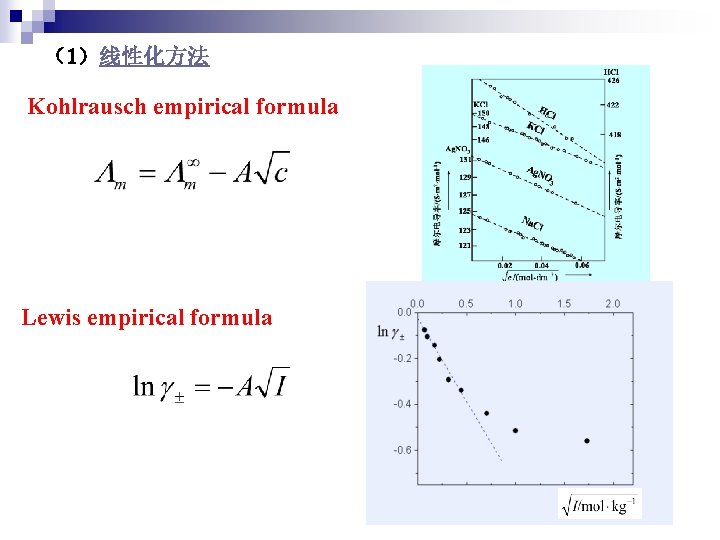
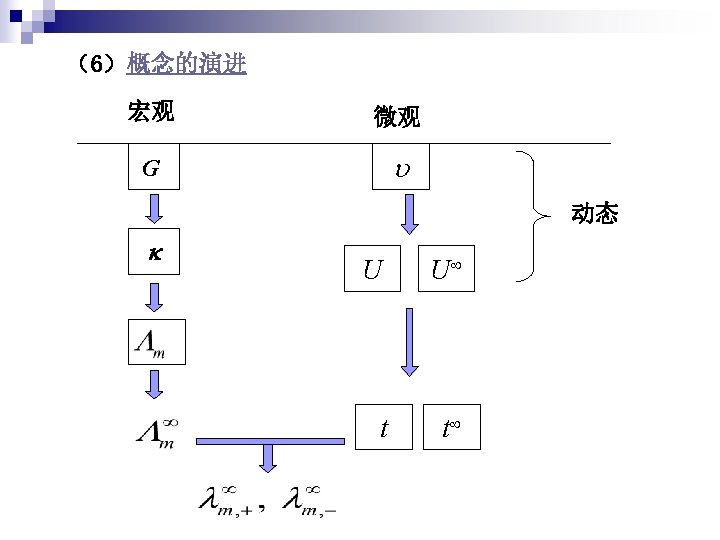
1 Kohlrausch empirical formula Lewis empirical formula log







