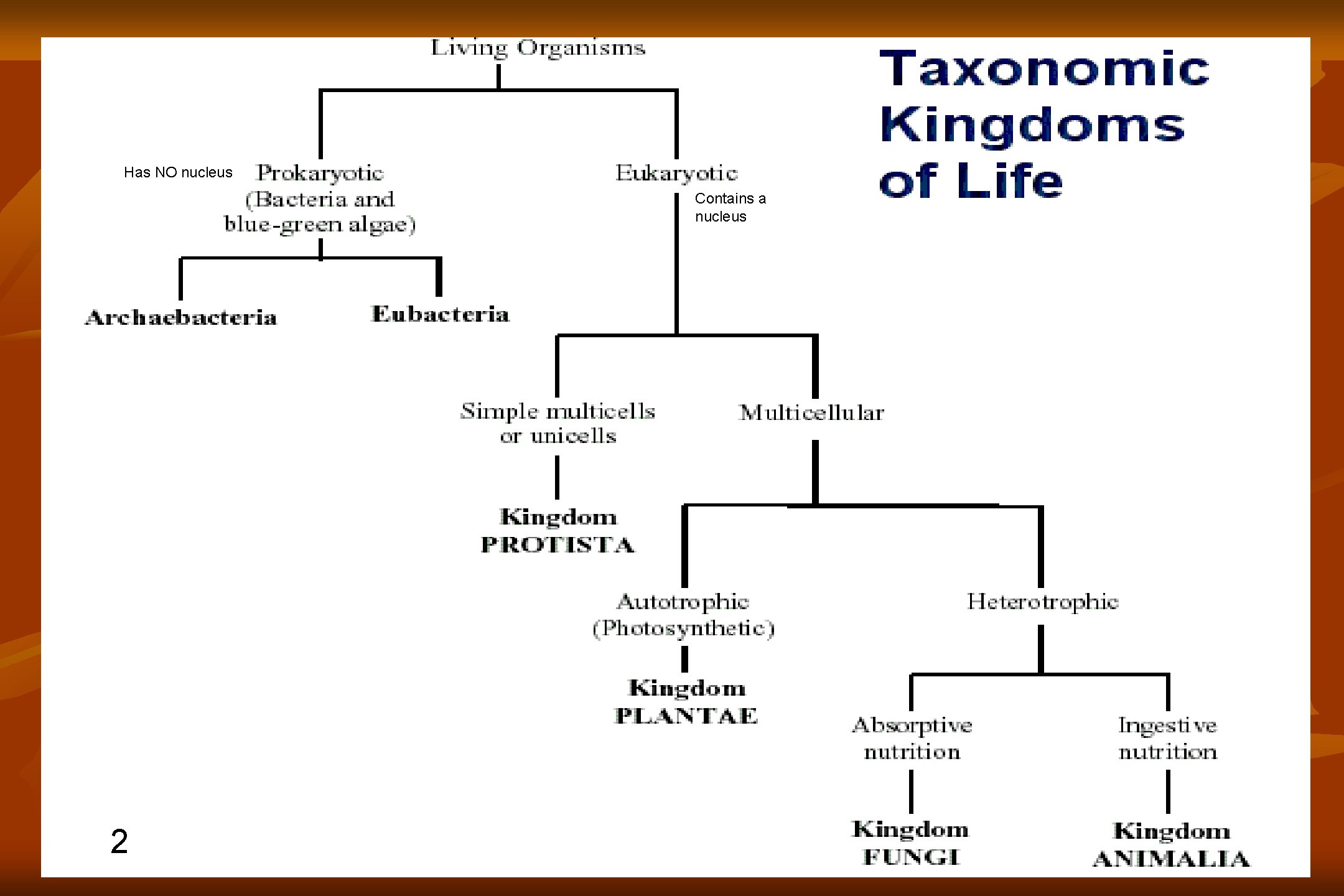
1 Has NO nucleus Contains a nucleus 2

















































- Slides: 108


1

Has NO nucleus Contains a nucleus 2

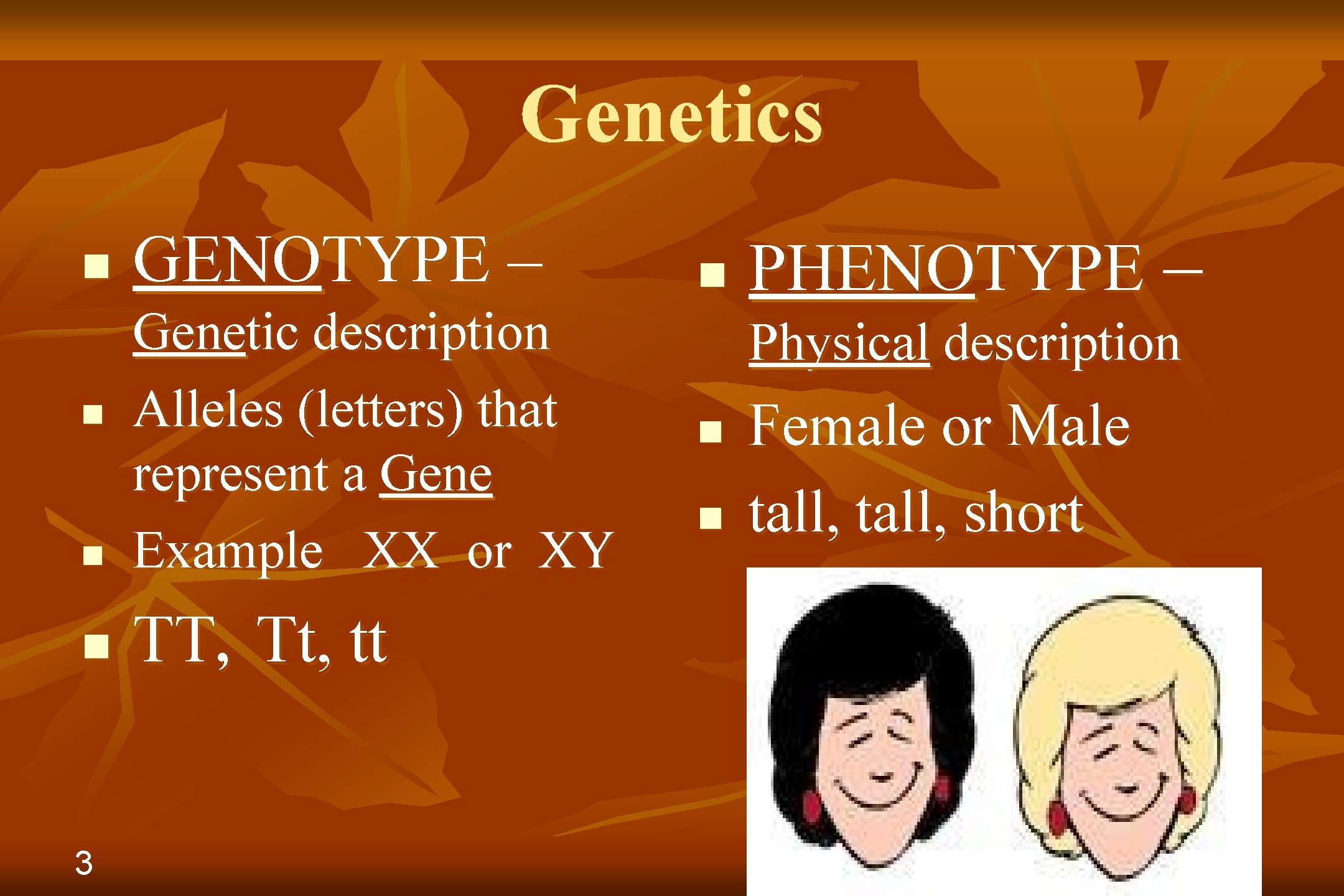
Genetics n GENOTYPE – n Genetic description Alleles (letters) that represent a Gene Example XX or XY n TT, Tt, tt n 3 n PHENOTYPE – Physical description n n Female or Male tall, short

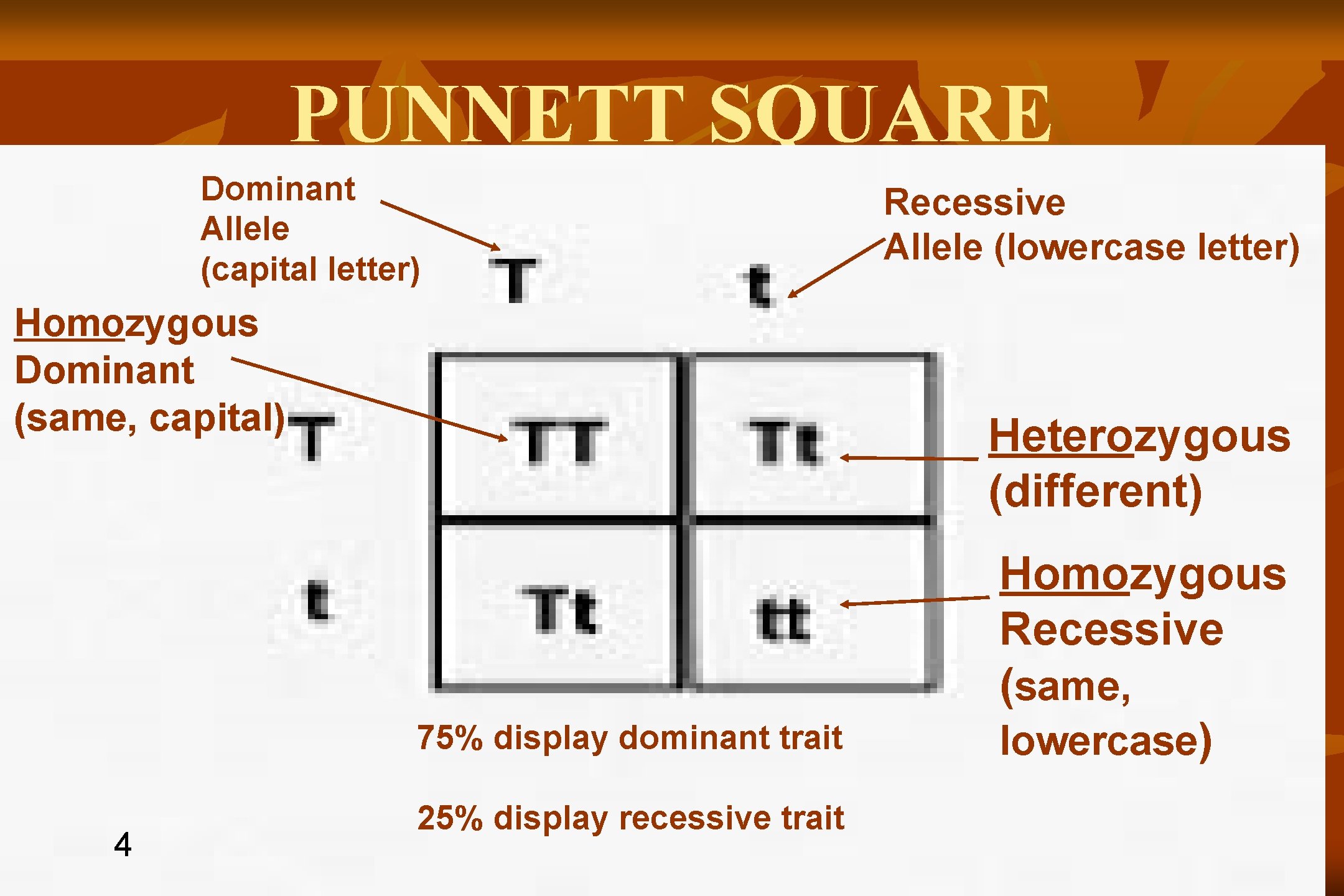
PUNNETT SQUARE Dominant Allele (capital letter) Homozygous Dominant (same, capital) Heterozygous (different) 75% display dominant trait 4 Recessive Allele (lowercase letter) 25% display recessive trait Homozygous Recessive (same, lowercase)

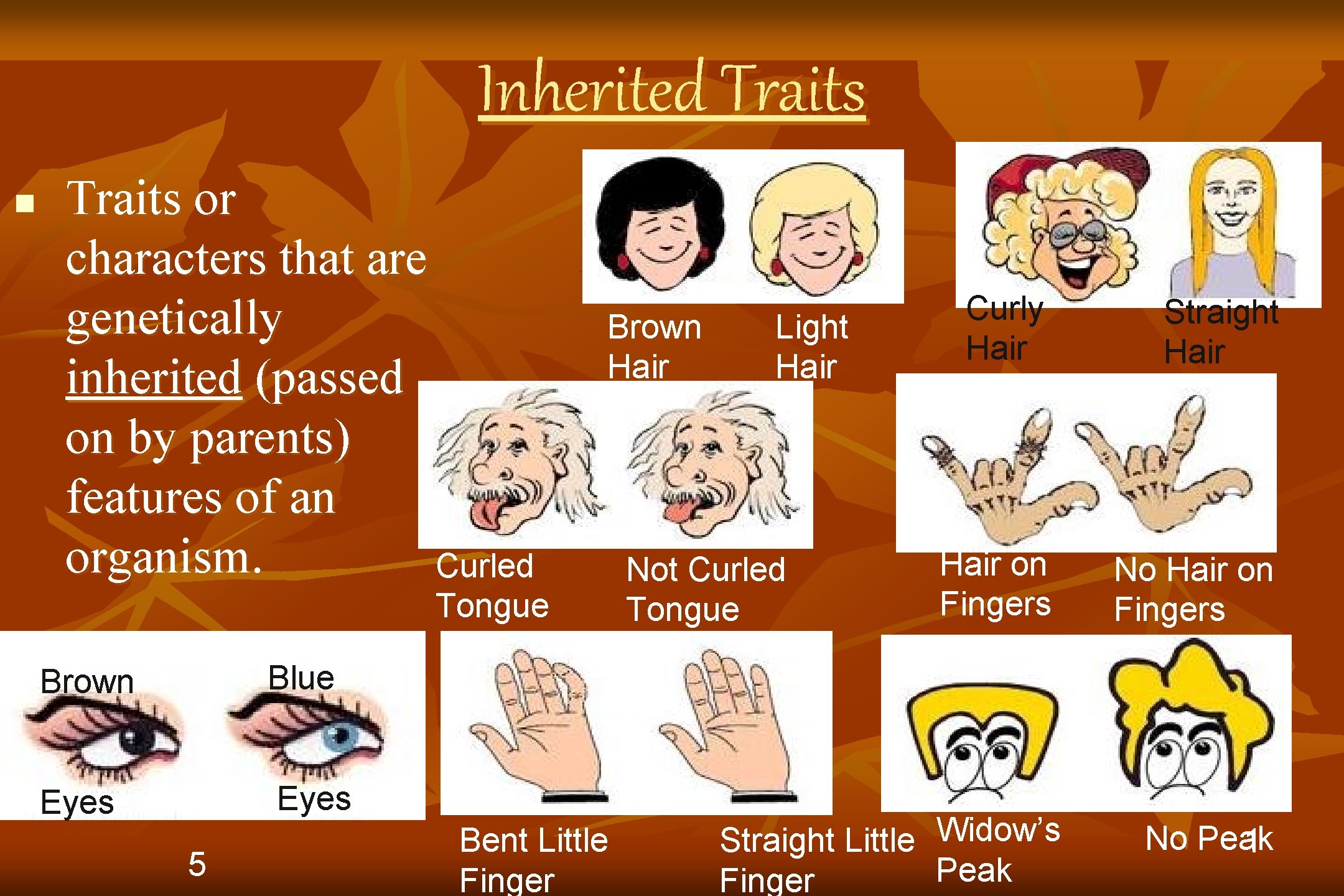
Inherited Traits n Traits or characters that are genetically inherited (passed on by parents) features of an organism. Curled Brown Hair Tongue Brown Blue Eyes 5 Bent Little Finger Curly Hair Straight Hair on Fingers No Hair on Fingers Widow’s Straight Little Peak Finger No Peak 1 Light Hair Not Curled Tongue

Traits affected by environment n n n Weight gain Tanning your skin Chemicals Light Temperature 6

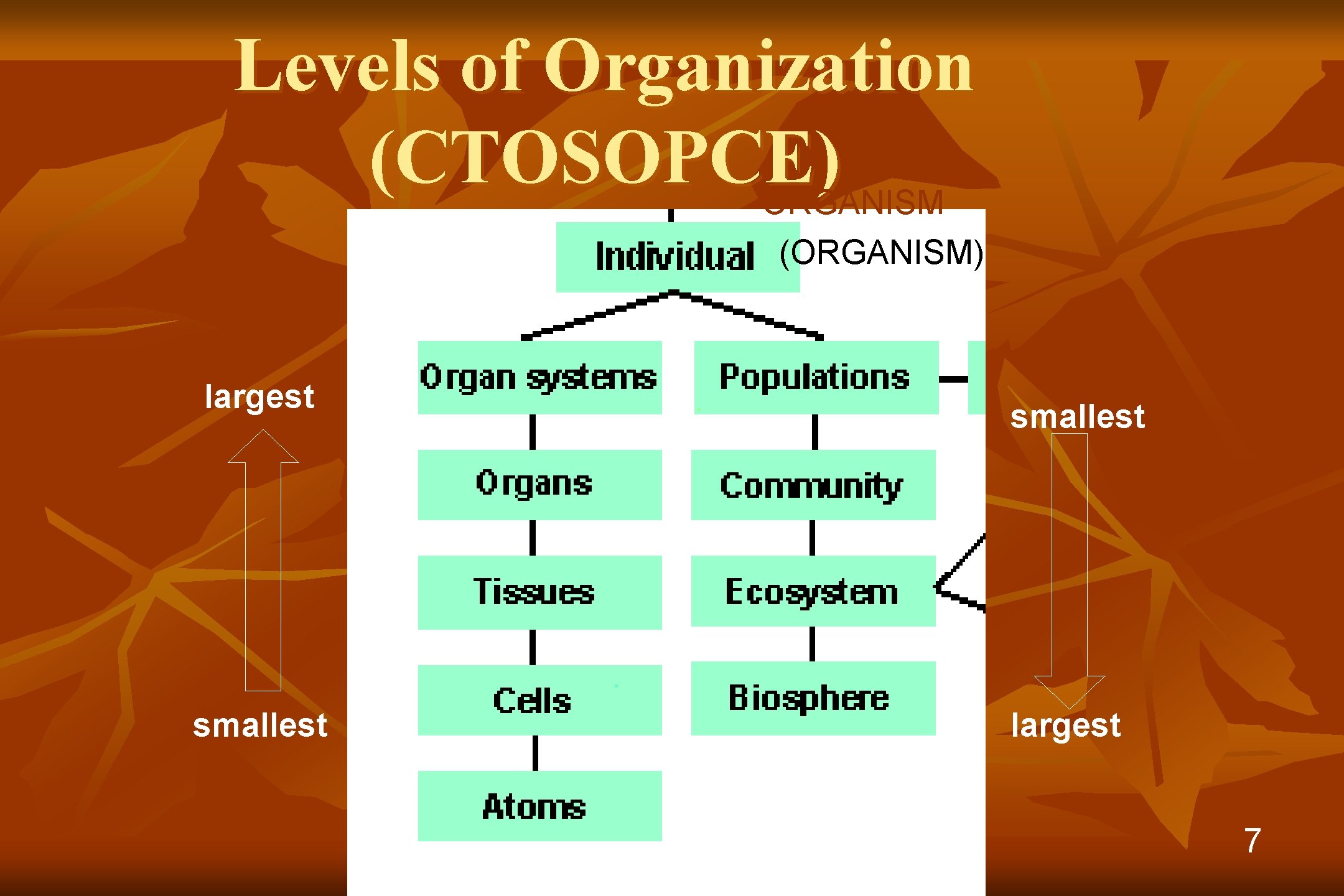
Levels of Organization (CTOSOPCE) ORGANISM (ORGANISM) largest smallest largest 7

