2008
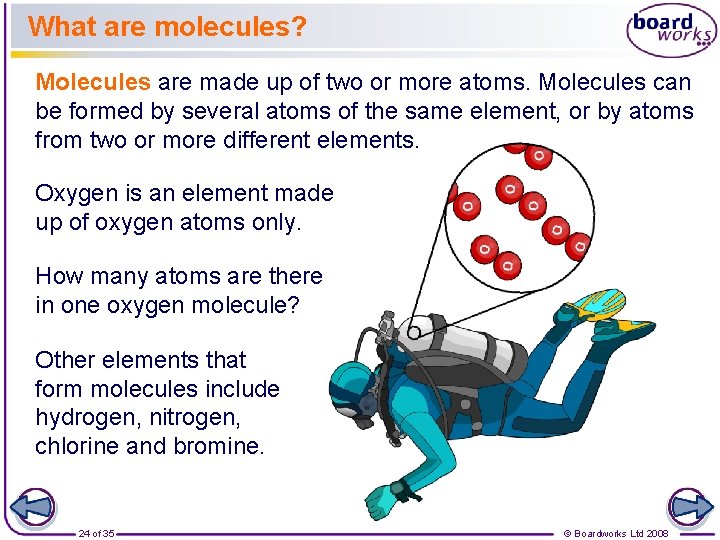
What are molecules? Molecules are made up of two or more atoms. Molecules can be formed by several atoms of the same element, or by atoms from two or more different elements. Oxygen is an element made up of oxygen atoms only. How many atoms are there in one oxygen molecule? Other elements that form molecules include hydrogen, nitrogen, chlorine and bromine. 24 of 35 © Boardworks Ltd 2008
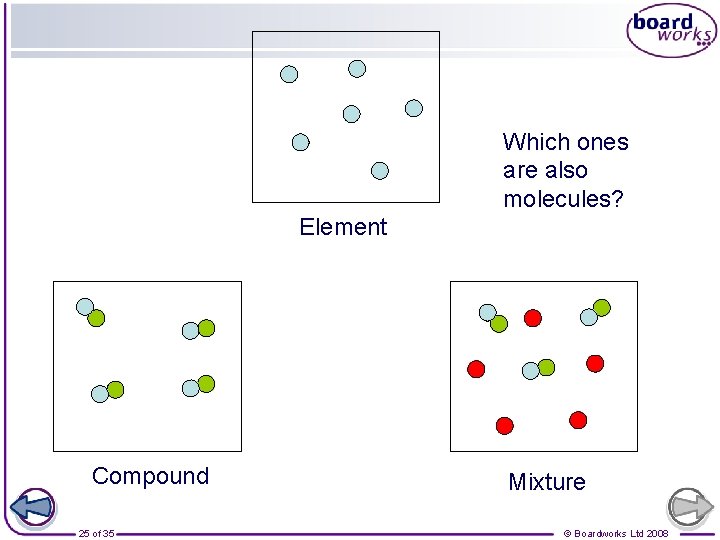
Which ones are also molecules? Element Compound 25 of 35 Mixture © Boardworks Ltd 2008
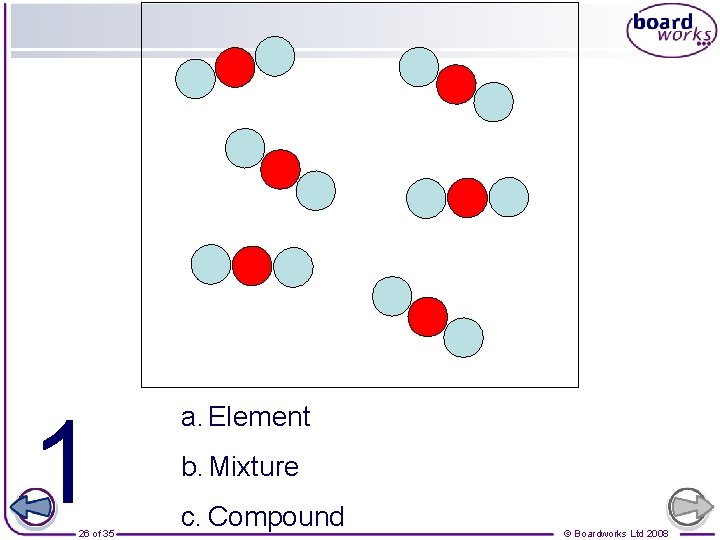
1 26 of 35 a. Element b. Mixture c. Compound © Boardworks Ltd 2008
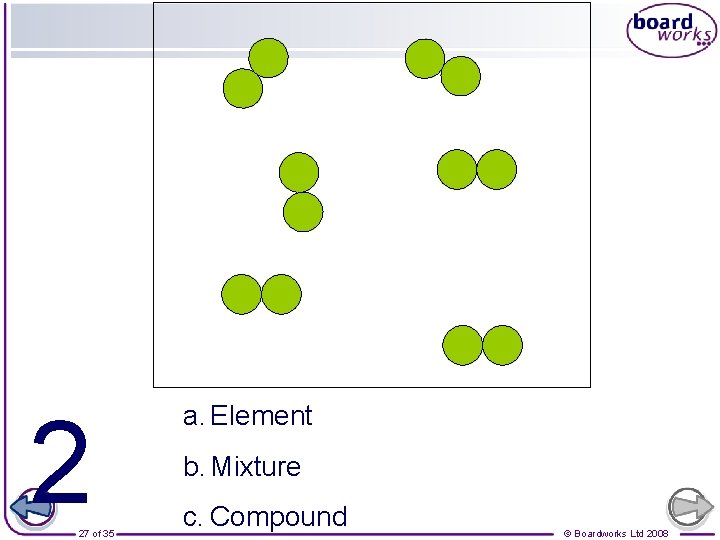
2 27 of 35 a. Element b. Mixture c. Compound © Boardworks Ltd 2008
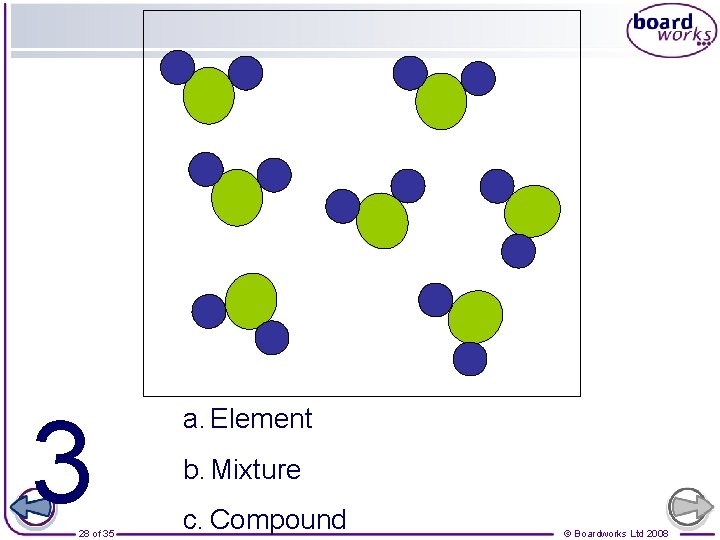
3 28 of 35 a. Element b. Mixture c. Compound © Boardworks Ltd 2008
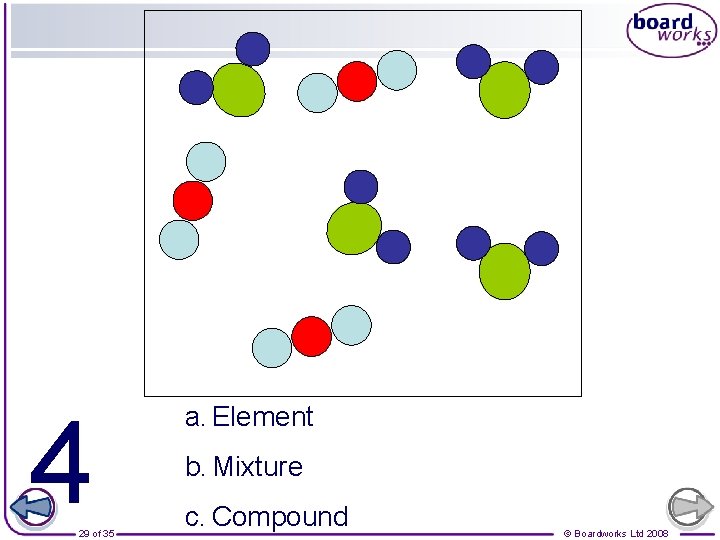
4 29 of 35 a. Element b. Mixture c. Compound © Boardworks Ltd 2008
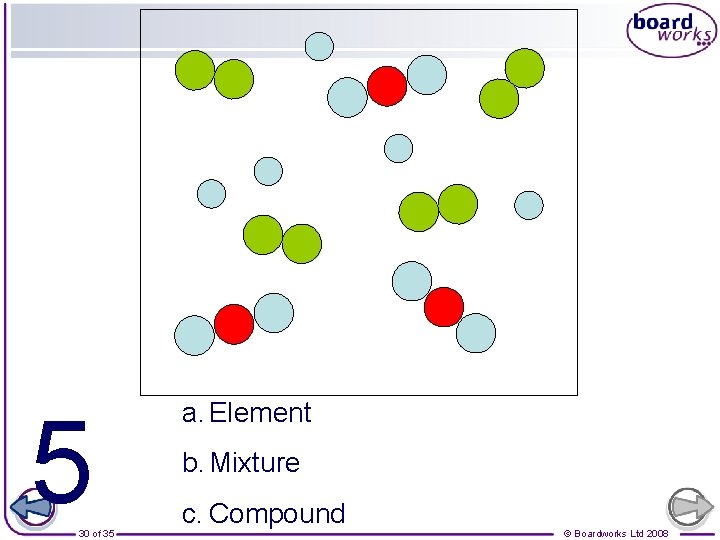
5 30 of 35 a. Element b. Mixture c. Compound © Boardworks Ltd 2008