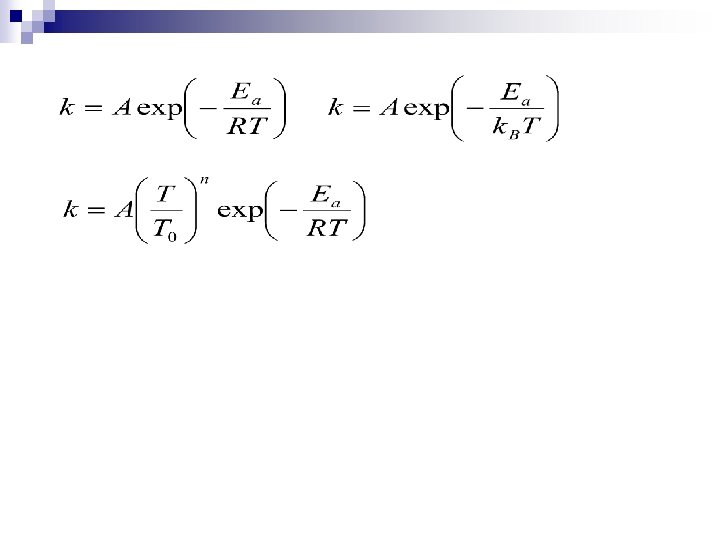
- Slides: 30





(1)线性化方法 Kohlrausch empirical formula Lewis empirical formula

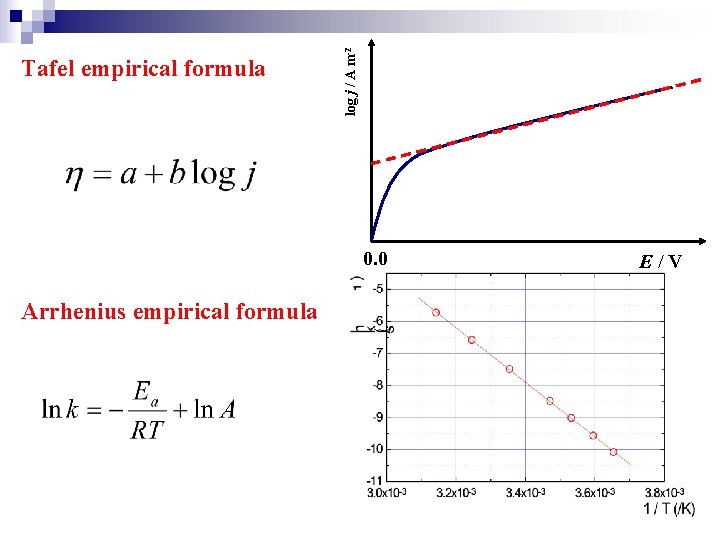
log j / A m-2 Tafel empirical formula 0. 0 Arrhenius empirical formula E/V


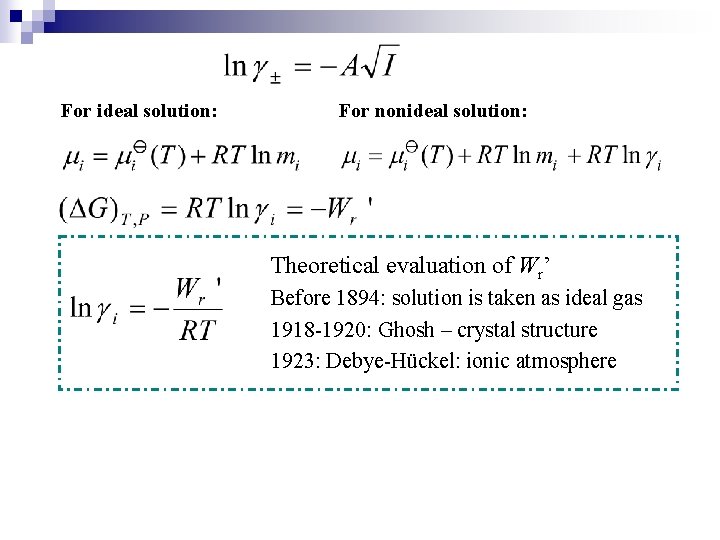
For ideal solution: For nonideal solution: Theoretical evaluation of Wr’ Before 1894: solution is taken as ideal gas 1918 -1920: Ghosh – crystal structure 1923: Debye-Hückel: ionic atmosphere

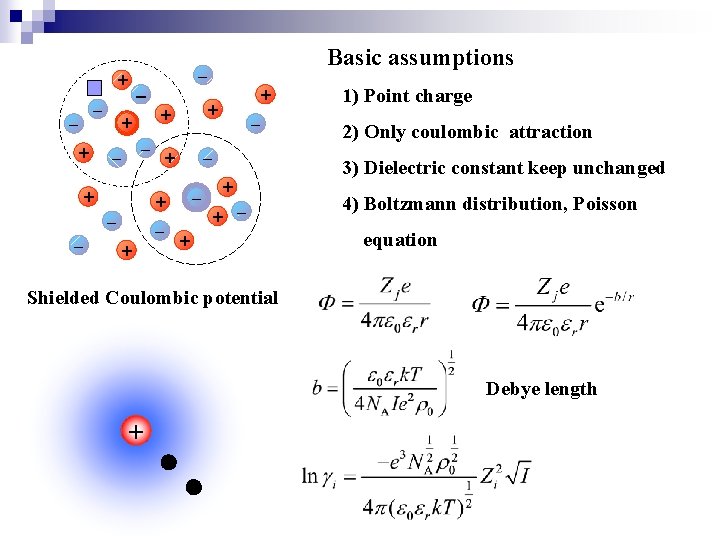
+ + + + + Basic assumptions + + 1) Point charge 2) Only coulombic attraction 3) Dielectric constant keep unchanged 4) Boltzmann distribution, Poisson equation Shielded Coulombic potential Debye length +