8 PLANT n n n CELLS Cell wall Contains chloroplast Larger vacuoles n n ANIMAL Flagellum to help with movement, No cell wall or chloroplast 1

Cell Parts n n n n CELL WALL - (found only in plant cells) CHLOROPLAST - (found only in plant cells) where glucose/sugar is made, the process of Photosynthesis CELL MEMBRANE – the outer boundary that only allows certain materials to move into or out of the cell CYTOPLASM – gel-like material that contains water and nutrients NUCLEUS – contains the chromosomes with the DNA MITOCHONDRIA – breaks down food and releases energy VACUOLES – storage areas for cells 9

CELL THEORY n n n All organisms are composed of cells. All cells come from pre-existing cells Cells carry similar functions such as extracting energy from food to sustain life. 10

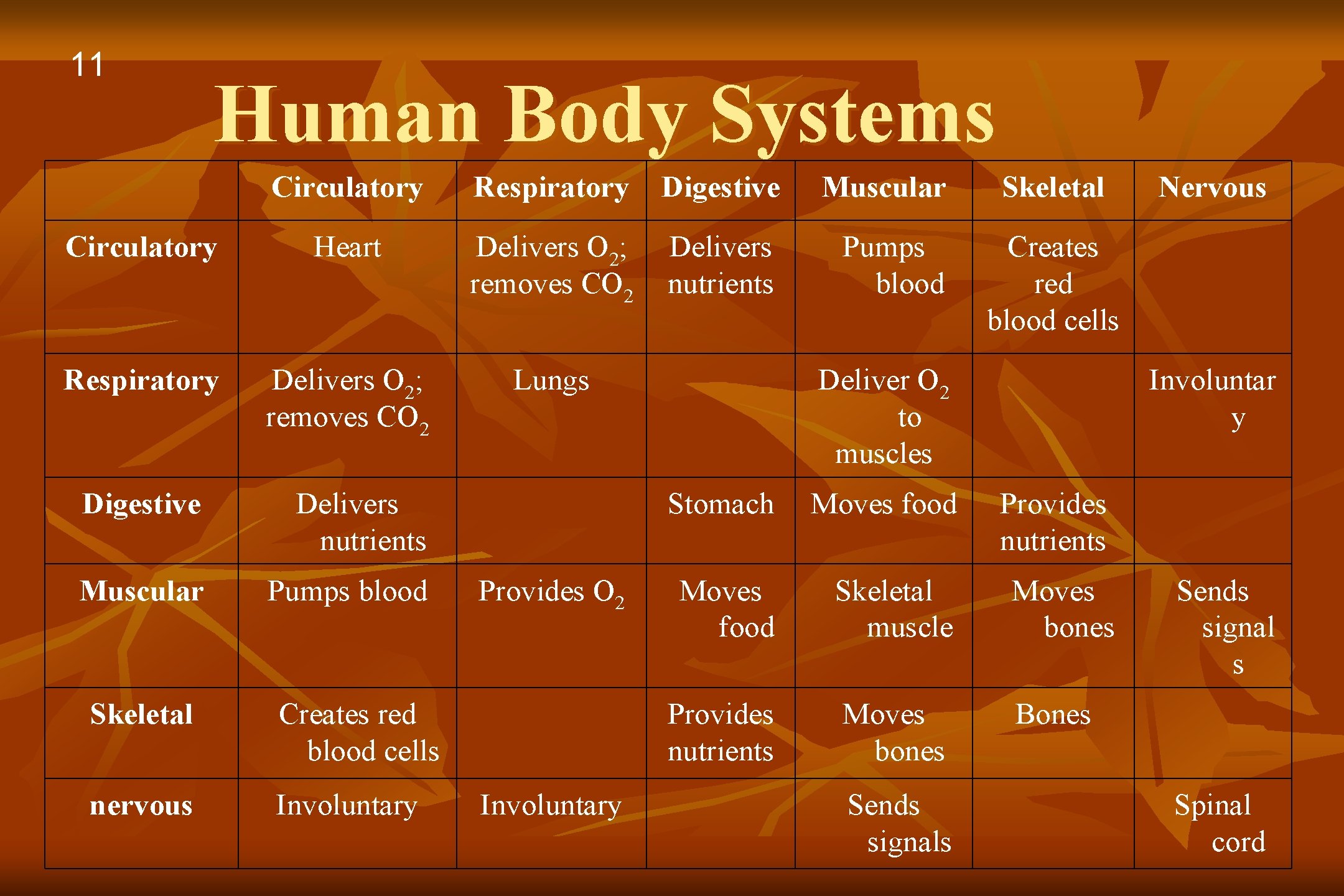
11 Human Body Systems Circulatory Respiratory Digestive Muscular Circulatory Heart Delivers O 2; removes CO 2 Delivers nutrients Pumps blood Respiratory Delivers O 2; removes CO 2 Lungs Digestive Delivers nutrients Muscular Pumps blood Skeletal Creates red blood cells nervous Involuntary Provides O 2 Involuntary Skeletal Creates red blood cells Deliver O 2 to muscles Involuntar y Stomach Moves food Provides nutrients Moves food Skeletal muscle Moves bones Provides nutrients Moves bones Bones Sends signals Nervous Sends signal s Spinal cord

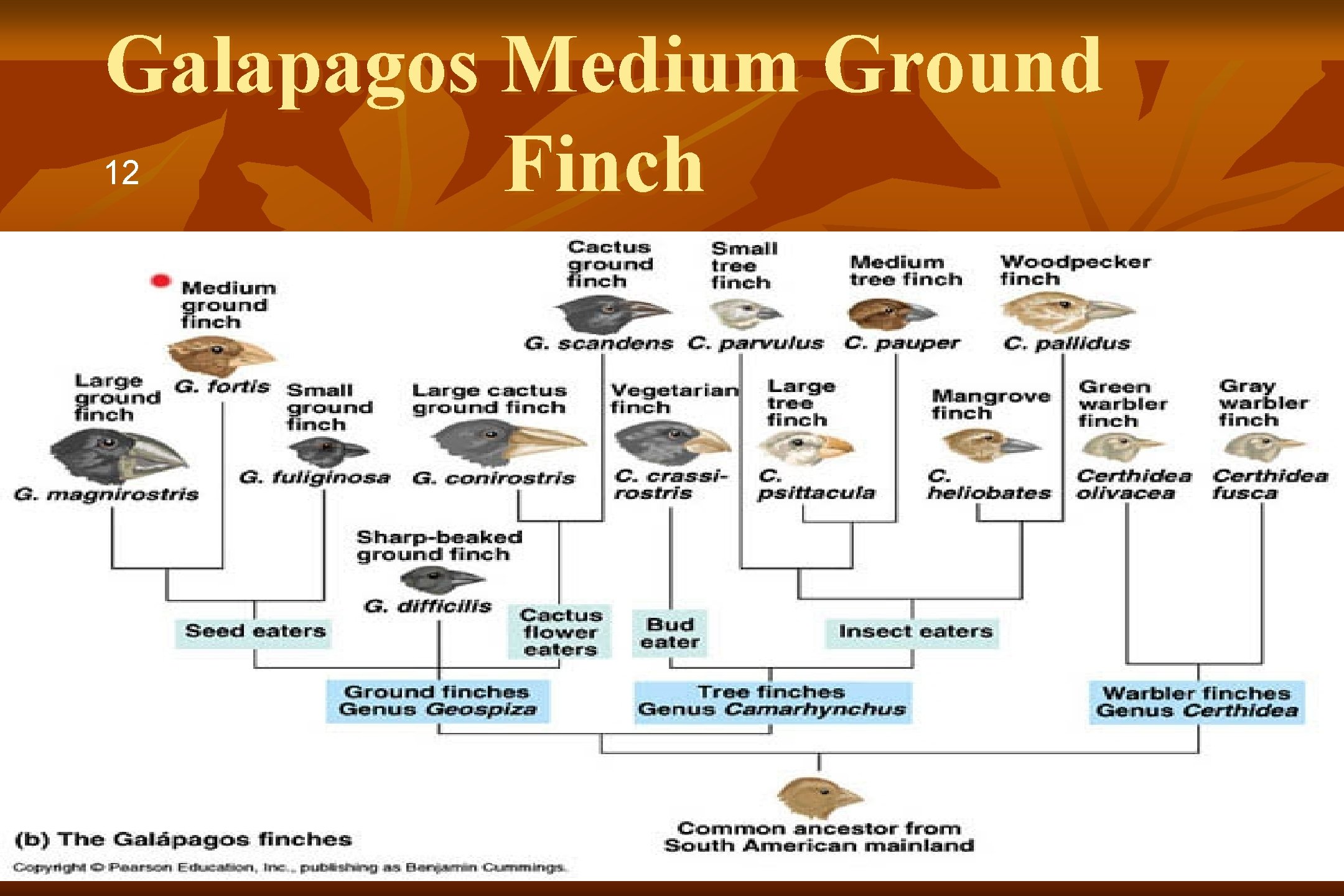
Galapagos Medium Ground 12 Finch 1

Species and Adaptations n n SPECIES – group of similar organisms that can mate and produce fertile offspring ADAPTATIONS – genetic trait that helps an organism survive and reproduce EVOLUTION – change in a species over time NATURAL SELECTION – process by which individuals better adapted to their environment are more likely to survive and reproduce 13

Dichotomous Keys for Identification n A dichotomous key is a tool that allows the user to determine the identity of items in the natural world, such as trees, wildflowers, mammals, reptiles, rocks, and fish. Keys consist of a series of choices that lead the user to the correct name of a given item. "Dichotomous" means "divided into two parts". Therefore, dichotomous keys always give two choices in each step. Basically it’s a series of questions that lead you to the correct IDENTITY of the organism 14

Dichotomous Key Example n n n 1. a. Organism is living. . . go to 4 1. b. Organism is nonliving. . . go to 2 2. a. Object is metallic. . . go to 3. 2. b. Object is nonmetallic. . . ROCK. 3. a. Object has wheels. . . BICYCLE. 3. b. Object does not have wheels. . . . TIN CAN. 4. a. Organism is microscopic. . . . . PARAMECIUM. 4. b. Organism is macroscopic. . . . . go to 5. 5. a. Organism is a plant. . . go to 6. 5. b. Organism is an animal. . . . . go to 8. 6. a. Plant has a woody stem. . . . . go to 7. 6. b. Plant has a herbaceous stem. . . . . DANDELION. n n n n n 7. a. Tree has needle like leaves. . . PINE TREE. 7. b. Tree has broad leaves. . . . OAK TREE. 8. a. Organism lives on land. . . . . go to 9. 8. b. Organism lives in water. . . . . CLAM. 9. a. Organism has 4 legs or fewer. . . . go to 10. 9. b. Organism has more than 4 legs. . . . ANT. 10 a. Organism has fur. . . go to 11. 10 b. Organism has feathers. . . . . ROBIN. 11 a. Organism has hooves. . . DEER. 11 b. Organism has no hooves. . . . . MOUSE. 15

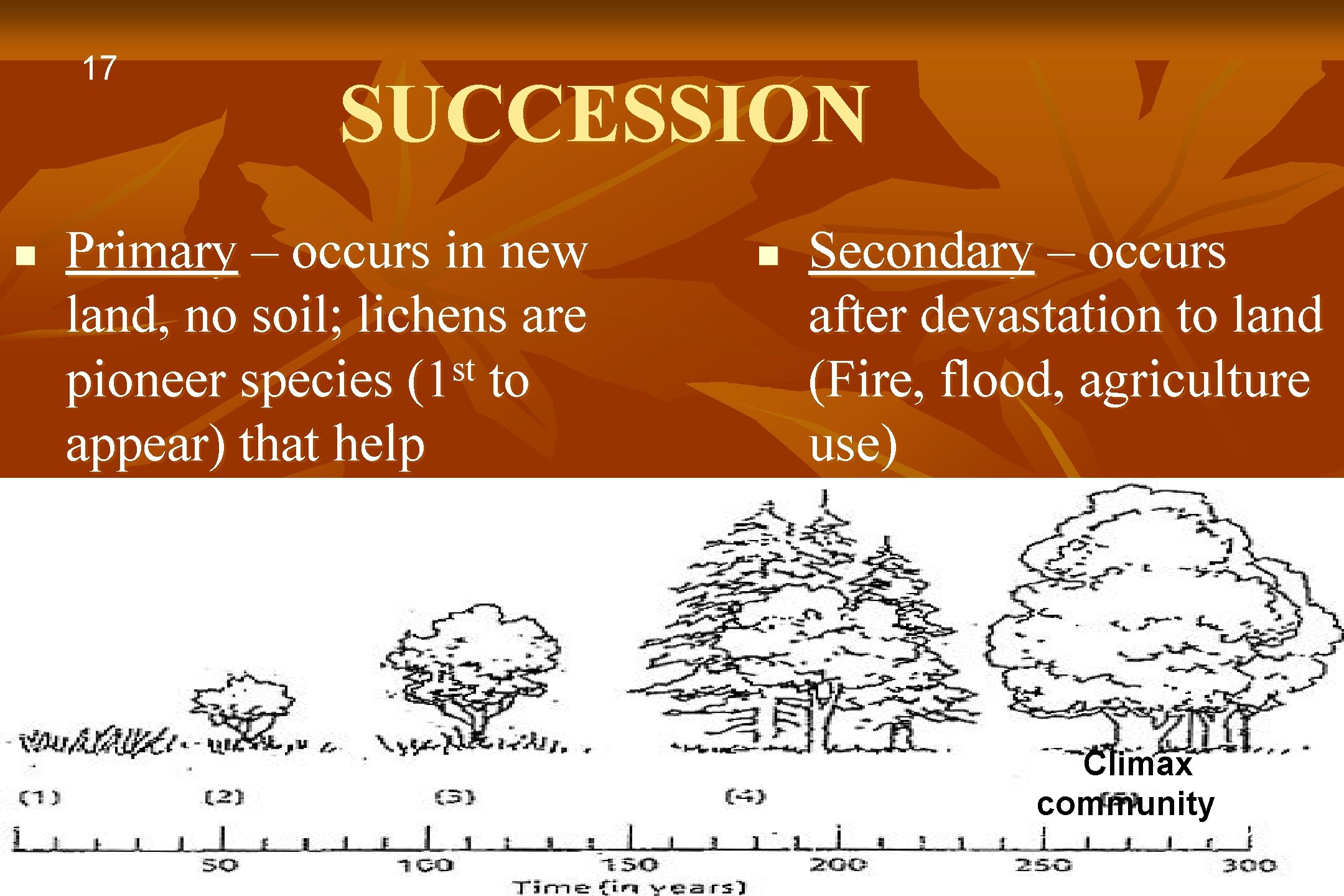
SUCCESSION Primary succession n begins with bare rock exposed by geologic activity (new land when volcano erupts n example: rock -> lichen > moss > grass -> shrub > trees > oak hickory forest Secondary succession n n begins on soil from which previous community has been removed (by fire, agriculture, etc. ) old field succession example: grass -> shrub > trees > oak hickory forest secondary succession can proceed much faster because the soil has been prepared by the previous community 16

17 n SUCCESSION Primary – occurs in new land, no soil; lichens are st pioneer species (1 to appear) that help develop soil n Secondary – occurs after devastation to land (Fire, flood, agriculture use) Climax community 1

