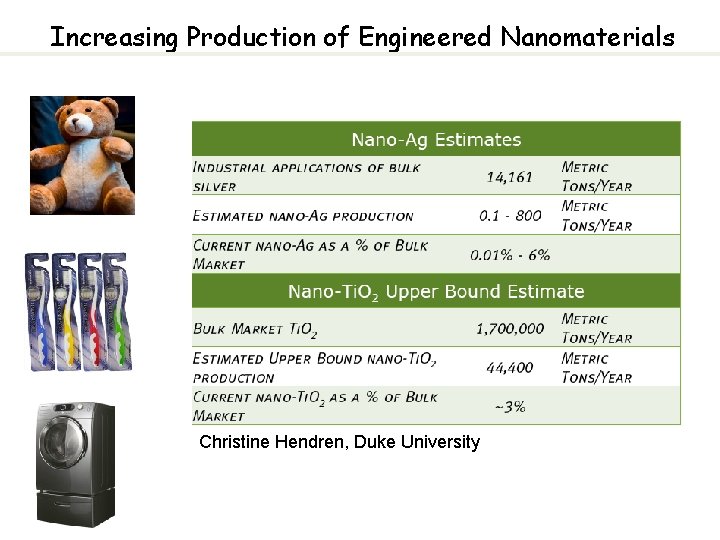
1 Increasing Production of Engineered Nanomaterials Christine Hendren
















- Slides: 34

1

Increasing Production of Engineered Nanomaterials Christine Hendren, Duke University

Egyptian Eye Makeup and Hair Dye (~ 2000 BC): Fabrication of quantum dots in your hair!! The composition and supramolecular organization of keratins can control Pb. S nanocrystal growth inside a hair Nano Lett. , 2006, 6 (10), pp 2215– 2219

Maya Blue Paint (2000 BC – AD 250): An hybrid organic-inorganic composite nanomaterial Resistance to acid and biocorrosion, color retention after centuries in the extreme conditions of the rain forest Science 273, 223 (1996)

The Lycurgus Cup (~ 4 AD): Most sophisticated glass objects before the modern era www. nanowerk. com/spotlight/ NPs are silver-gold alloy, with a ratio of silver to gold of about 7: 3, containing in addition about 10% copper.

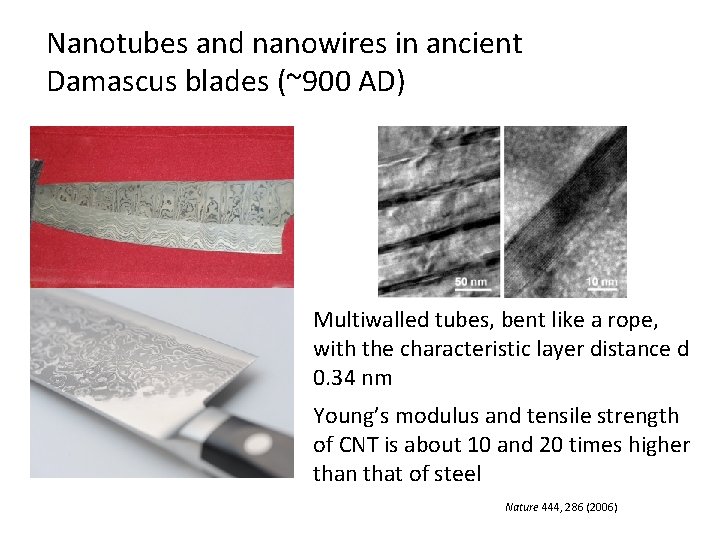
Nanotubes and nanowires in ancient Damascus blades (~900 AD) Multiwalled tubes, bent like a rope, with the characteristic layer distance d 0. 34 nm Young’s modulus and tensile strength of CNT is about 10 and 20 times higher than that of steel Nature 444, 286 (2006)

Nanoparticles are Ubiquitous in Water and Wastewater The Clark Fork River in Western Montana Source: Hochella et al. , 2007 Mine Drainage Hydrothermal Vent Sediment Porewater