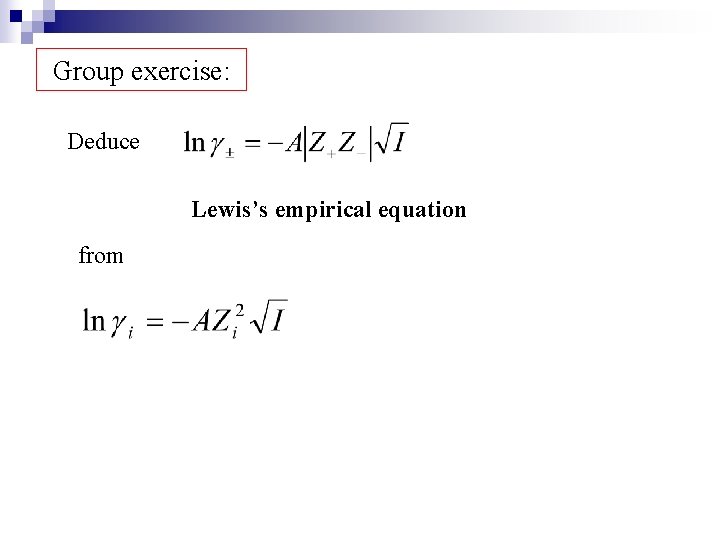
Group exercise: Deduce Lewis’s empirical equation from

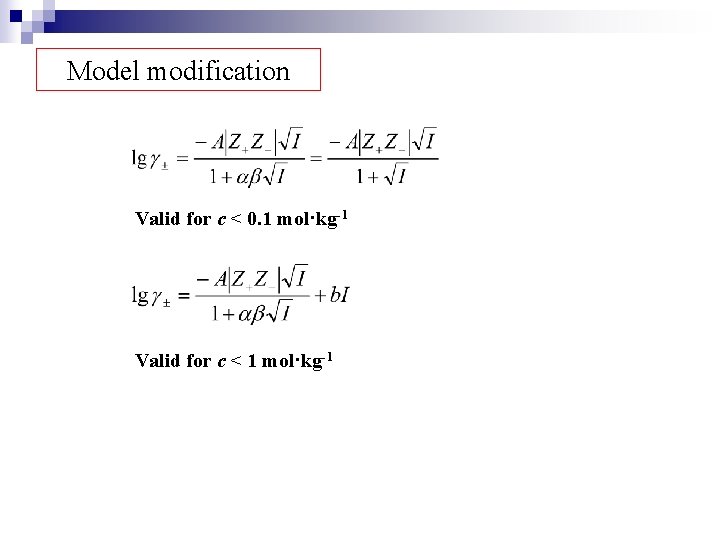
Model modification Valid for c < 0. 1 mol·kg-1 Valid for c < 1 mol·kg-1