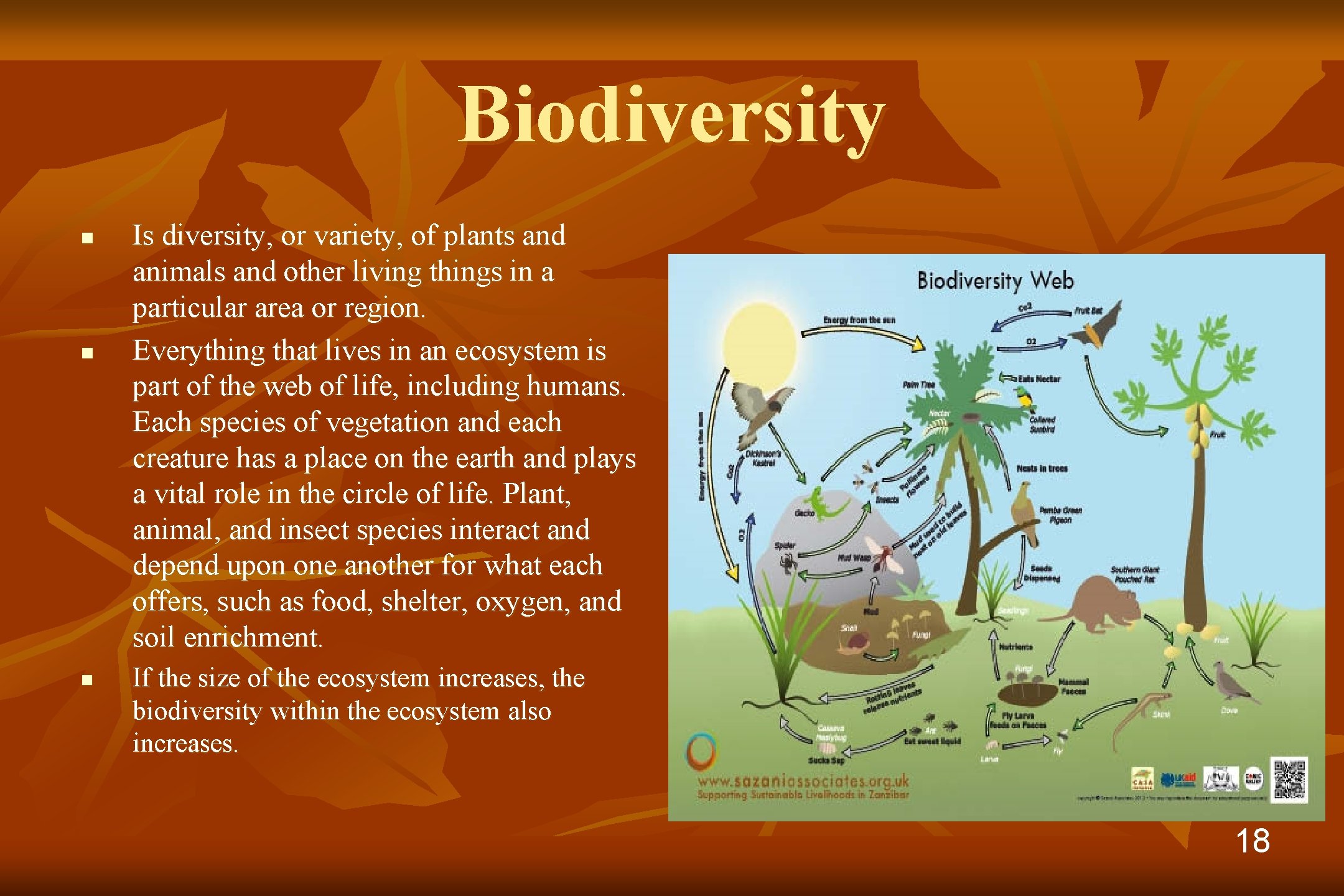
Biodiversity n n n Is diversity, or variety, of plants and animals and other living things in a particular area or region. Everything that lives in an ecosystem is part of the web of life, including humans. Each species of vegetation and each creature has a place on the earth and plays a vital role in the circle of life. Plant, animal, and insect species interact and depend upon one another for what each offers, such as food, shelter, oxygen, and soil enrichment. If the size of the ecosystem increases, the biodiversity within the ecosystem also increases. 18

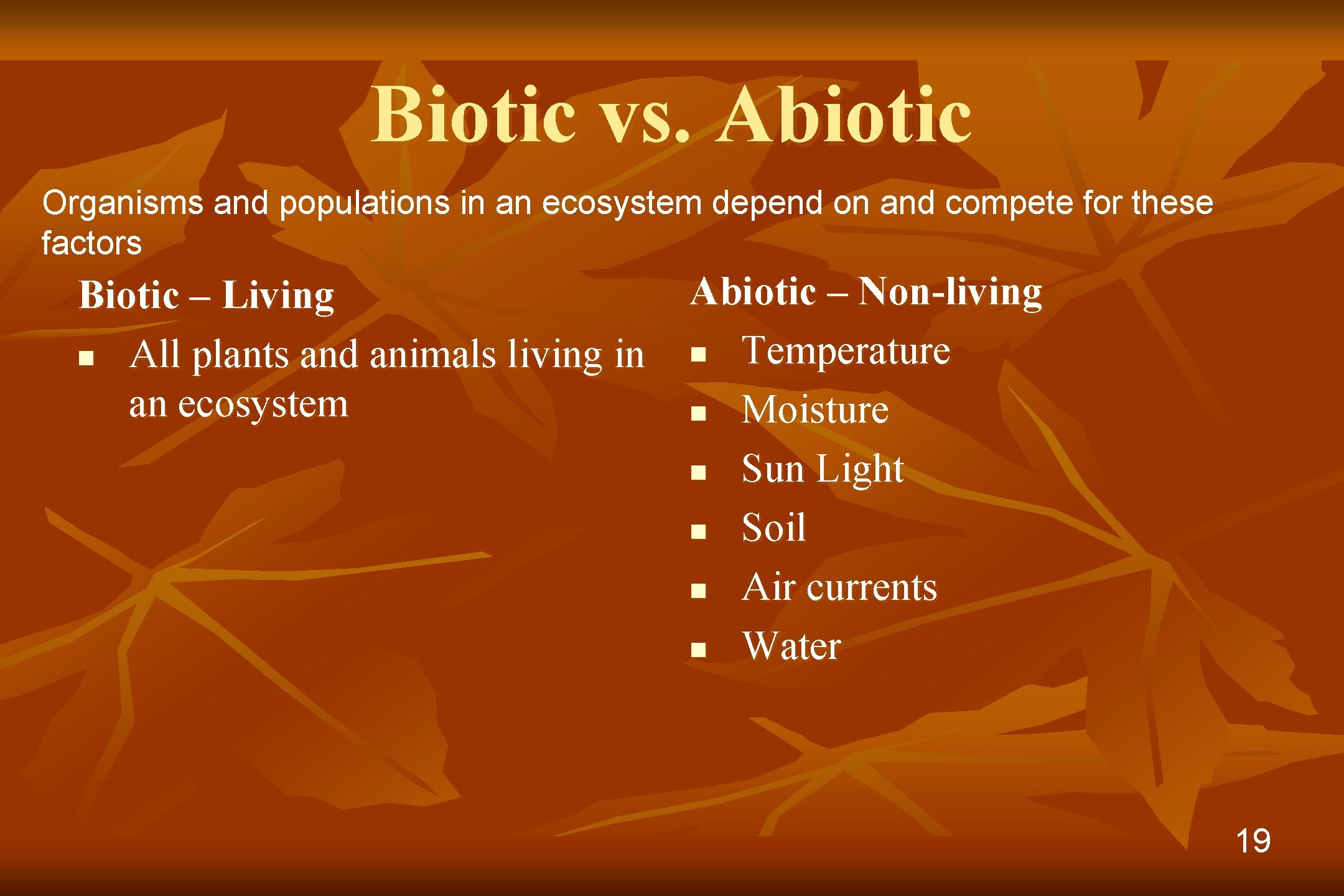
Biotic vs. Abiotic Organisms and populations in an ecosystem depend on and compete for these factors Biotic – Living n All plants and animals living in an ecosystem Abiotic – Non-living n Temperature n Moisture n Sun Light n Soil n Air currents n Water 19

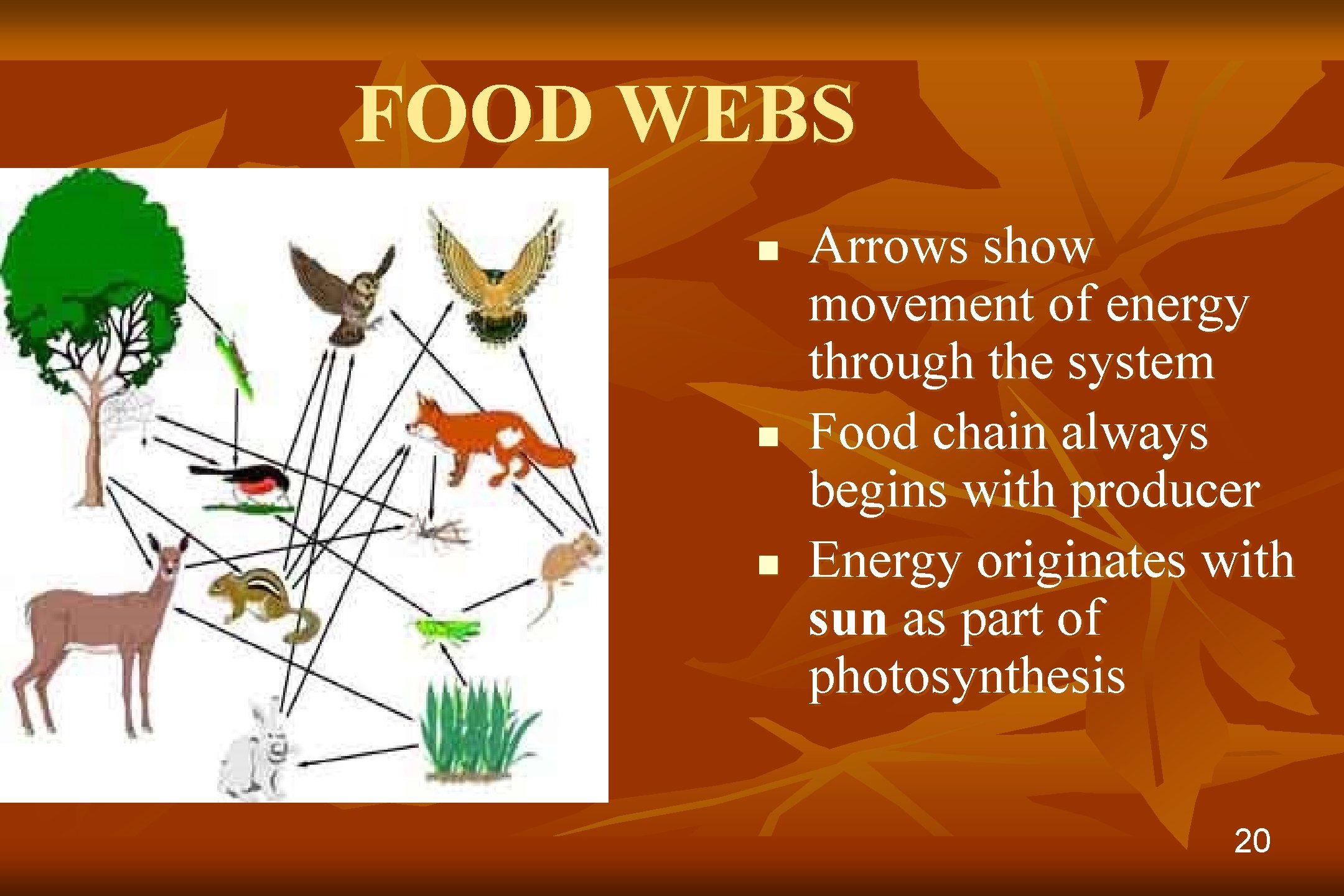
FOOD WEBS n n n Arrows show movement of energy through the system Food chain always begins with producer Energy originates with sun as part of photosynthesis 20

Ecological Relationships Producer or Autotroph/ Consumer Example: Plant / Deer n Predator / Example: Fox / n Parasite Example: Fleas n Prey Rabbit / / Host Dog 21

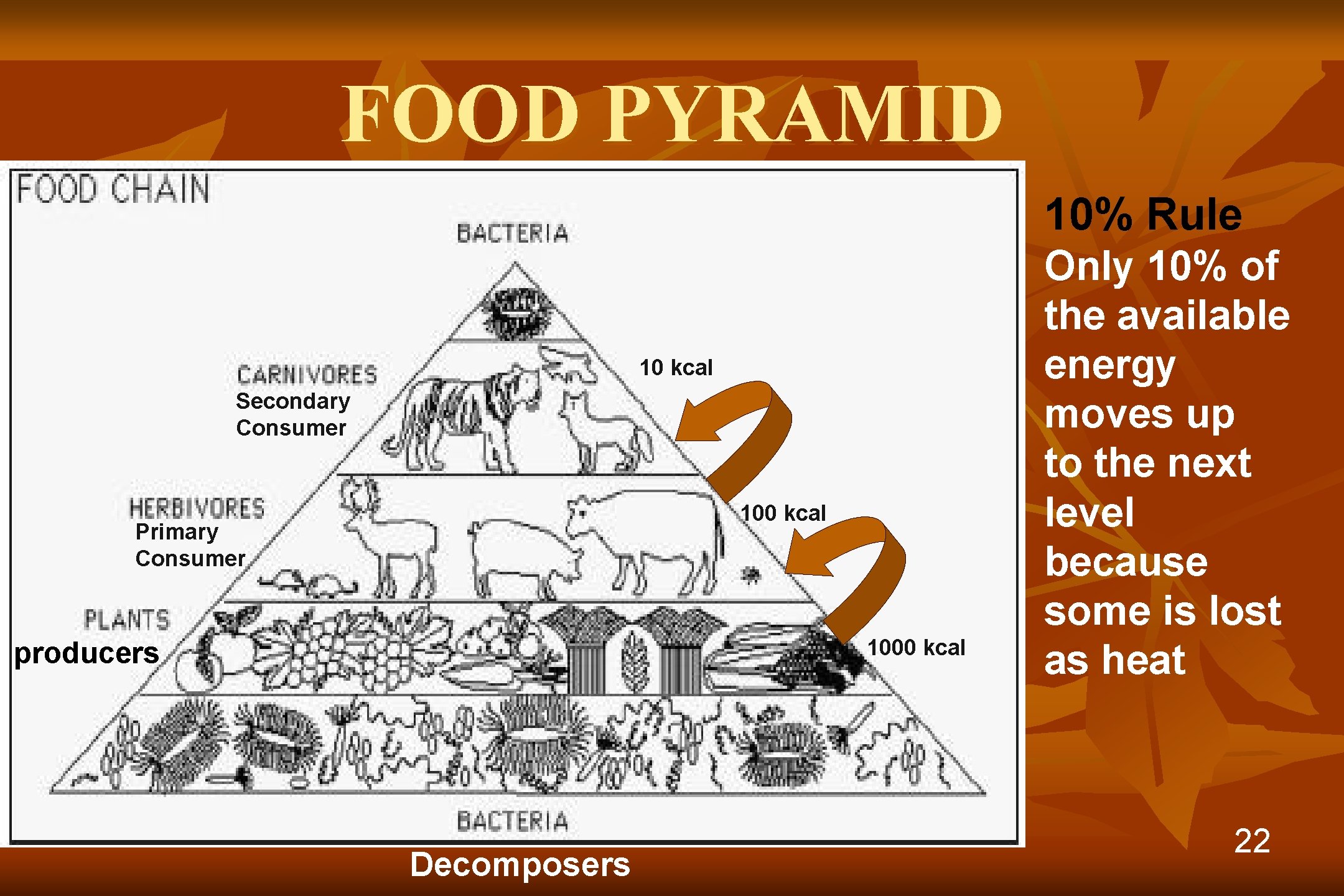
FOOD PYRAMID 10% Rule 10 kcal Secondary Consumer 100 kcal Primary Consumer 1000 kcal producers Decomposers Only 10% of the available energy moves up to the next level because some is lost as heat 22

PHOTOSYNTHESIS CO 2 + H 2 O + sunlight C 6 H 12 O 6 + O 2 Carbon dioxide + water + sunlight glucose + oxygen 23