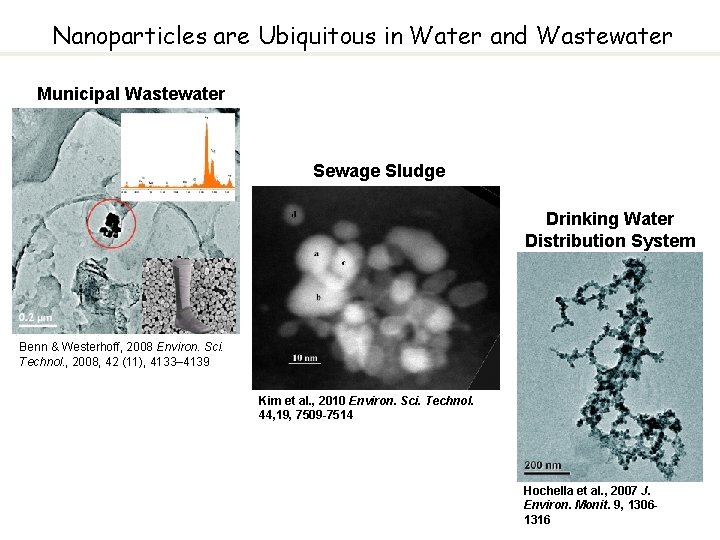
Nanoparticles are Ubiquitous in Water and Wastewater Municipal Wastewater Sewage Sludge Drinking Water Distribution System Benn & Westerhoff, 2008 Environ. Sci. Technol. , 2008, 42 (11), 4133– 4139 Kim et al. , 2010 Environ. Sci. Technol. 44, 19, 7509 -7514 Hochella et al. , 2007 J. Environ. Monit. 9, 13061316

9

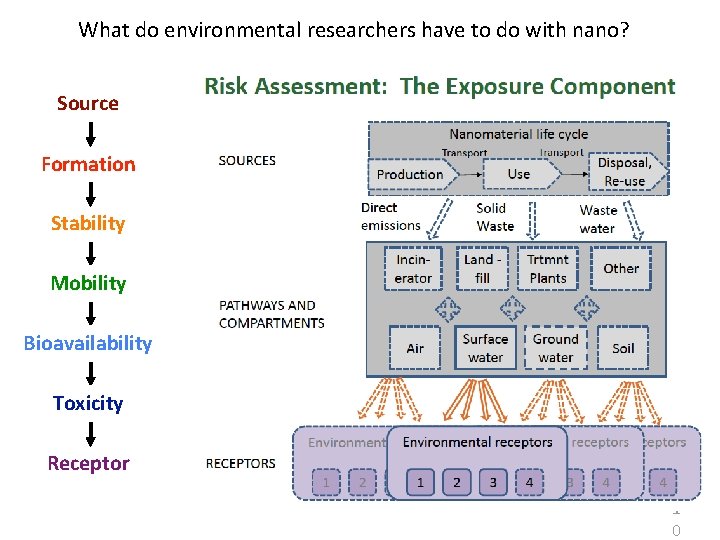
What do environmental researchers have to do with nano? Source Formation Stability Mobility Bioavailability Toxicity Receptor 1 0


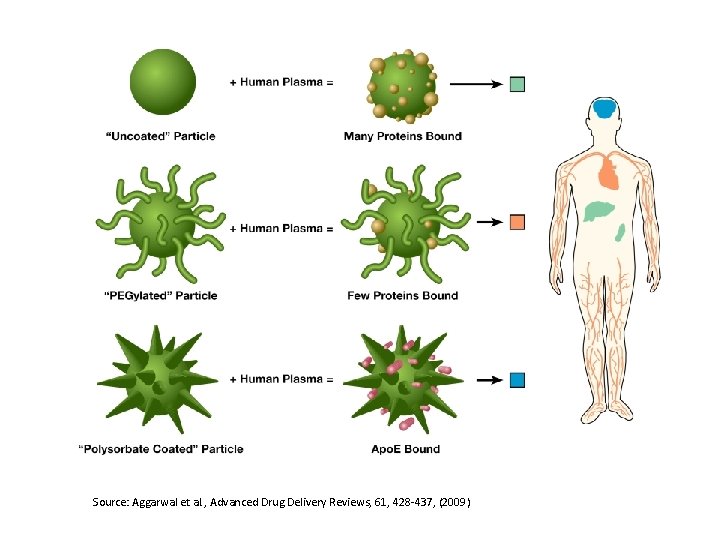
Source: Aggarwal et al. , Advanced Drug Delivery Reviews, 61, 428 -437, (2009)