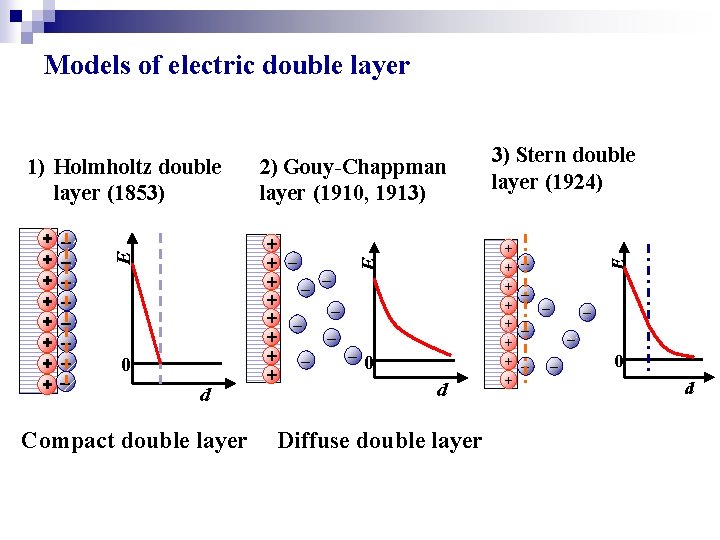
Models of electric double layer + + + 0 + 0 d Compact double layer d Diffuse double layer 3) Stern double layer (1924) + + + + E 2) Gouy-Chappman layer (1910, 1913) E + + + + E 1) Holmholtz double layer (1853) 0 d


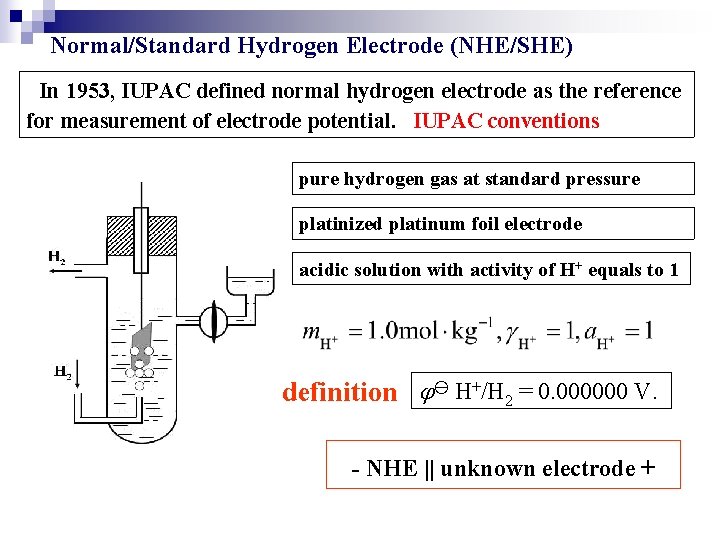
Normal/Standard Hydrogen Electrode (NHE/SHE) In 1953, IUPAC defined normal hydrogen electrode as the reference for measurement of electrode potential. IUPAC conventions pure hydrogen gas at standard pressure platinized platinum foil electrode acidic solution with activity of H+ equals to 1 definition H+/H 2 = 0. 000000 V. - NHE || unknown electrode +

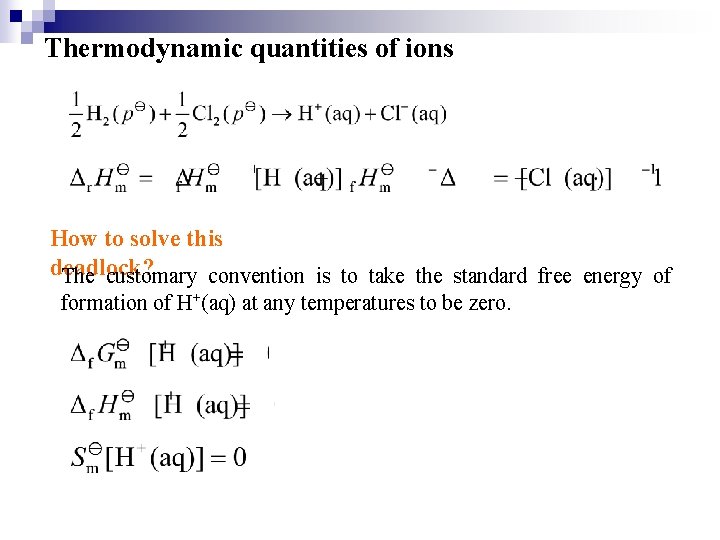
Thermodynamic quantities of ions How to solve this deadlock? The customary convention is to take the standard free energy of formation of H+(aq) at any temperatures to be zero.