Chemical Equation for Photosynthesis 24

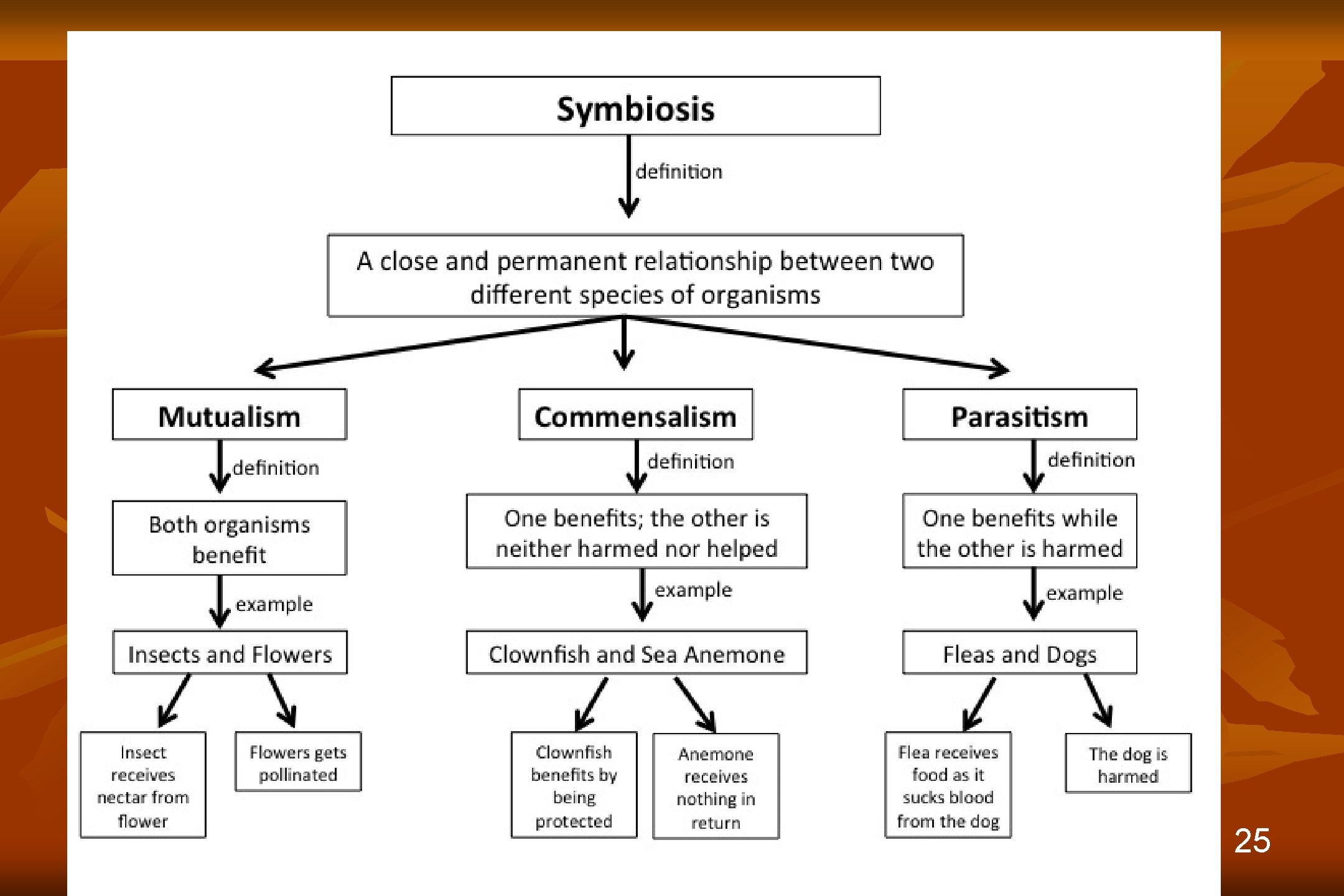
25

1 a

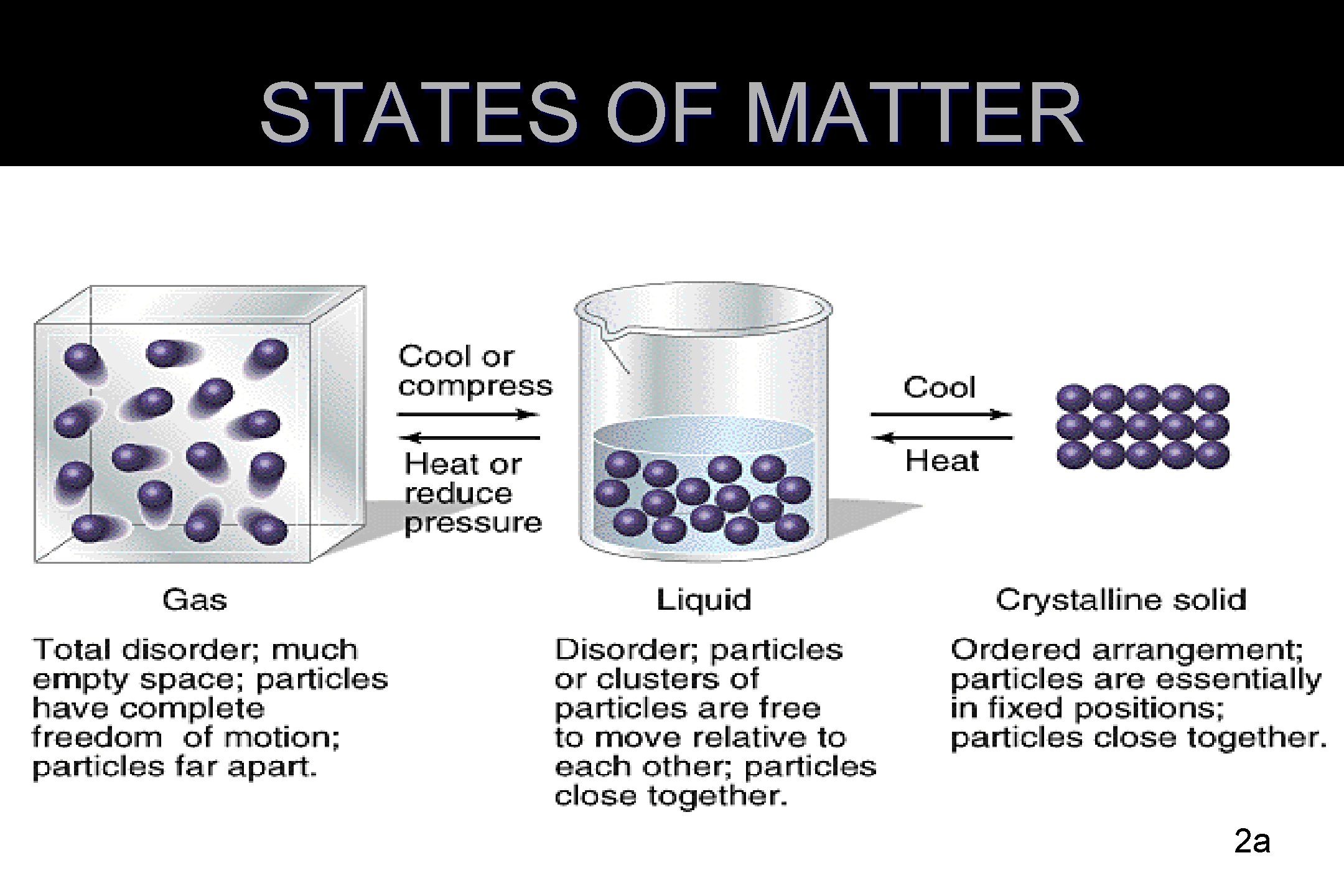
STATES OF MATTER 2 a

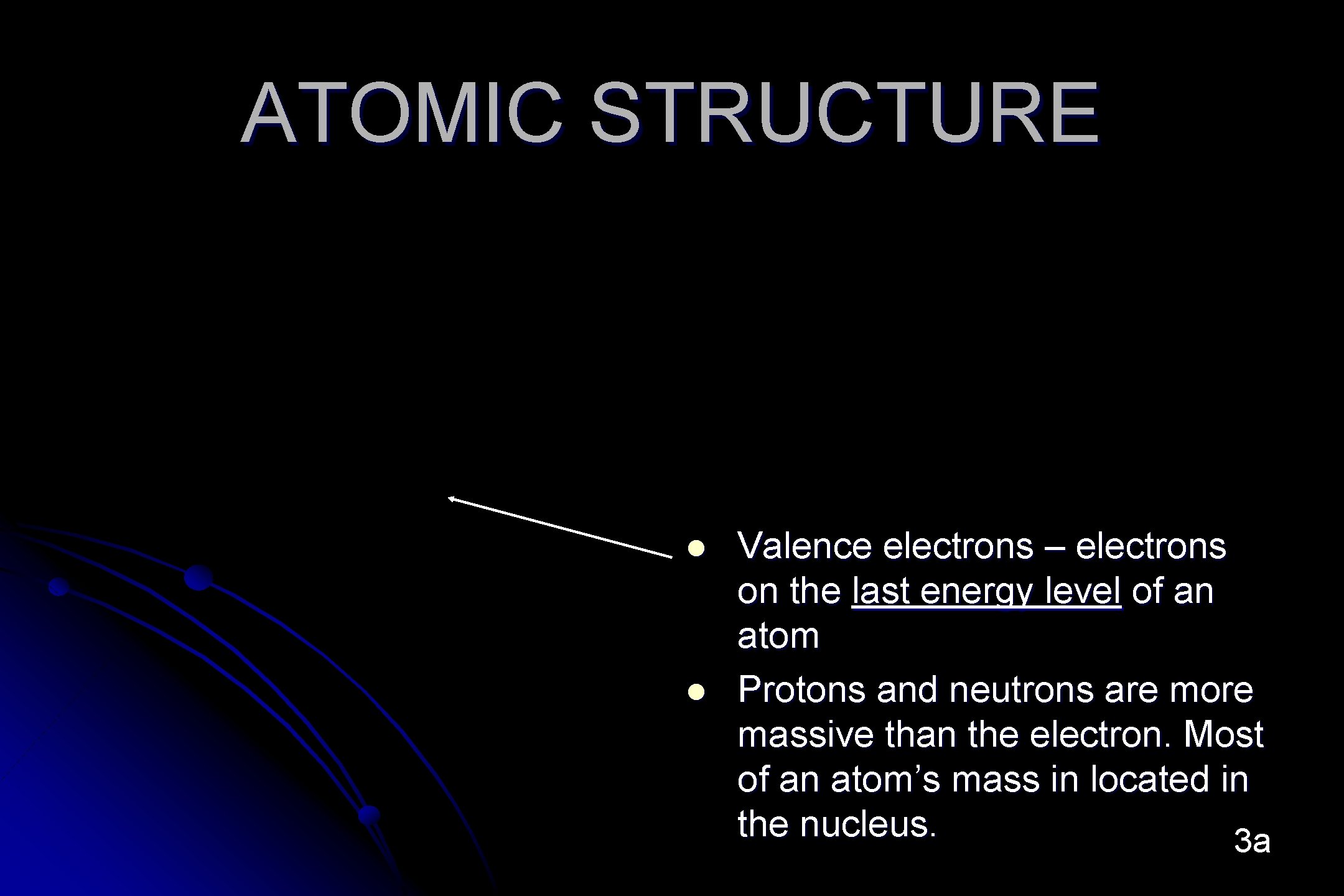
ATOMIC STRUCTURE l l Valence electrons – electrons on the last energy level of an atom Protons and neutrons are more massive than the electron. Most of an atom’s mass in located in the nucleus. 3 a

ELEMENT INFORMATION Hint: all elements are considered neutral because # of protons= # of electrons And Number of Electrons in a neutral atom 4 a

PERIODIC TABLE Group # – columns, up & down - elements have similar chemical, reactivity and physical properties - Group # gives # of Valence electrons Noble gases Right side (orange) Period #(rows, across) - # of energy levels, (clouds/Shells in an Atom Left side (green) Stair case(purple) 5 a Most Reactive Inert, nonreactive, stable, valence shell full of electrons (8) except for Helium (He) which has 2 Least Reactive

Physical properties METALS- good conductors of electricity, malleable, ductile, shiny, Mostly Solids l METALLOIDS- semi-conductors, characteristics of both metals and nonmetals l NON-METALS- poor conductors of electricity, brittle (breaks easily), Mostly Gases l 6 a

ATOM VS. MOLECULE 7 a

Organic compounds contain Carbon(C) and other elements such as hydrogen(H), oxygen(O), nitrogen(N), phosphorus(P), or sulfur(S) REMEMBER: CHONPS l CHONS – Proteins (meat, beans, eggs) l CHO – Carbohydrates(starch) (bread, rice, cereal) l CHONP – Lipids(fats) (oil, butter) *Note: If the compounds DOES NOT contain CARBON(C), the compound is NOT ORGANIC l 8 a

COUNTING ATOMS l l Subscript – # below letters; tells # of atoms Coefficient – # in front of element or compound; tells number of molecules; multiplied by subscript to get the total # of atoms for each element 9 a

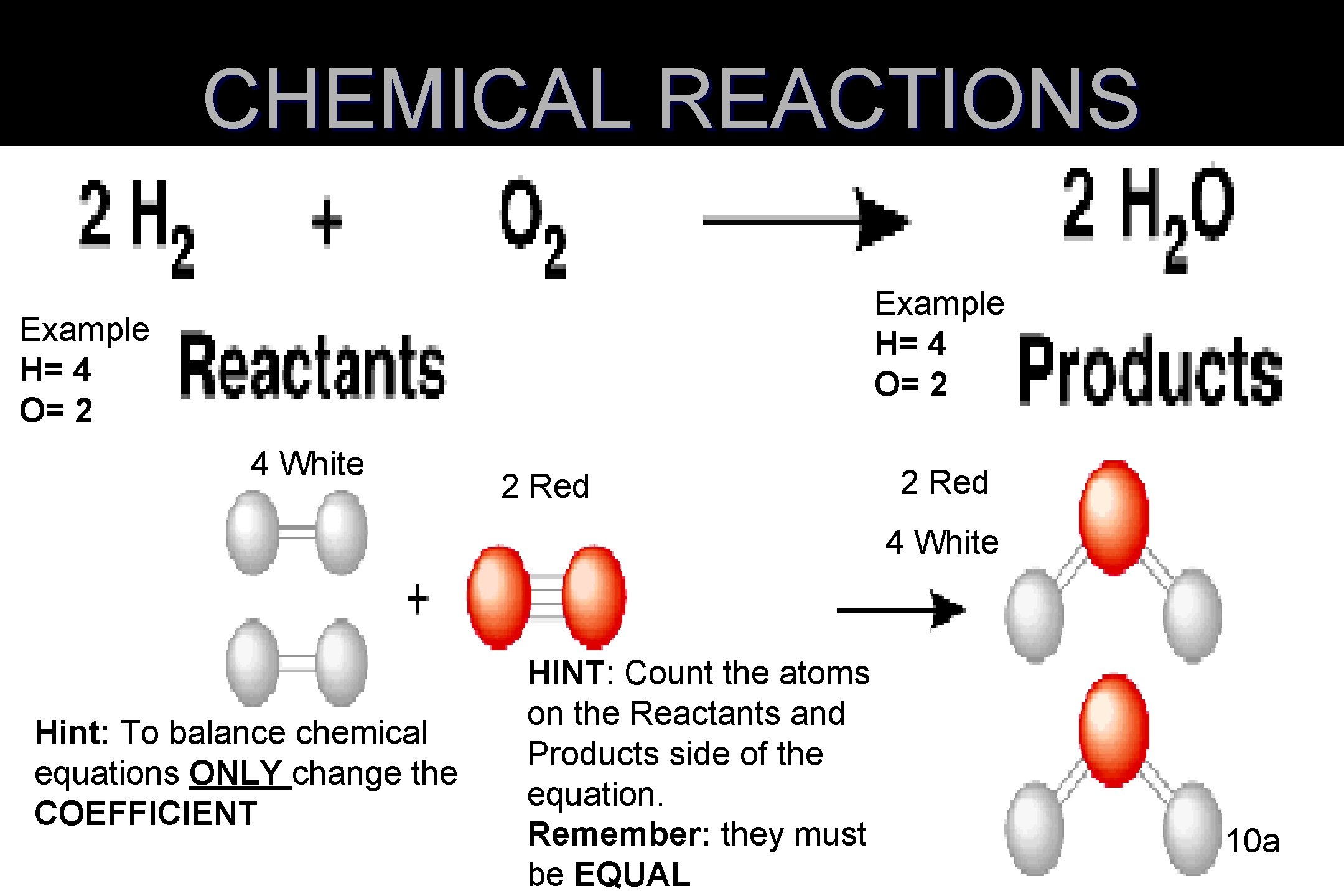
CHEMICAL REACTIONS Example H= 4 O= 2 4 White 2 Red 4 White Hint: To balance chemical equations ONLY change the COEFFICIENT HINT: Count the atoms on the Reactants and Products side of the equation. Remember: they must be EQUAL 10 a

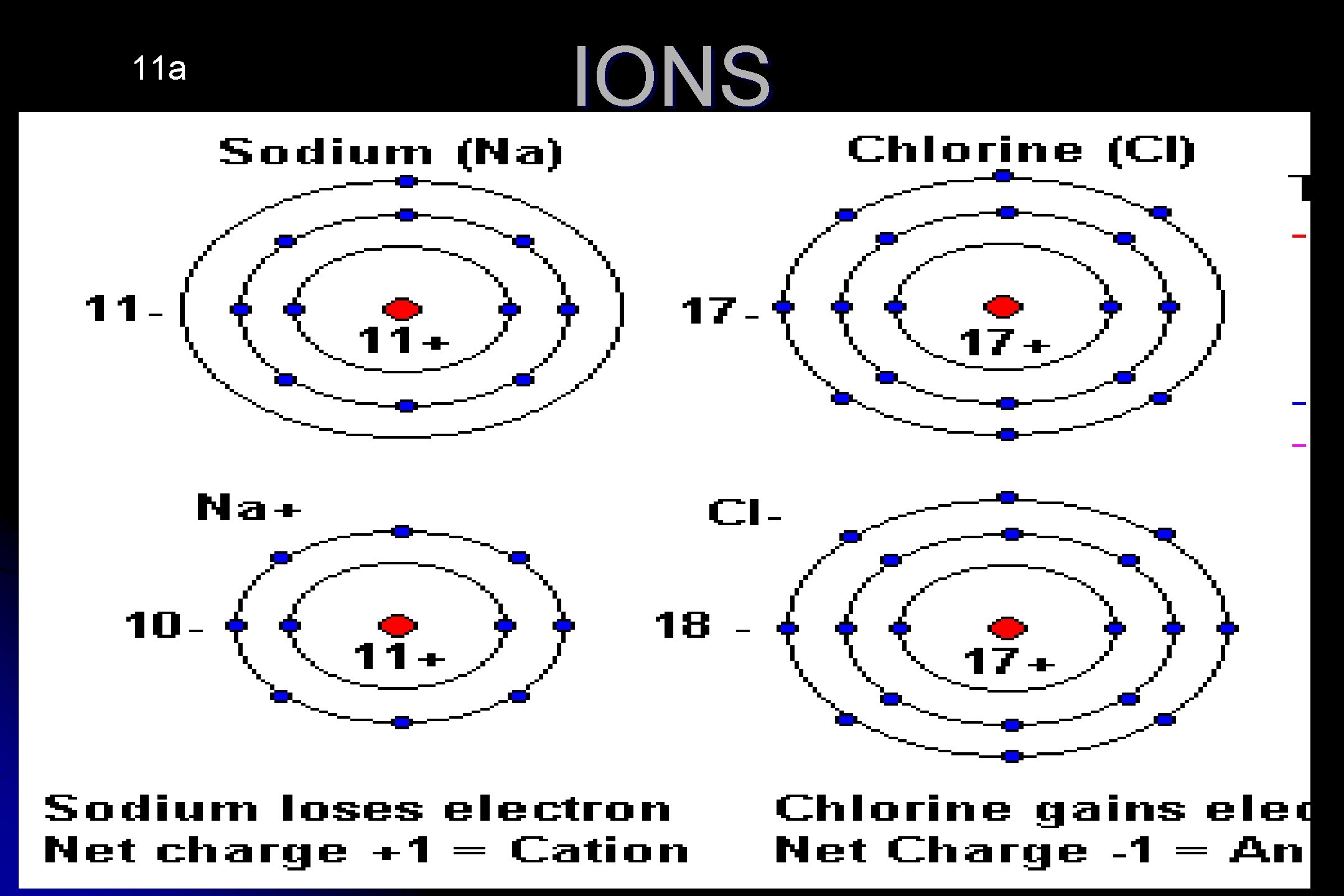
11 a IONS

IONS • An atom in which the # of Electrons DOES NOT Equal the # of protons giving the atom a positive or negative charge. Example: Atoms with a positive charge has lost electrons. + • Na - Atomic # 11, Protons 11, Electrons 10 +2 • Be - Atomic # 4, Protons 4, Electrons 2 Atoms with a negative charge has gained electrons • Cl – Atomic # 17, Protons 17, Electrons 18 -2 • O - Atomic # 8, Protons 8, Electrons 10 12 a