1 4

1 5

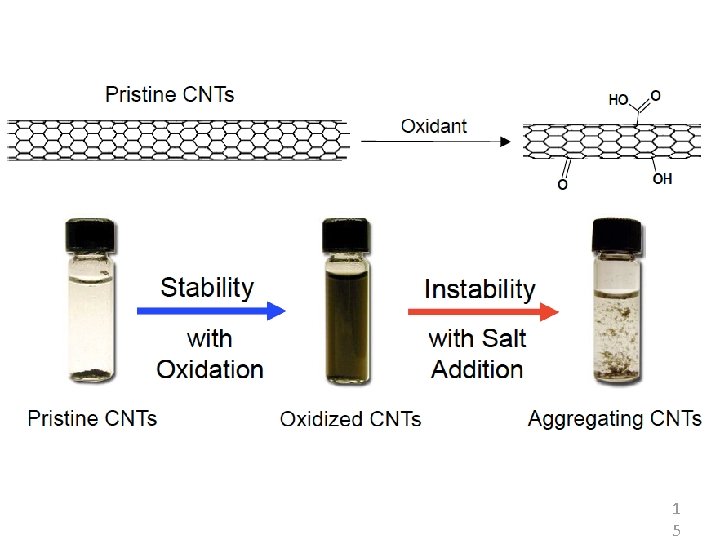
Levard et al. , ES&T, 2012, 46 (13), pp 6900– 6914

Kaegi et al. Environ. Sci. Technol. 2011, 45, 3902– 3908. 1 7

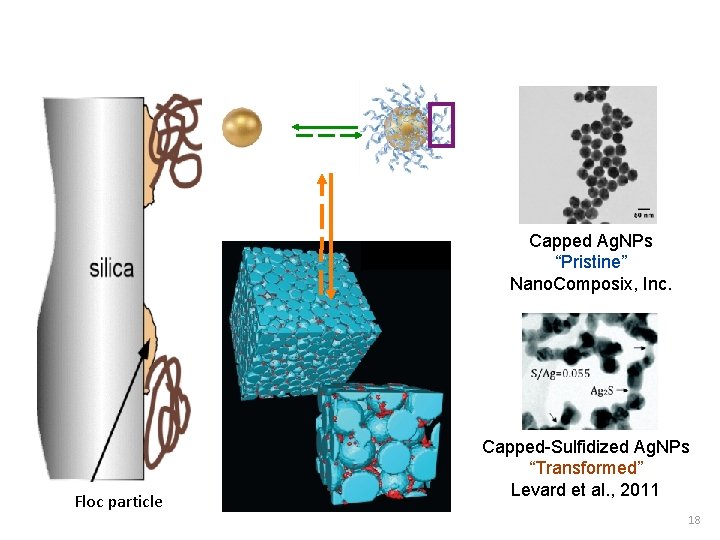
Capped Ag. NPs “Pristine” Nano. Composix, Inc. Floc particle Capped-Sulfidized Ag. NPs “Transformed” Levard et al. , 2011 18