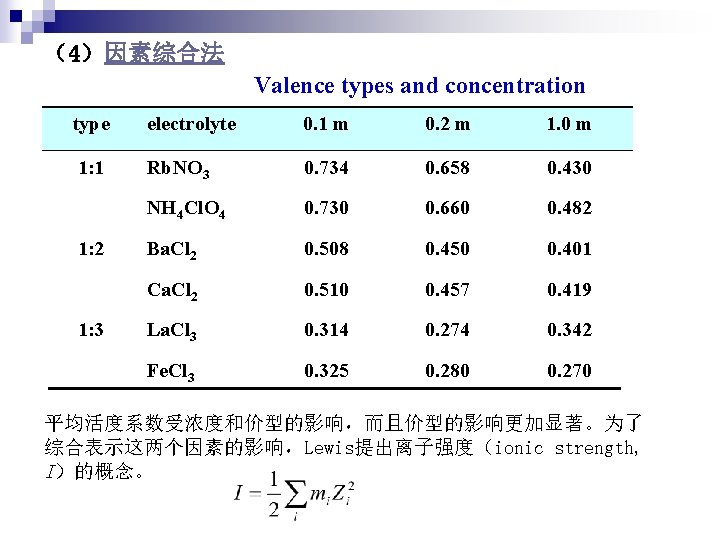
(4)因素综合法 Valence types and concentration type electrolyte 0. 1 m 0. 2 m 1. 0 m 1: 1 Rb. NO 3 0. 734 0. 658 0. 430 NH 4 Cl. O 4 0. 730 0. 660 0. 482 Ba. Cl 2 0. 508 0. 450 0. 401 Ca. Cl 2 0. 510 0. 457 0. 419 La. Cl 3 0. 314 0. 274 0. 342 Fe. Cl 3 0. 325 0. 280 0. 270 1: 2 1: 3 平均活度系数受浓度和价型的影响,而且价型的影响更加显著。为了 综合表示这两个因素的影响,Lewis提出离子强度(ionic strength, I)的概念。

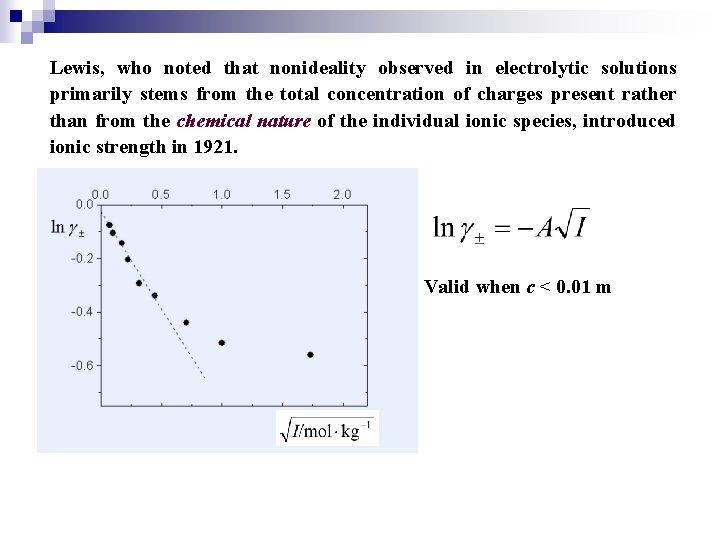
Lewis, who noted that nonideality observed in electrolytic solutions primarily stems from the total concentration of charges present rather than from the chemical nature of the individual ionic species, introduced ionic strength in 1921. Valid when c < 0. 01 m