Elements 31 of 35 © Boardworks Ltd 2008

Who discovered the elements? Some elements, such as silver and gold, have been known about and used by people for centuries. However, many of the elements were only discovered in the 18 th and 19 th century. For example, British scientist Joseph Priestley discovered oxygen when he experimented with heating gases. Other scientists, such as Humphrey Davy, used electrolysis to isolate elements such as sodium and potassium for the first time. At the start of the 20 th century, Marie Curie and other scientists discovered radioactive elements like polonium and francium. Which countries were these elements named after? 32 of 35 © Boardworks Ltd 2008

How many elements are there? There are currently 118 elements that have been discovered, 91 of which are naturally occurring. The remaining 27 elements only exist under laboratory conditions. How many naturally-occurring elements can you name? 33 of 35 © Boardworks Ltd 2008

How are artificial elements made? The first element to be artificially created was technetium, which was discovered in 1937 by Italian scientists working with the naturally-occurring element molybdenum. Since then, other artificial elements have been made in particle accelerators. CERN is one of the world’s largest particle accelerators. It is situated underground on the French. Swiss border and is run by scientists from all over Europe. Most artificial elements are very unstable and usually only exist for milliseconds before they decompose. 34 of 35 © Boardworks Ltd 2008
Symbols for elements Each element can be represented by a symbol. For many elements, the symbol is the start of the name, for example H = hydrogen or Li = lithium. Can you think of any other symbols like this? However, some of the symbols are not always as you might expect; for example, Pb = lead. Can you think of any other elements with unexpected symbols? The first letter of an element’s symbol is always a capital letter, e. g. N (not n) for nitrogen. If there are two letters in the element’s symbol, the second letter is always a small letter, e. g. Co (not CO) for cobalt. 35 of 35 © Boardworks Ltd 2008