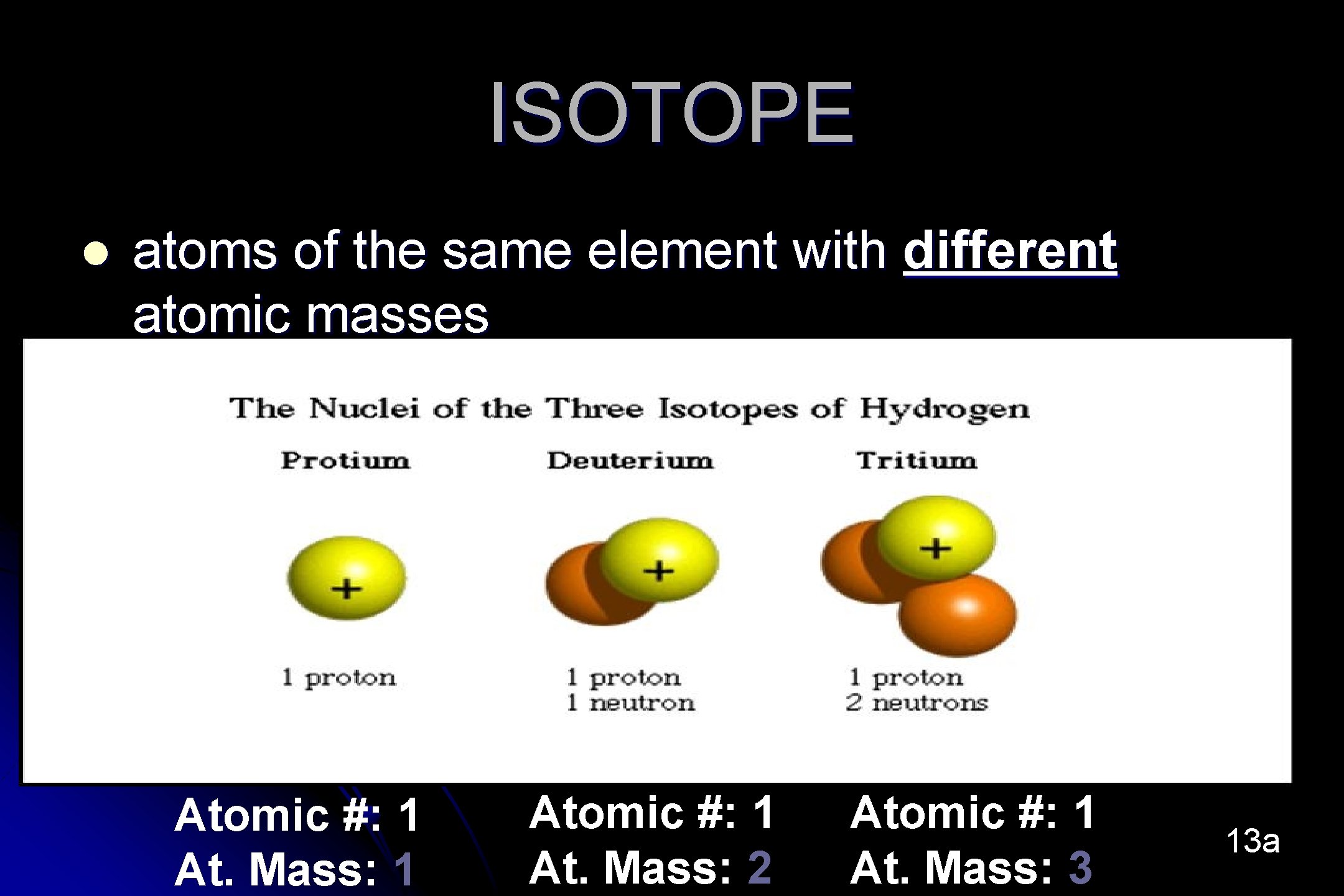
ISOTOPE l atoms of the same element with different atomic masses Atomic #: 1 At. Mass: 1 Atomic #: 1 At. Mass: 2 Atomic #: 1 At. Mass: 3 13 a

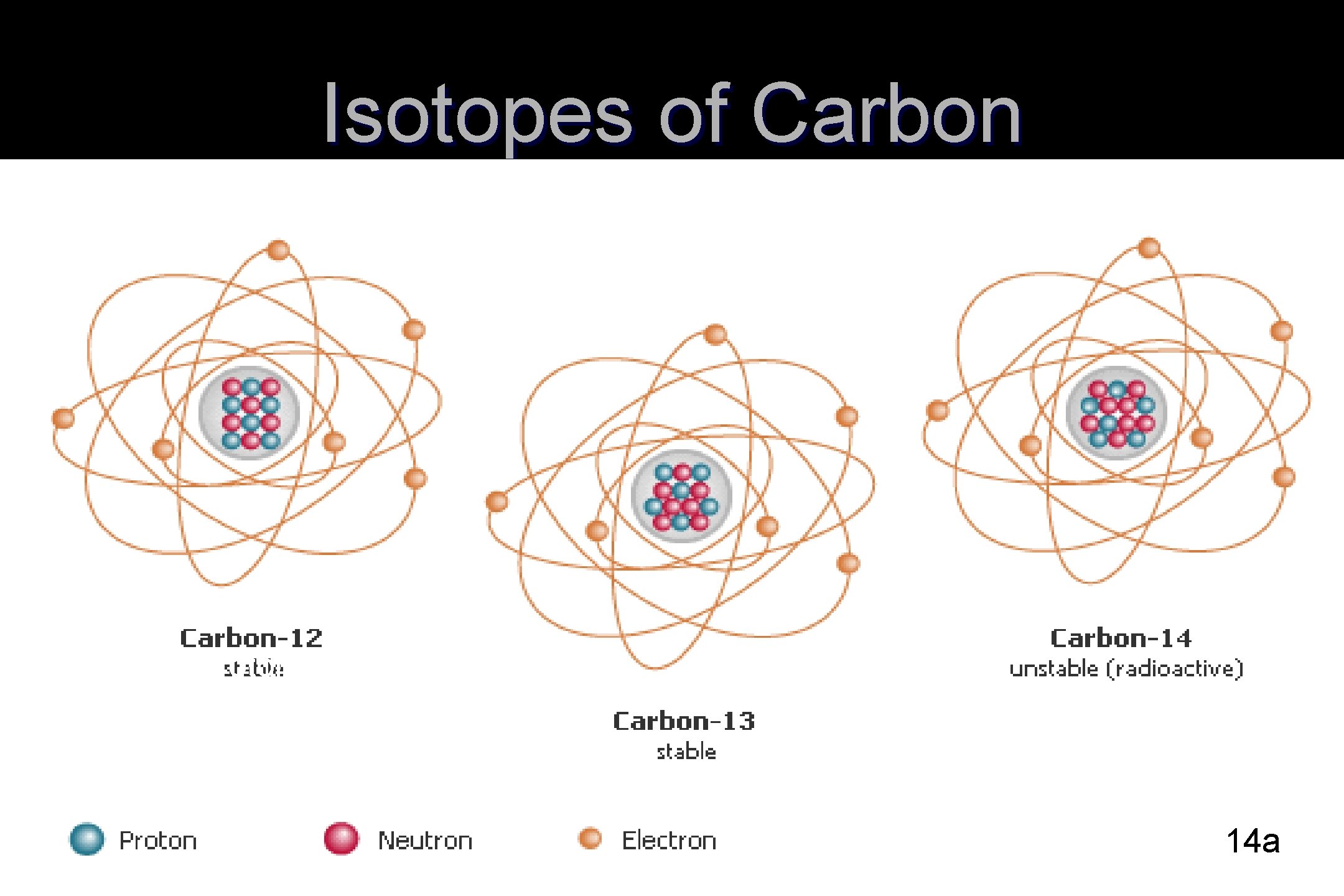
Isotopes of Carbon 6 protons, 6 neutrons 6 protons 7 neutron 14 a

Law of Conservation of Mass of reactants and mass of products will remain the same You cannot create nor can you destroy matter; you can only rearrange it! l 15 a

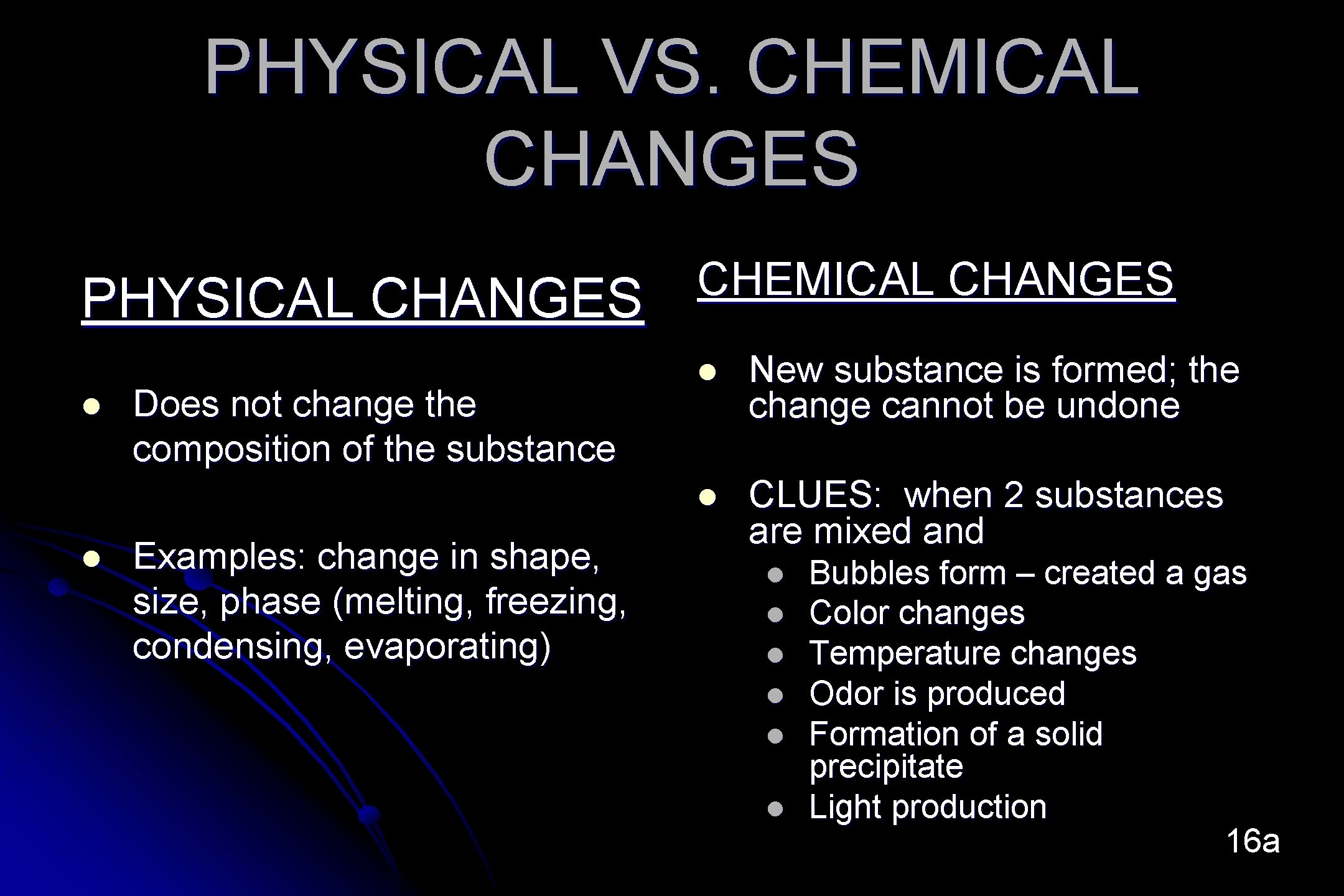
PHYSICAL VS. CHEMICAL CHANGES PHYSICAL CHANGES l l Does not change the composition of the substance Examples: change in shape, size, phase (melting, freezing, condensing, evaporating) CHEMICAL CHANGES l New substance is formed; the change cannot be undone l CLUES: when 2 substances are mixed and l l l Bubbles form – created a gas Color changes Temperature changes Odor is produced Formation of a solid precipitate Light production 16 a

SPECIFIC HEAT l HIGH SPECIFIC HEAT l Takes more energy to change temperature l Slower temperature change l Example: water, nonmetals l LOW SPECIFIC HEAT l Takes less energy to change temperature l Quicker temperature change l Example: sand, metals 17 a

1 b

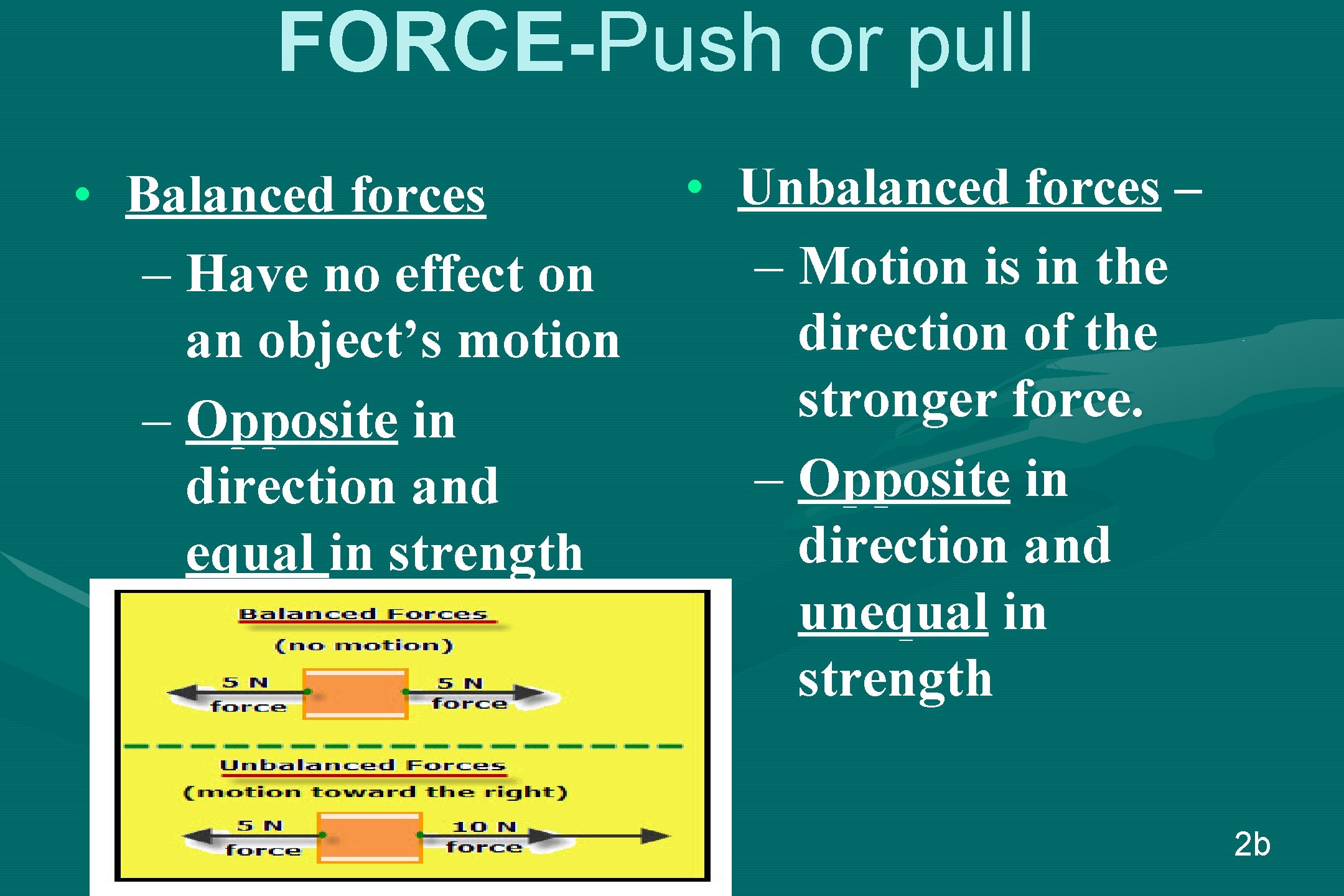
FORCE-Push or pull • Balanced forces – Have no effect on an object’s motion – Opposite in direction and equal in strength • Unbalanced forces – – Motion is in the direction of the stronger force. – Opposite in direction and unequal in strength 2 b

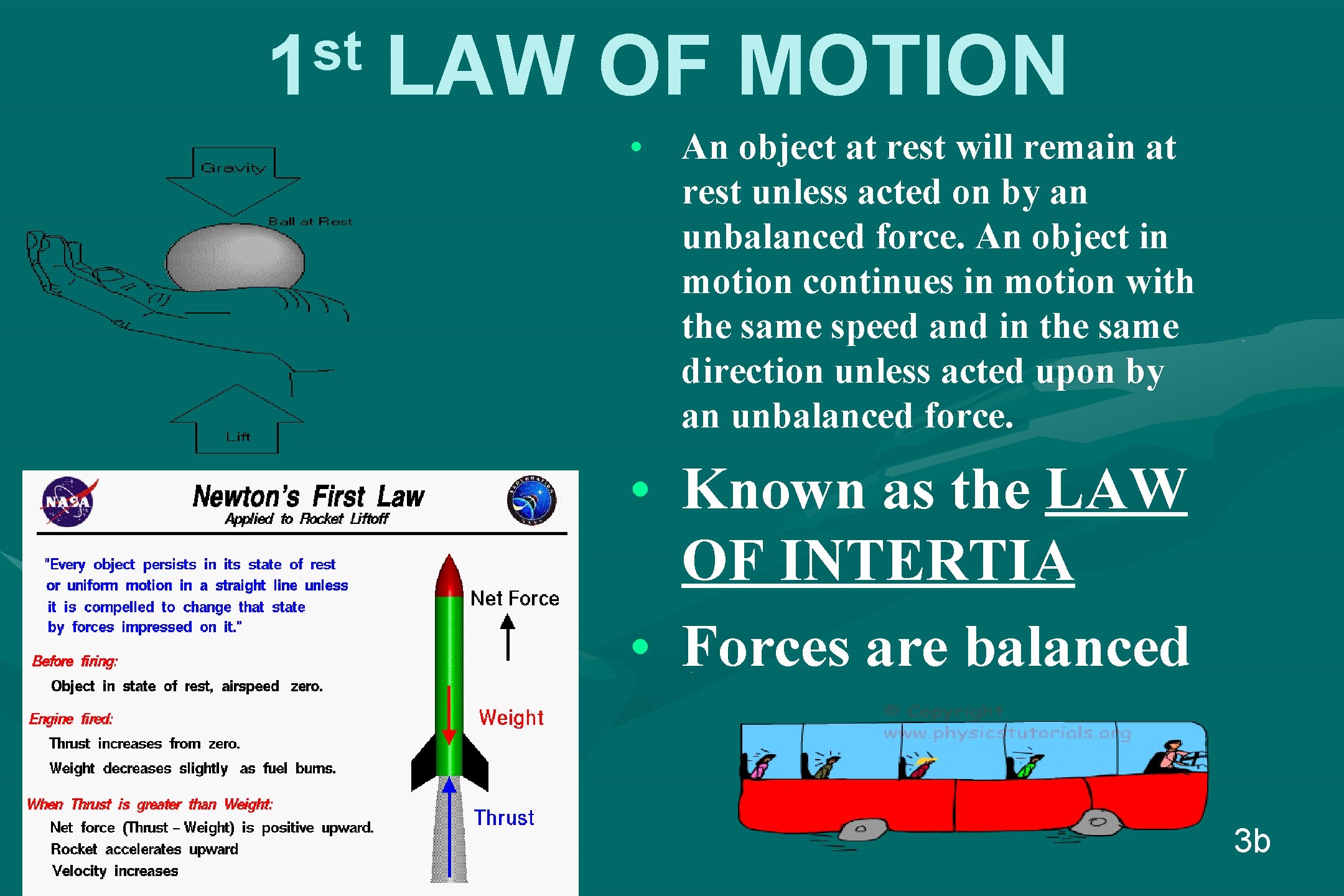
st 1 LAW OF MOTION • An object at rest will remain at rest unless acted on by an unbalanced force. An object in motion continues in motion with the same speed and in the same direction unless acted upon by an unbalanced force. • Known as the LAW OF INTERTIA • Forces are balanced 3 b