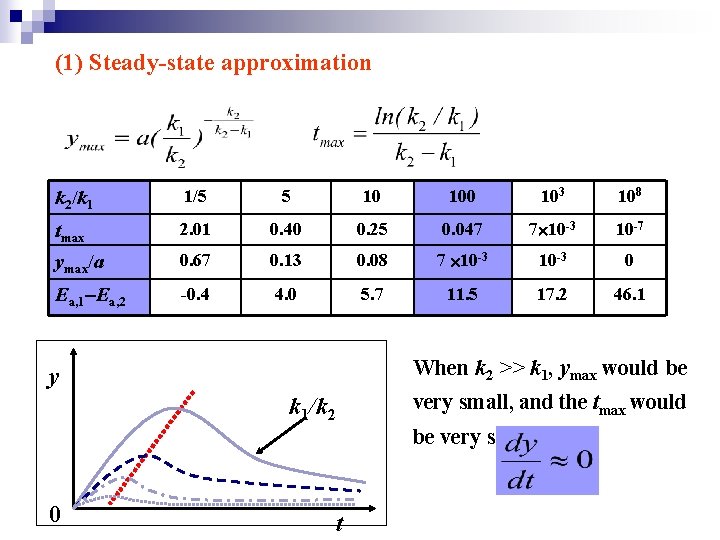
(1) Steady-state approximation k 2/k 1 1/5 5 10 103 108 tmax 2. 01 0. 40 0. 25 0. 047 7 10 -3 10 -7 ymax/a 0. 67 0. 13 0. 08 7 10 -3 0 Ea, 1 Ea, 2 -0. 4 4. 0 5. 7 11. 5 17. 2 46. 1 When k 2 >> k 1, ymax would be y very small, and the tmax would k 1/k 2 be very short. 0 t

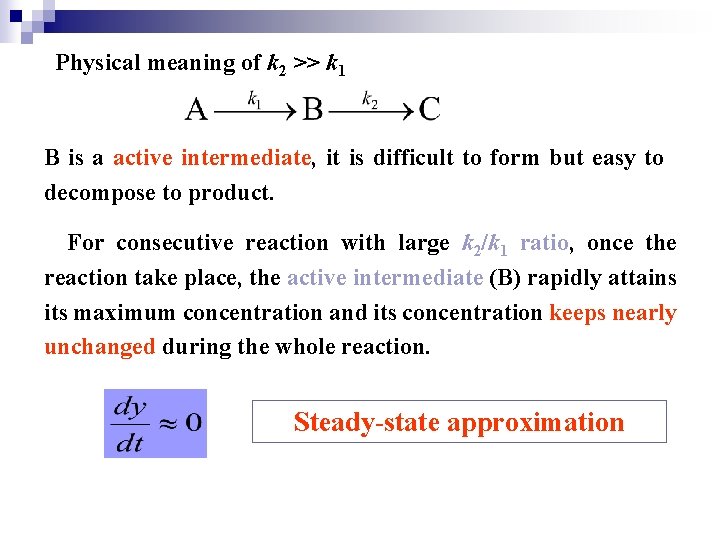
Physical meaning of k 2 >> k 1 B is a active intermediate, it is difficult to form but easy to decompose to product. For consecutive reaction with large k 2/k 1 ratio, once the reaction take place, the active intermediate (B) rapidly attains its maximum concentration and its concentration keeps nearly unchanged during the whole reaction. Steady-state approximation

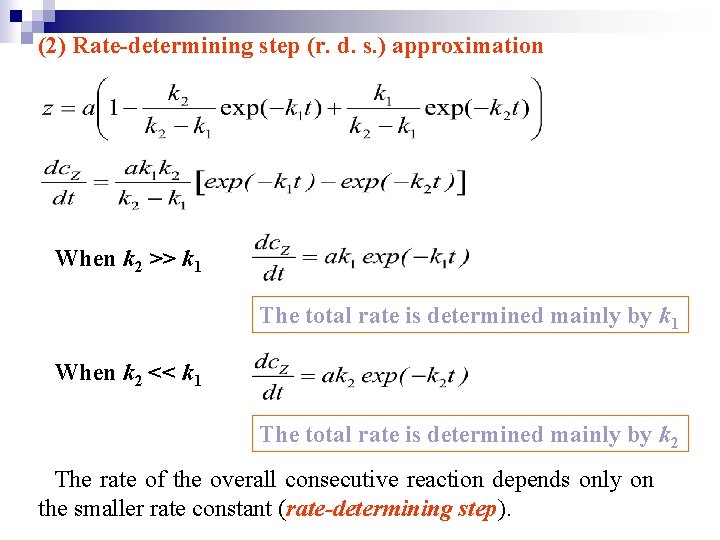
(2) Rate-determining step (r. d. s. ) approximation When k 2 >> k 1 The total rate is determined mainly by k 1 When k 2 << k 1 The total rate is determined mainly by k 2 The rate of the overall consecutive reaction depends only on the smaller rate constant (rate-determining step).