p. H 7 5 m. M Na. NO 3 19

p. H 7 5 m. M Na. NO 3 20

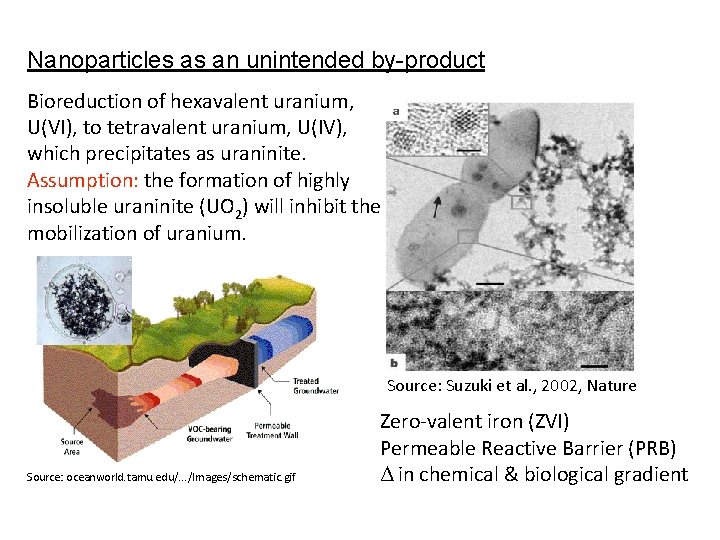
Nanoparticles as an unintended by-product Bioreduction of hexavalent uranium, U(VI), to tetravalent uranium, U(IV), which precipitates as uraninite. Assumption: the formation of highly insoluble uraninite (UO 2) will inhibit the mobilization of uranium. Source: Suzuki et al. , 2002, Nature Source: oceanworld. tamu. edu/. . . /Images/schematic. gif Zero-valent iron (ZVI) Permeable Reactive Barrier (PRB) Δ in chemical & biological gradient

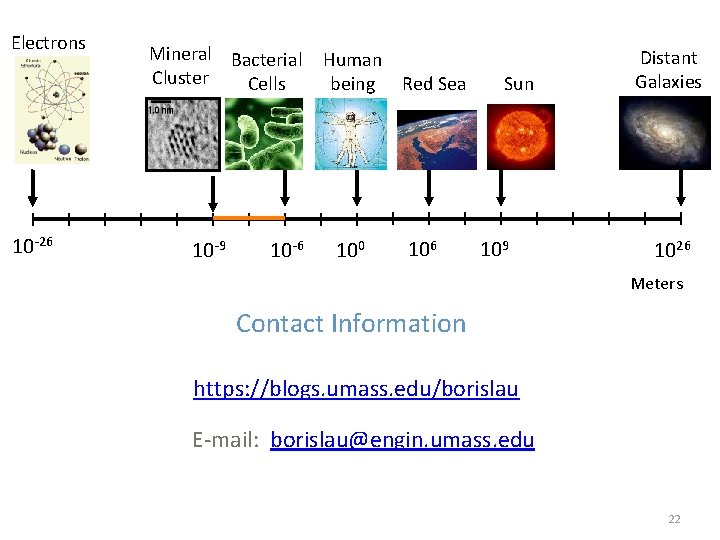
Electrons 10 -26 Mineral Bacterial Human Cluster Cells being Red Sea 10 -9 10 -6 100 106 Sun 109 Distant Galaxies 1026 Meters Contact Information https: //blogs. umass. edu/borislau E-mail: borislau@engin. umass. edu 22

2 3

2 4

2 5

2 6

Source: Andrew Maynard, Woodrow Wilson Center, Project on Emerging Nanotechnologies

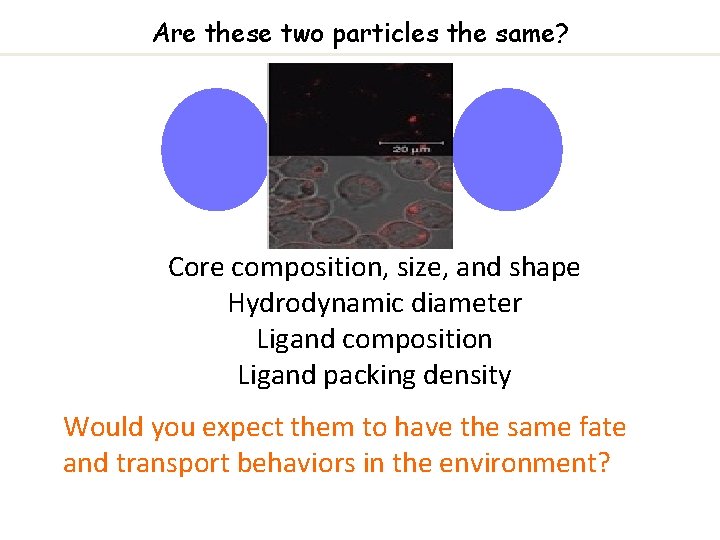
Are these two particles the same? Core composition, size, and shape Hydrodynamic diameter Ligand composition Ligand packing density Would you expect them to have the same fate and transport behaviors in the environment?