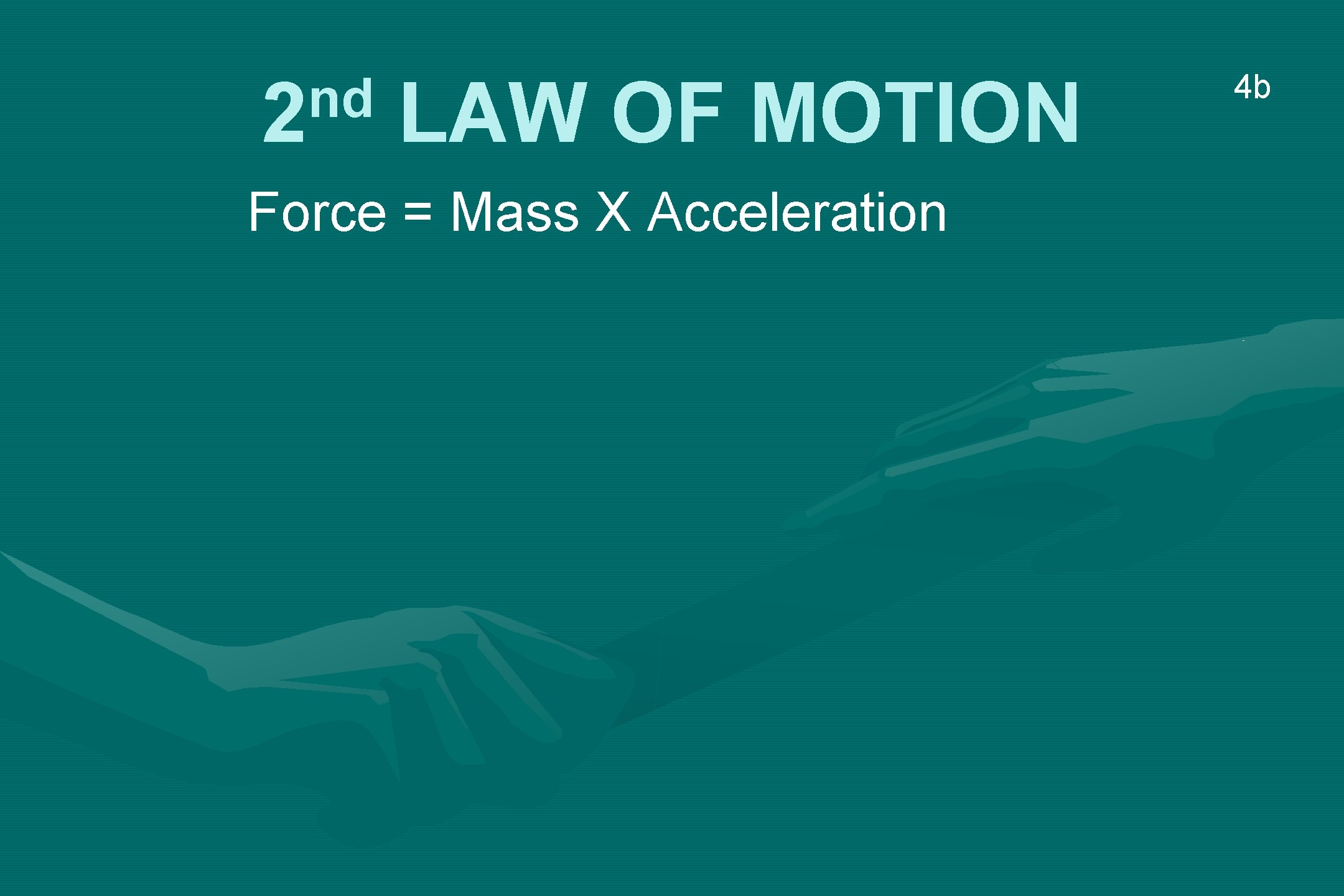
nd 2 LAW OF MOTION Force = Mass X Acceleration 4 b

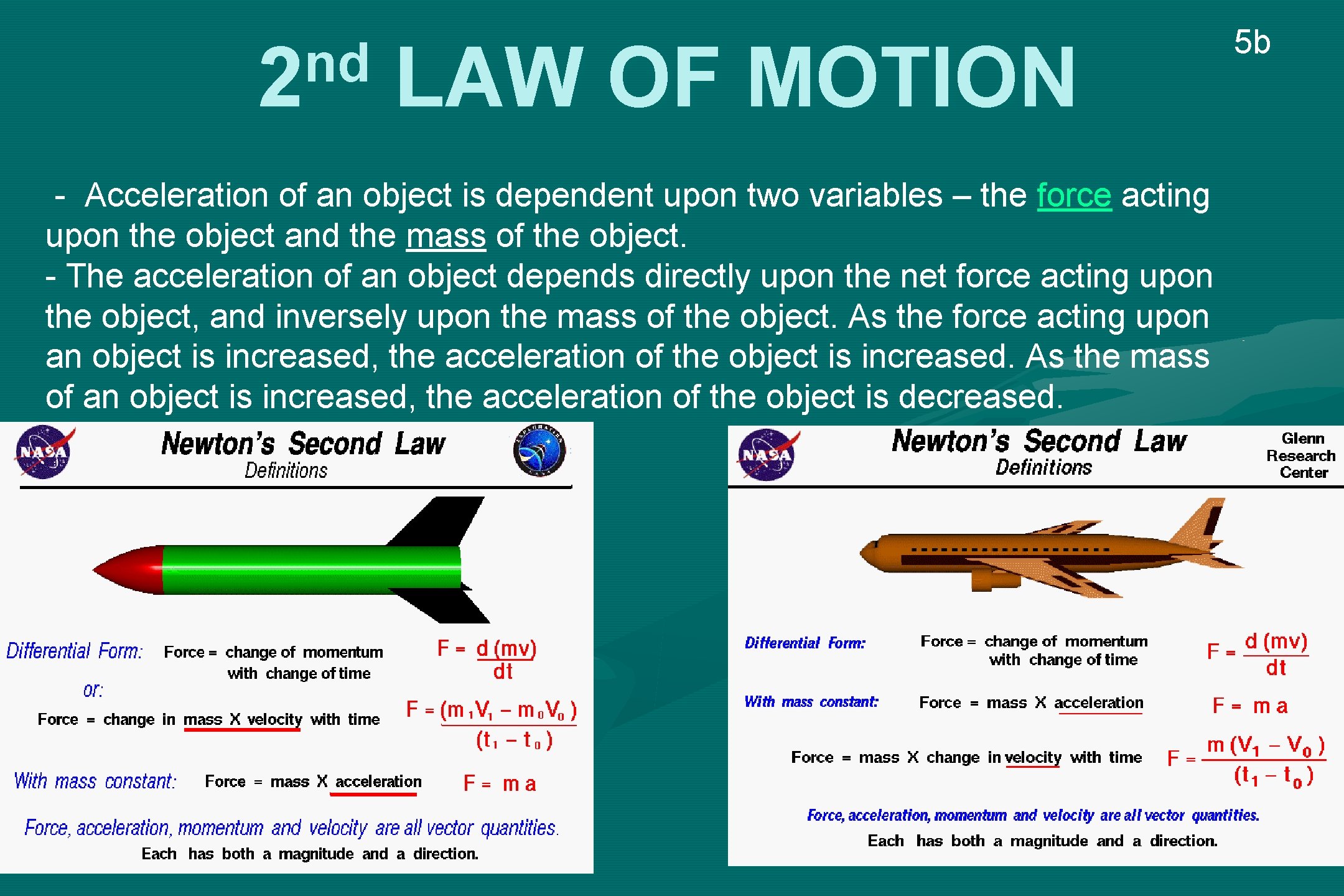
nd 2 LAW OF MOTION - Acceleration of an object is dependent upon two variables – the force acting upon the object and the mass of the object. - The acceleration of an object depends directly upon the net force acting upon the object, and inversely upon the mass of the object. As the force acting upon an object is increased, the acceleration of the object is increased. As the mass of an object is increased, the acceleration of the object is decreased. 5 b

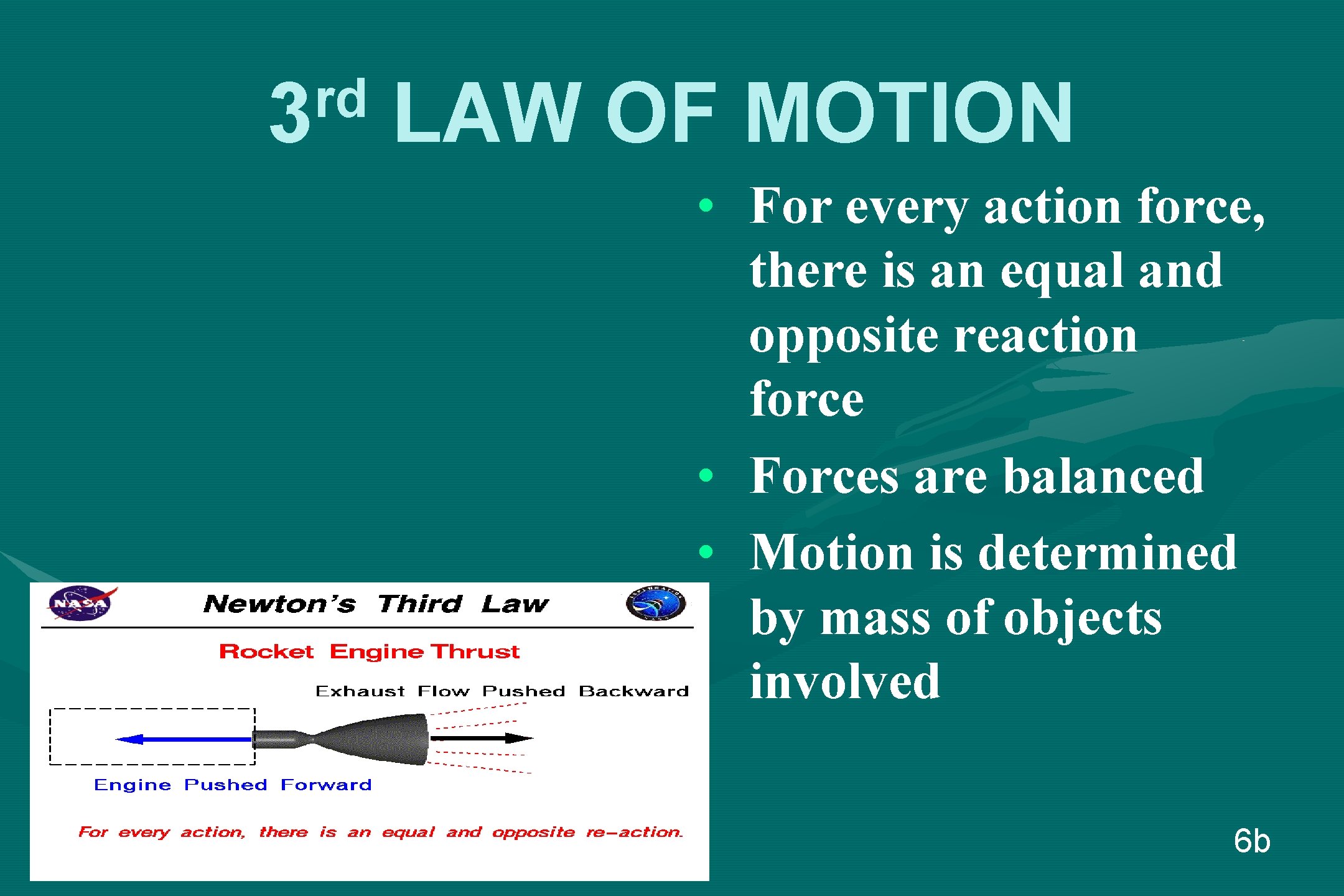
rd 3 LAW OF MOTION • For every action force, there is an equal and opposite reaction force • Forces are balanced • Motion is determined by mass of objects involved 6 b

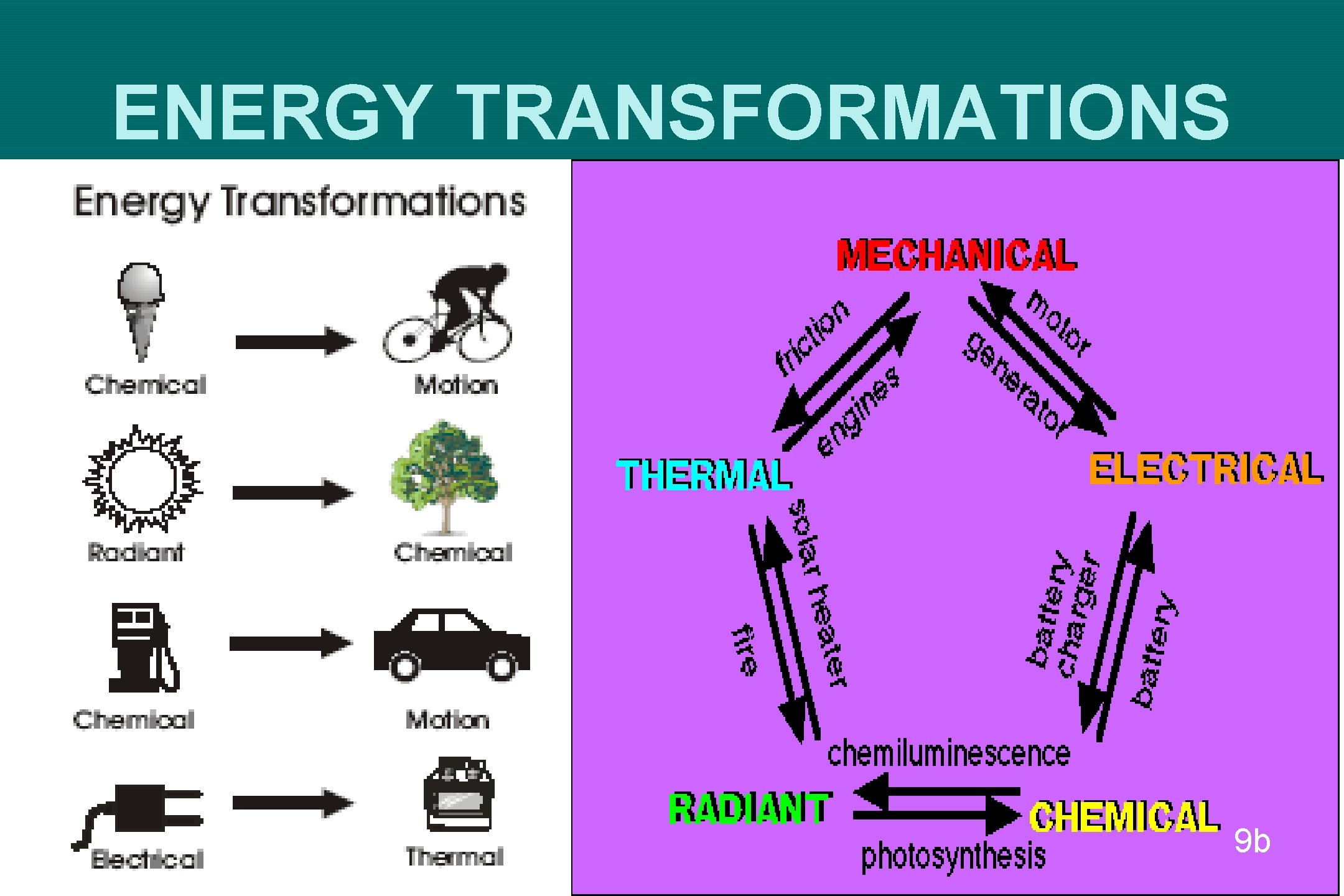
ENERGY – Ability to work or cause change – FORMS OF ENERGY: electrical, mechanical, chemical, thermal, potential, kinetic – LAW OF CONSERVATION OF ENERGY – cannot create nor destroy energy; energy may be transformed 7 b

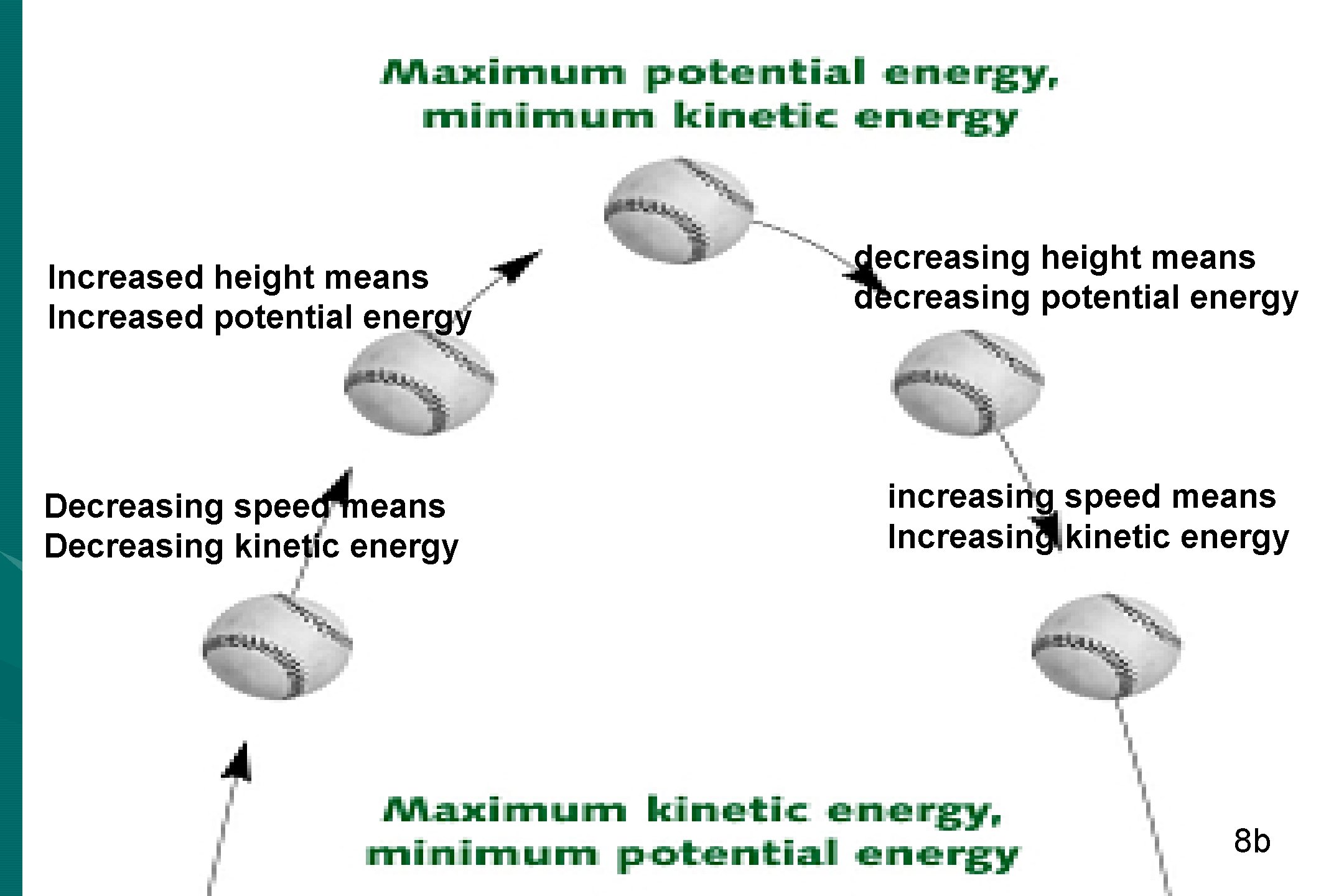
Increased height means Increased potential energy Decreasing speed means Decreasing kinetic energy decreasing height means decreasing potential energy increasing speed means Increasing kinetic energy 8 b