rate-determining step (r. d. s. ): the step with the slowest rate. patient ! It’s a r. d. s ? ? !!




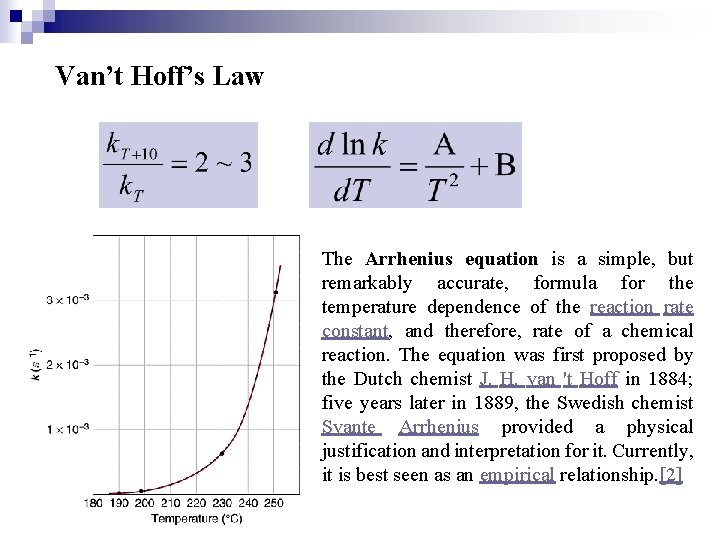
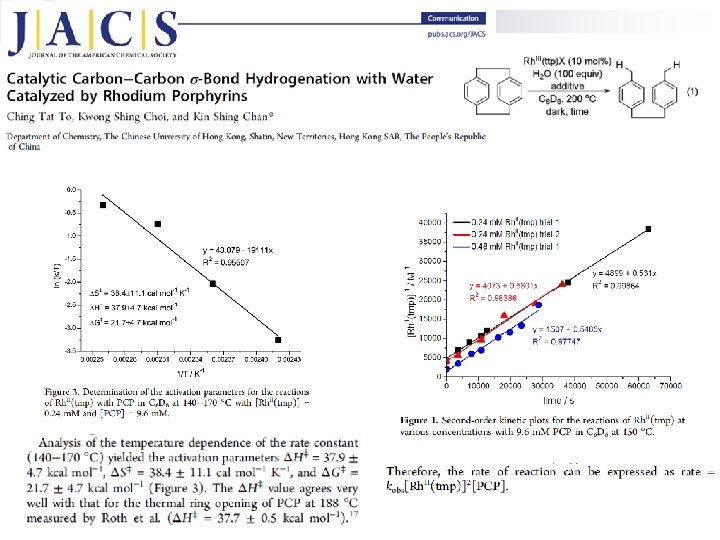
Van’t Hoff’s Law The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction. The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish chemist Svante Arrhenius provided a physical justification and interpretation for it. Currently, it is best seen as an empirical relationship. [2]