Why are symbols important? Why might scientists find it easier to use symbols for elements rather than names? l Elements have different names in different languages, e. g. in Portuguese, nitrogen is called ‘azote’, and iron is called ‘ferro’. l Symbols are quicker to write than names, and can be easily used in chemical formulae, diagrams and equations. The current system for naming elements and compounds was devised by the International Union of Pure and Applied Chemistry (IUPAC) so that scientists all around the world could communicate without confusion. 36 of 35 © Boardworks Ltd 2008
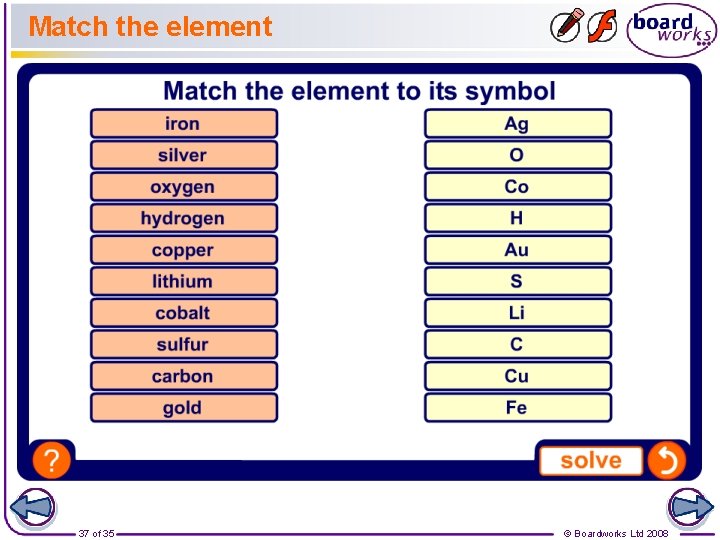
Match the element 37 of 35 © Boardworks Ltd 2008
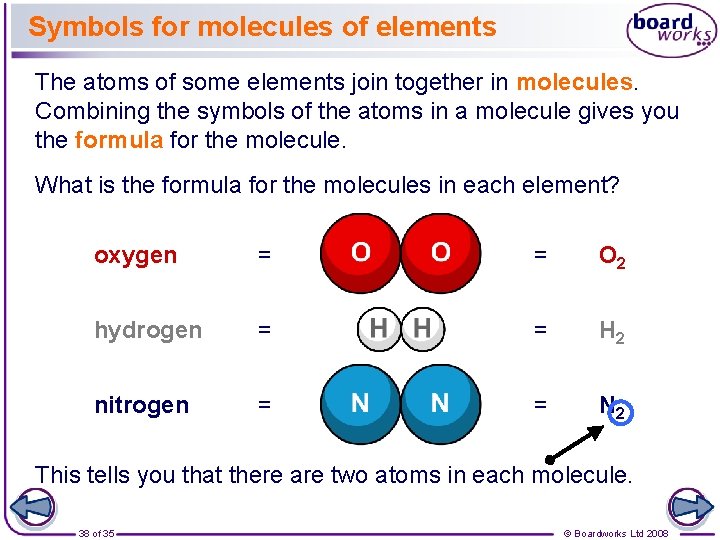
Symbols for molecules of elements The atoms of some elements join together in molecules. Combining the symbols of the atoms in a molecule gives you the formula for the molecule. What is the formula for the molecules in each element? oxygen = = O 2 hydrogen = = H 2 nitrogen = = N 2 This tells you that there are two atoms in each molecule. 38 of 35 © Boardworks Ltd 2008

Activity • Play element bingo 39 of 35 © Boardworks Ltd 2008
- Slides: 39