ENERGY TRANSFORMATIONS 9 b

SOURCES OF ENERGY • Inexhaustible: Wind, water, solar • • Renewable – readily available Nonrenewable – takes a long time to replenish • Fossil fuels (which form from decay of plants & 10 b

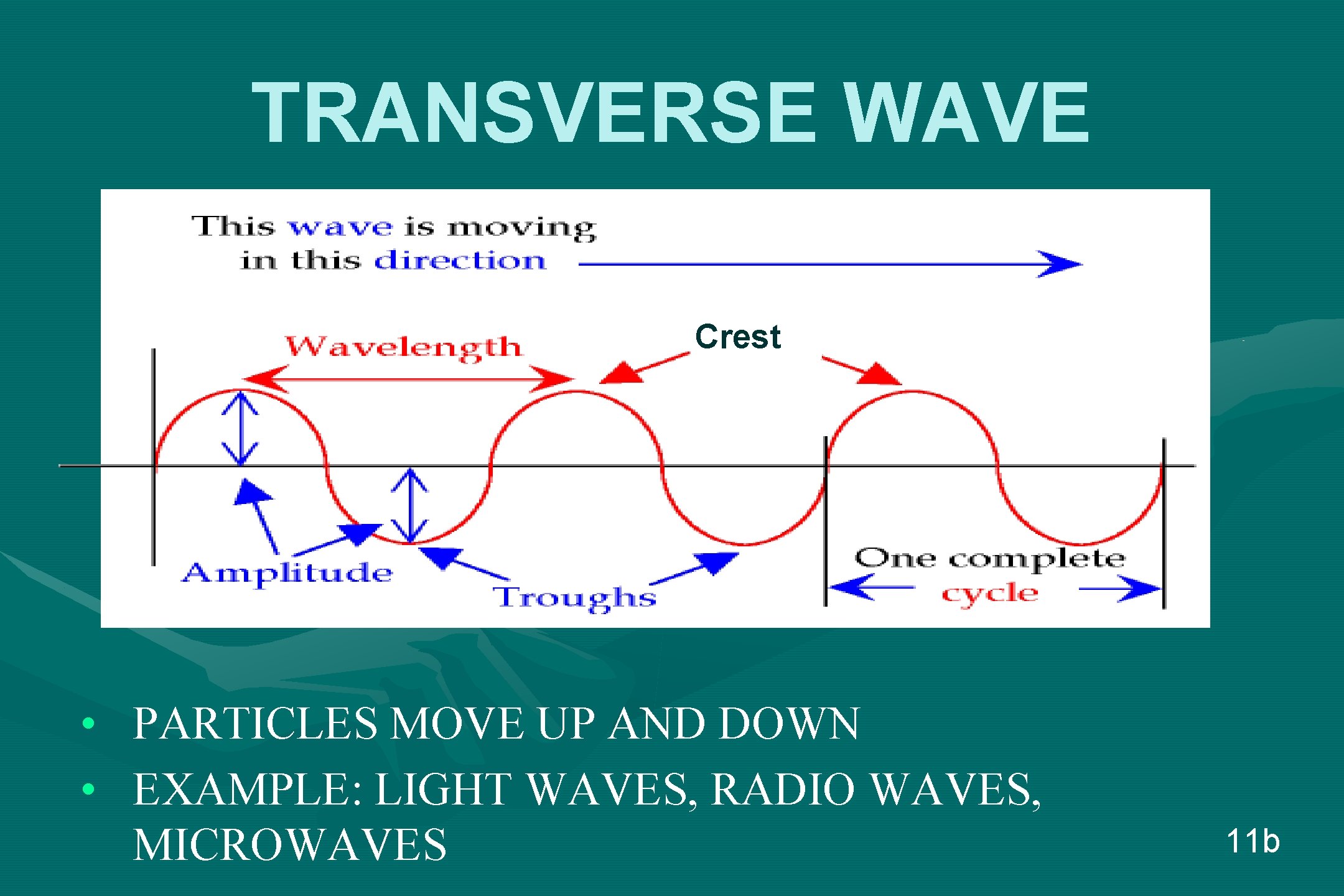
TRANSVERSE WAVE Crest • • PARTICLES MOVE UP AND DOWN EXAMPLE: LIGHT WAVES, RADIO WAVES, MICROWAVES 11 b

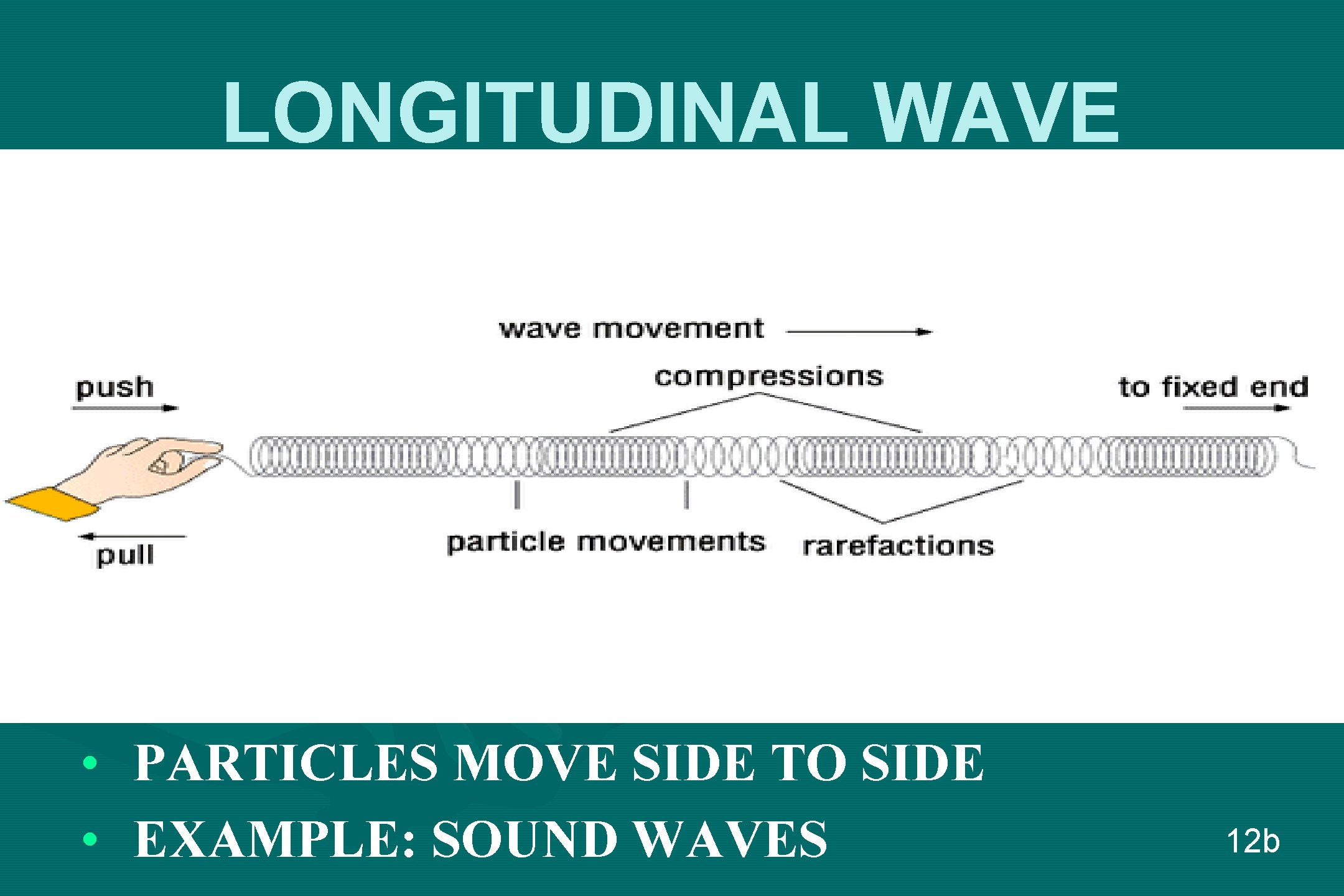
LONGITUDINAL WAVE • • PARTICLES MOVE SIDE TO SIDE EXAMPLE: SOUND WAVES 12 b

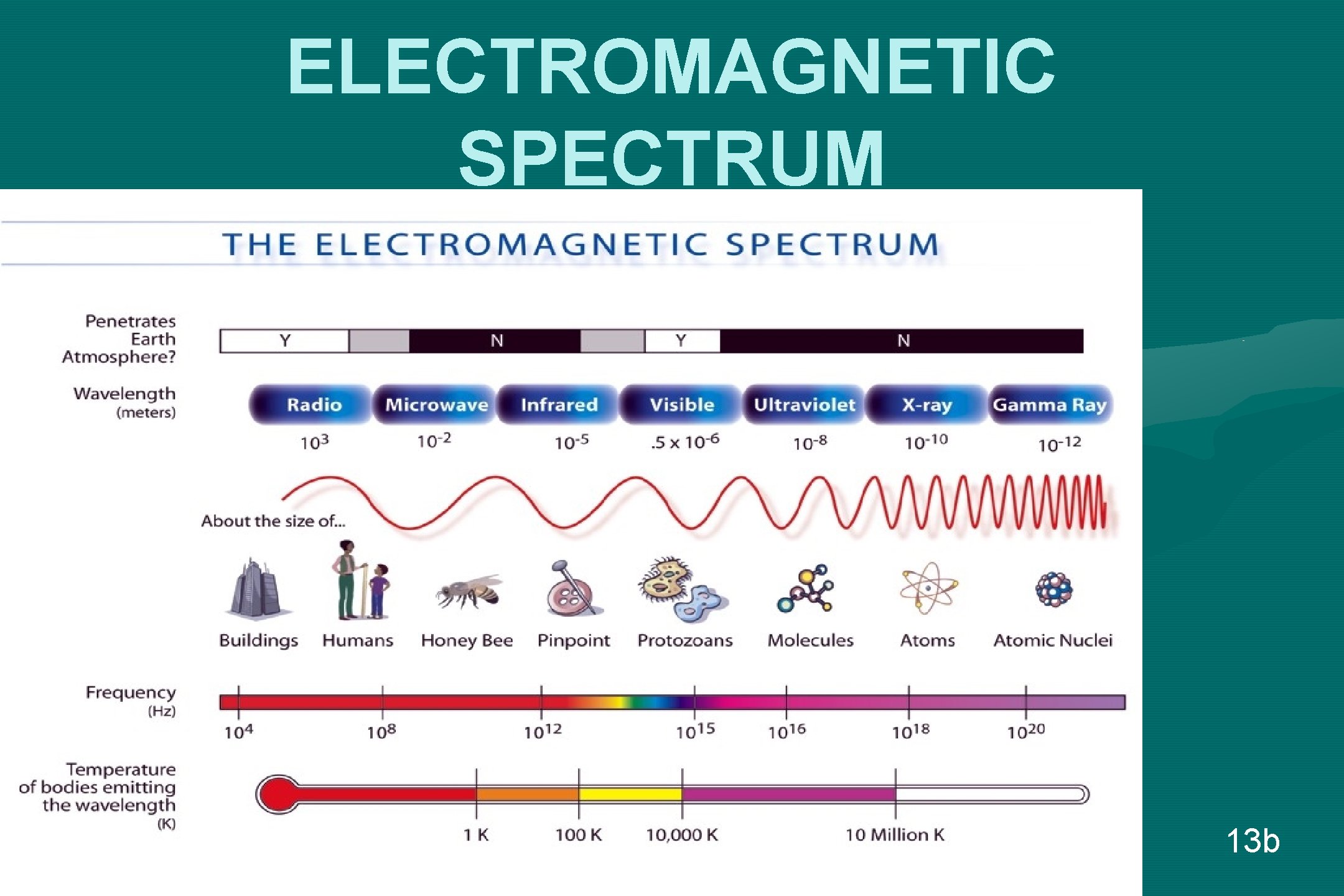
ELECTROMAGNETIC SPECTRUM 13 b

REFLECTION • Light bounces off of a surface and starts traveling in a different direction • Example: image in a mirror, echo 14 b

REFRACTION • Change in the direction of a wave as it passes from one medium to another; causes the wave to bend • Changes the speed and wavelength 15 b

DIFFRACTION • Wave direction changes as it passes through an opening • Will cause a straight wave front to bend and spread 16 b

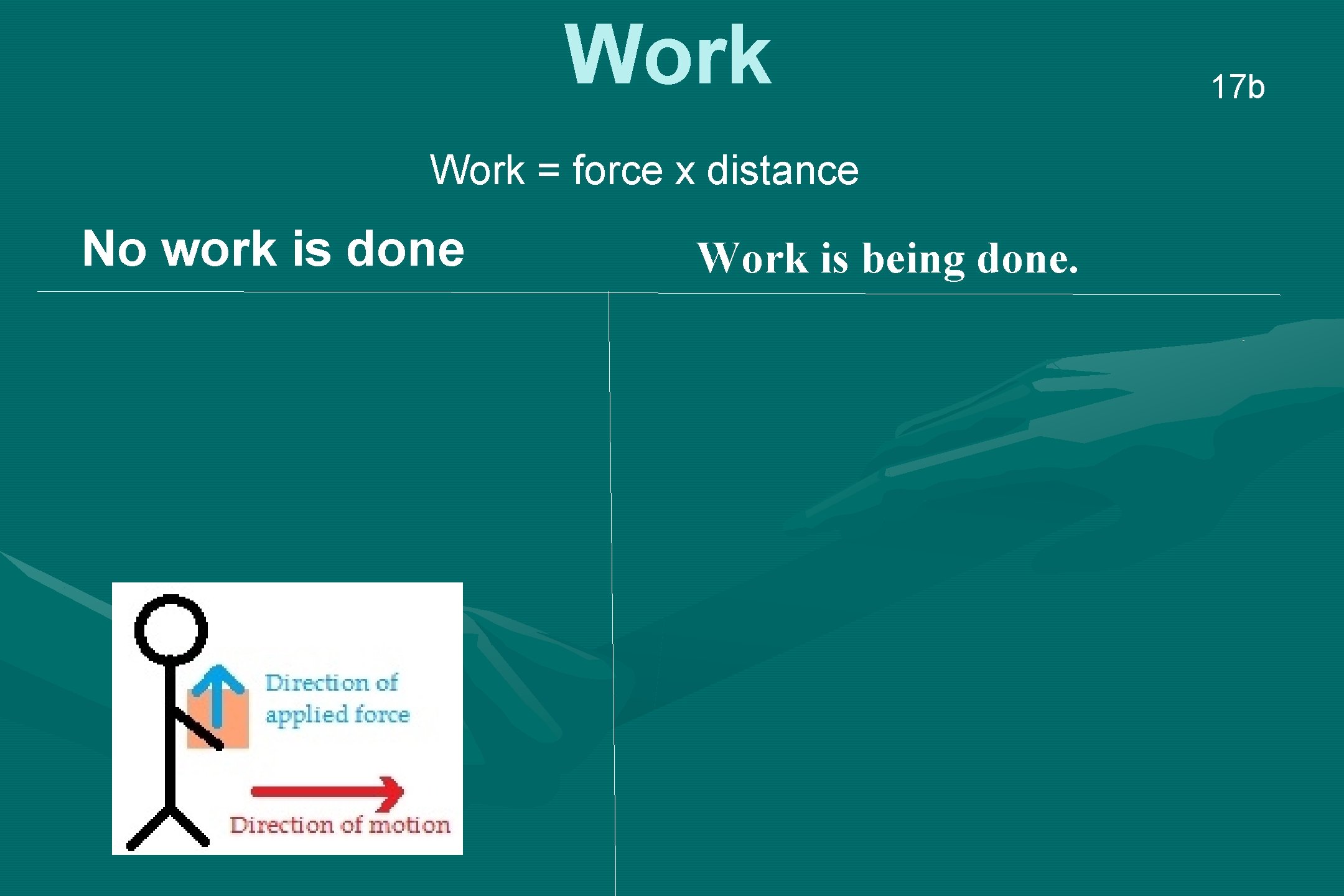
Work = force x distance No work is done Work is being done. 17 b

LEVERS BEST WHEN FULCRUM IS NEAR RESISTANCE FORCE BEST WHEN RESISTANCE FORCE IS NEAR FULCRUM NOT AN ADVANTAGE FORCE; ADVANTAGE IS IN DISTANCE GAINED 18 b

PULLEY • Changes direction of force • pulleys = less effort force; pull rope a longer distance 19 b