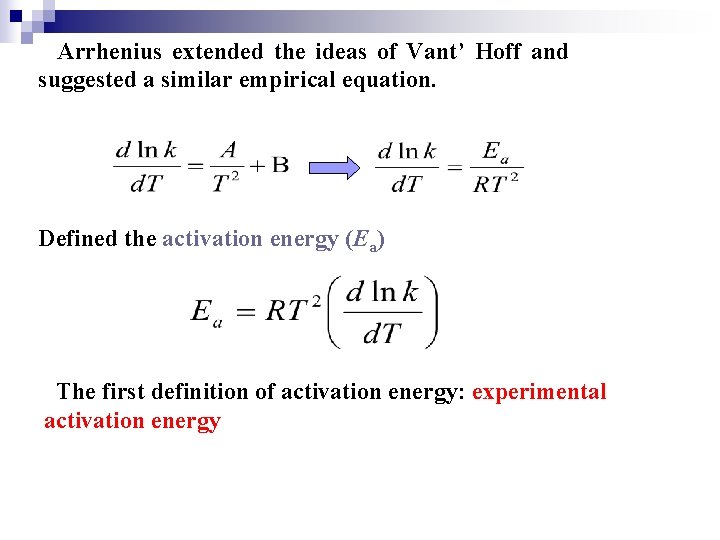
Arrhenius extended the ideas of Vant’ Hoff and suggested a similar empirical equation. Defined the activation energy (Ea) The first definition of activation energy: experimental activation energy
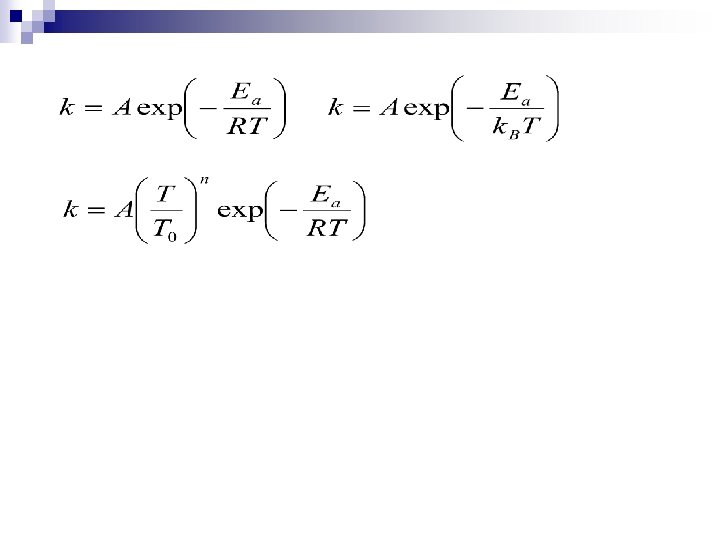

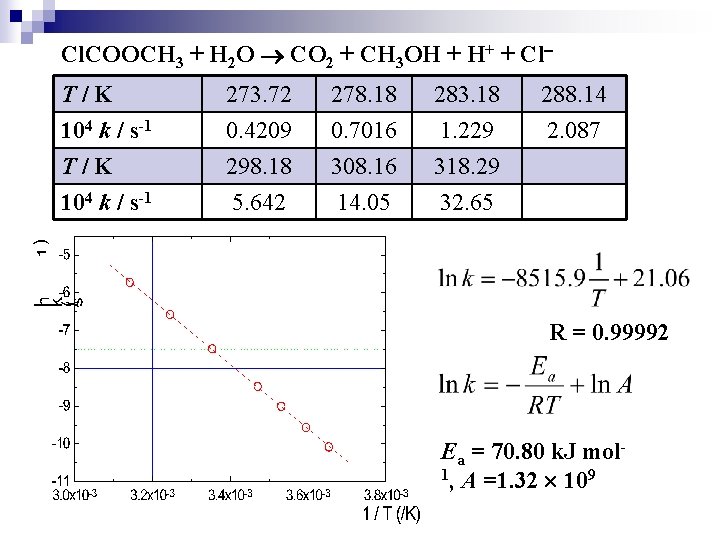
Cl. COOCH 3 + H 2 O CO 2 + CH 3 OH + H+ + Cl T/K 104 k / s-1 273. 72 0. 4209 298. 18 5. 642 278. 18 0. 7016 308. 16 14. 05 283. 18 1. 229 318. 29 32. 65 288. 14 2. 087 R = 0. 99992 Ea = 70. 80 k. J mol 1, A =1. 32 109