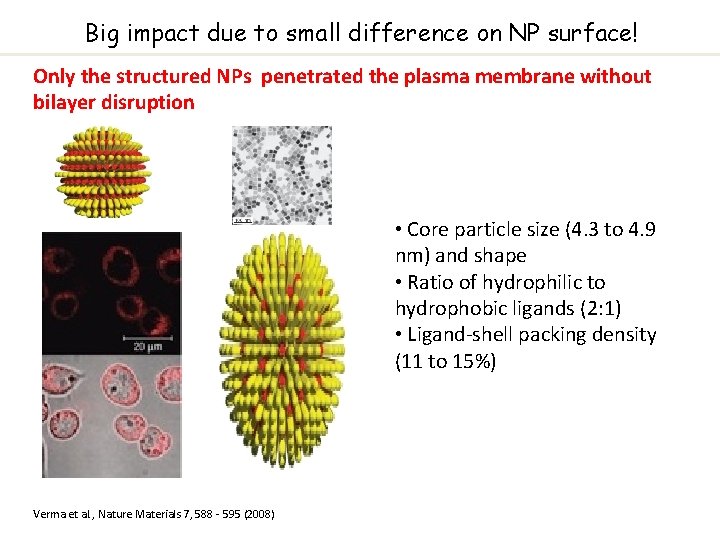
Big impact due to small difference on NP surface! Only the structured NPs penetrated the plasma membrane without bilayer disruption • Core particle size (4. 3 to 4. 9 nm) and shape • Ratio of hydrophilic to hydrophobic ligands (2: 1) • Ligand-shell packing density (11 to 15%) Verma et al. , Nature Materials 7, 588 - 595 (2008)

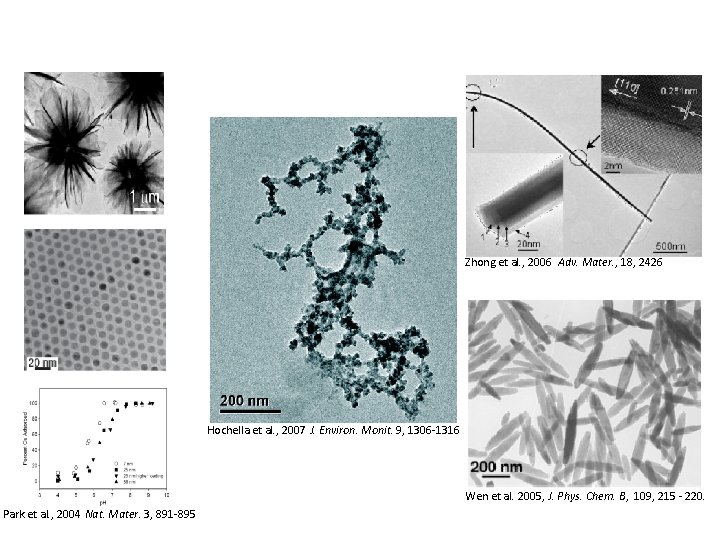
Zhong et al. , 2006 Adv. Mater. , 18, 2426 Hochella et al. , 2007 J. Environ. Monit. 9, 1306 -1316 Wen et al. 2005, J. Phys. Chem. B, 109, 215 - 220. Park et al. , 2004 Nat. Mater. 3, 891 -895

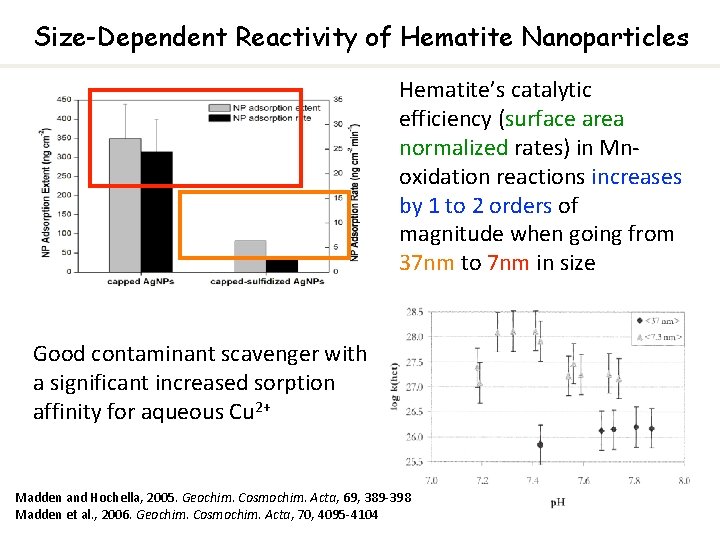
Size-Dependent Reactivity of Hematite Nanoparticles Hematite’s catalytic efficiency (surface area normalized rates) in Mnoxidation reactions increases by 1 to 2 orders of magnitude when going from 37 nm to 7 nm in size Good contaminant scavenger with a significant increased sorption affinity for aqueous Cu 2+ Madden and Hochella, 2005. Geochim. Cosmochim. Acta, 69, 389 -398 Madden et al. , 2006. Geochim. Cosmochim. Acta, 70, 4095 -4104

p. H 6 10 m. M Na. NO 3 32


3 4
 Simple cubic unit cell
Simple cubic unit cell Hardness of nanomaterials
Hardness of nanomaterials Magnetic properties of nanomaterials
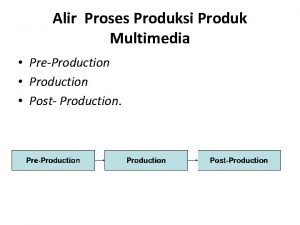
Magnetic properties of nanomaterials Apa itu pre-production?
Apa itu pre-production? Engineered wear products
Engineered wear products Engineered shading
Engineered shading Engineered castings
Engineered castings Unit 7 engineered wood products
Unit 7 engineered wood products Ellwood engineered castings
Ellwood engineered castings Which type of plan shows the layout of hvac system
Which type of plan shows the layout of hvac system Engineered pressure systems
Engineered pressure systems Engineered in germany
Engineered in germany What are engineered plans for motors pumps
What are engineered plans for motors pumps Laser engineered net shaping
Laser engineered net shaping Graphing transformations
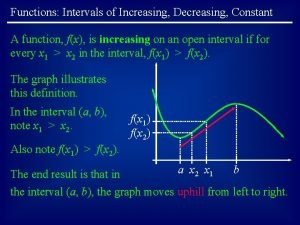
Graphing transformations Increasing intervals
Increasing intervals Economies of scale graph
Economies of scale graph Comparing fractions with different denominators
Comparing fractions with different denominators Increasing and decreasing recipes
Increasing and decreasing recipes Amine carboxylic acid reaction
Amine carboxylic acid reaction The law of increasing opportunity costs states that
The law of increasing opportunity costs states that Law of increasing opportunity cost
Law of increasing opportunity cost Bulk reducing industries examples
Bulk reducing industries examples Increasing intervals
Increasing intervals Increasing quantities
Increasing quantities Atomic size
Atomic size Electromagenetic spectrum
Electromagenetic spectrum Compared to a gear tooth on the rear sprocket
Compared to a gear tooth on the rear sprocket On the grasshopper and the cricket summary
On the grasshopper and the cricket summary Cognitive synonym
Cognitive synonym Graphs that compare distance and time are called
Graphs that compare distance and time are called What causes friction
What causes friction Increasing cost industry supply curve
Increasing cost industry supply curve Longest increasing subsequence
Longest increasing subsequence Is the act of increasing the knowledge
Is the act of increasing the knowledge