INCLINED PLANE 20 b

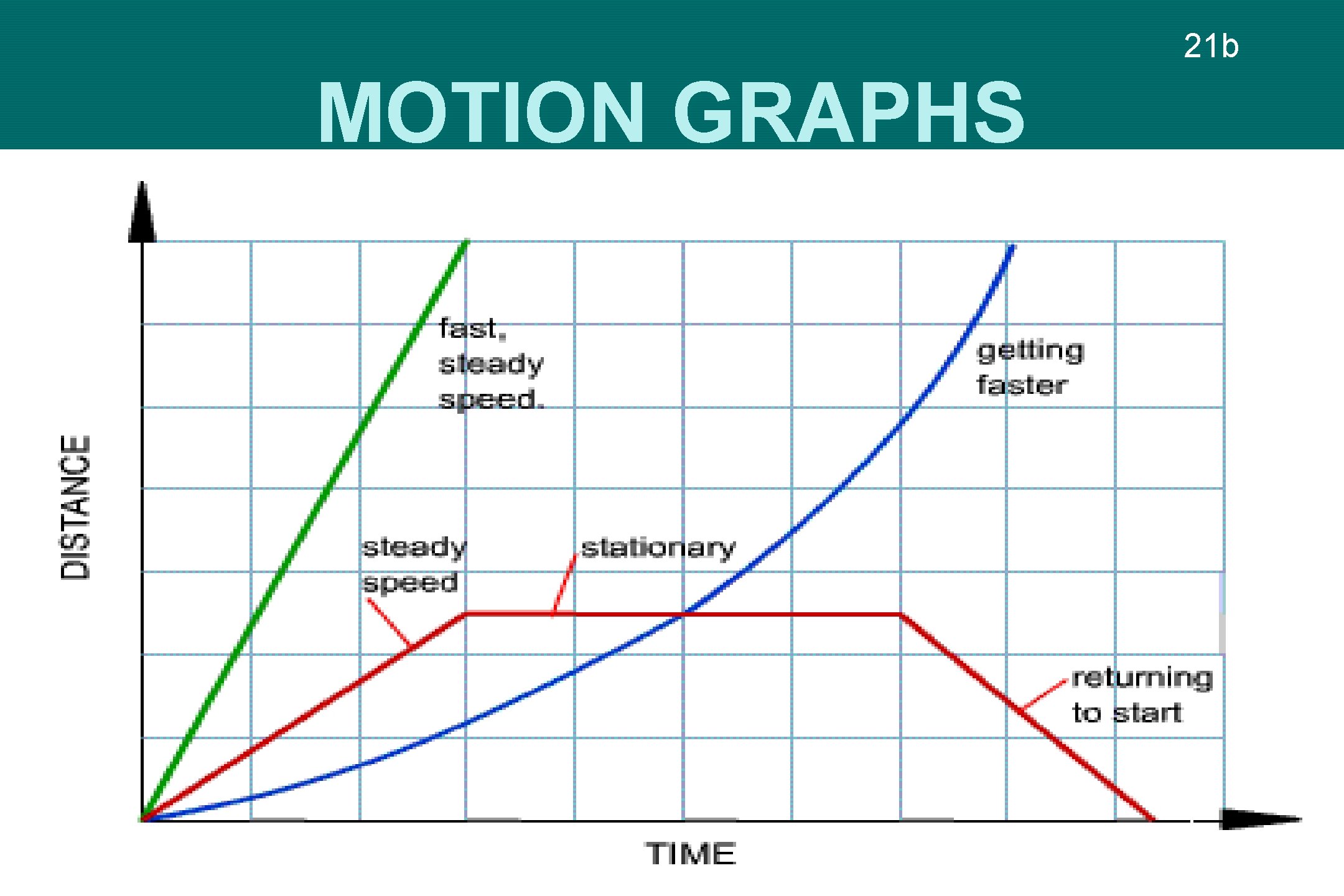
21 b MOTION GRAPHS

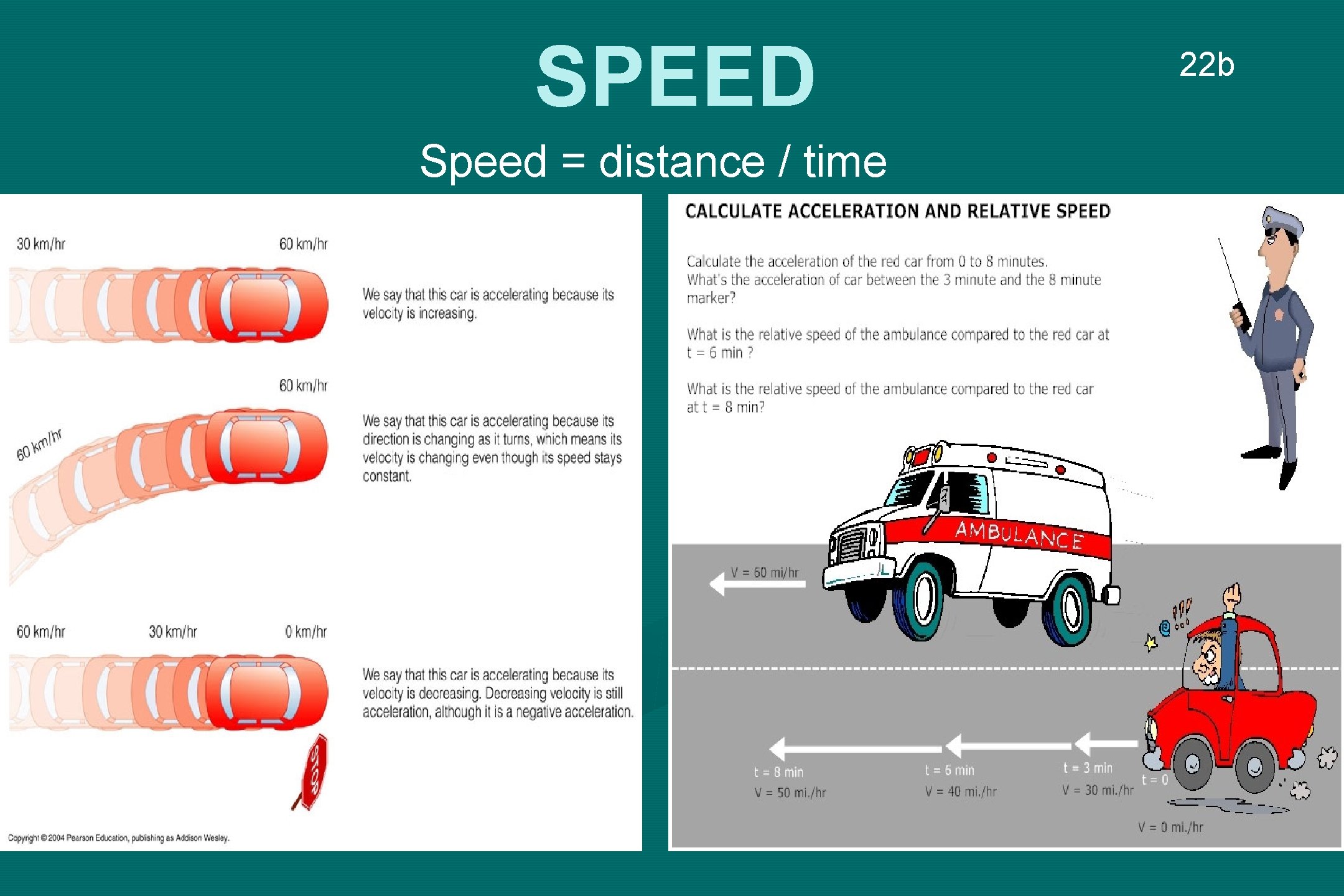
SPEED Speed = distance / time 22 b

23 b

1 c

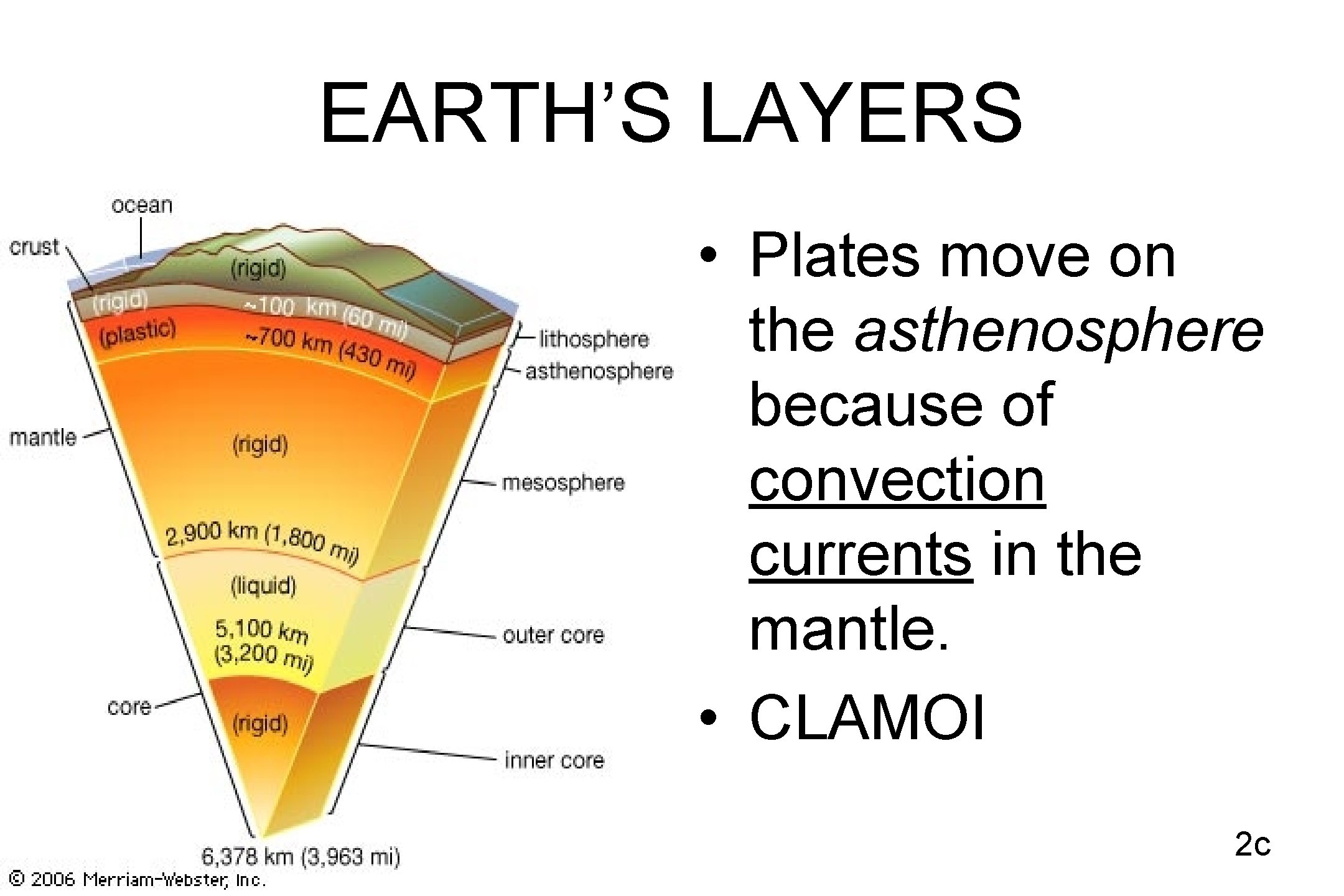
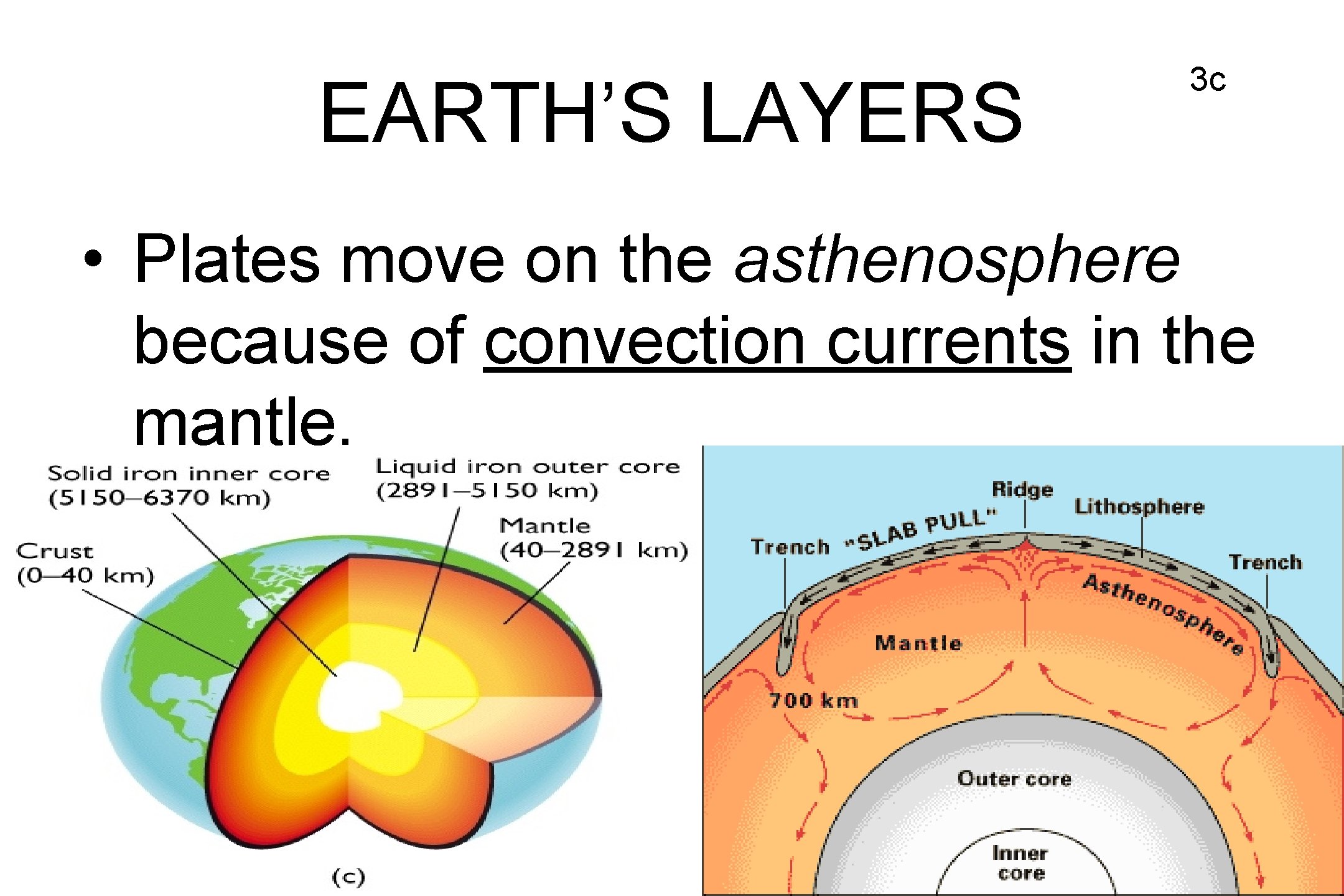
EARTH’S LAYERS • Plates move on the asthenosphere because of convection currents in the mantle. • CLAMOI 2 c

EARTH’S LAYERS 3 c • Plates move on the asthenosphere because of convection currents in the mantle.

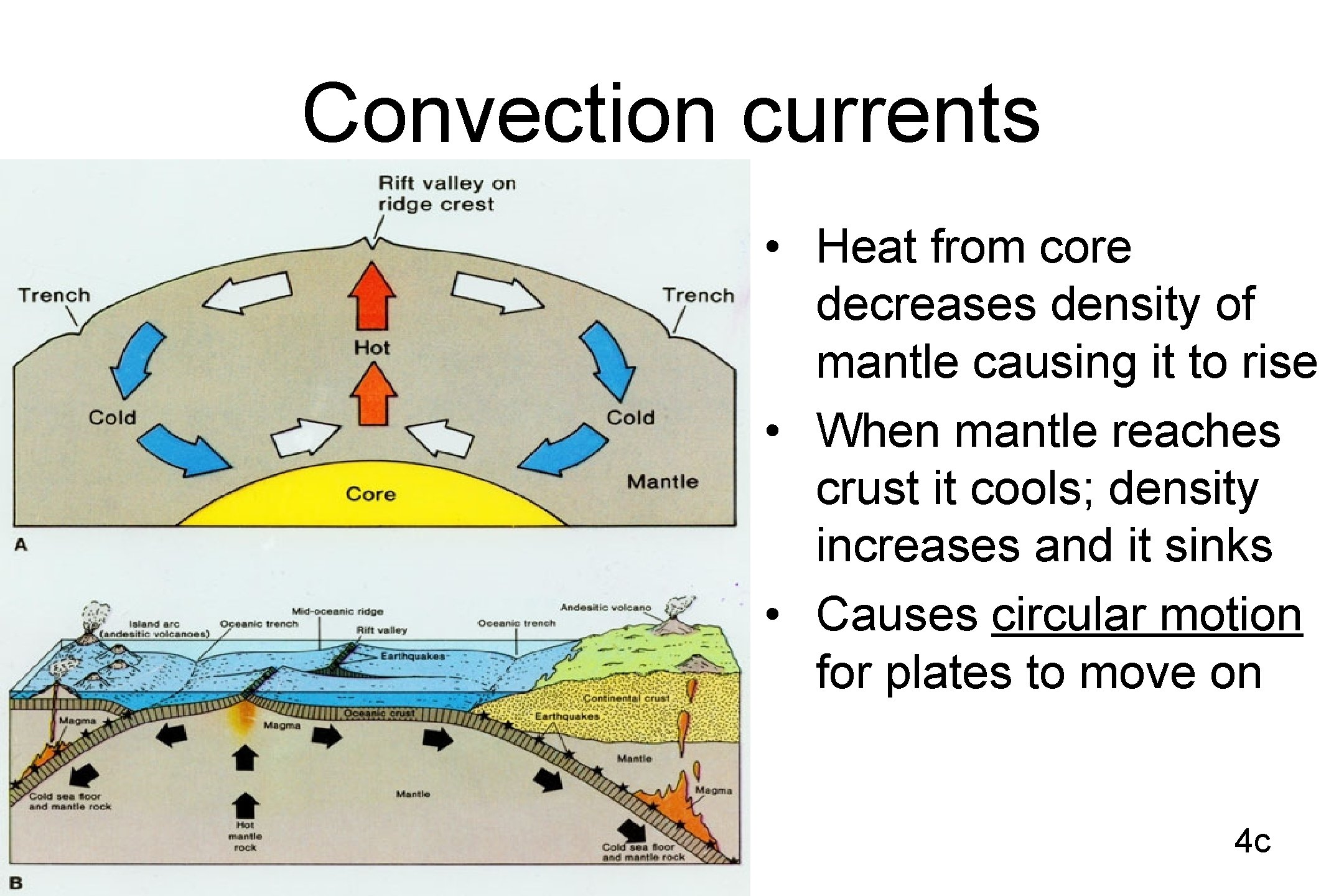
Convection currents • Heat from core decreases density of mantle causing it to rise • When mantle reaches crust it cools; density increases and it sinks • Causes circular motion for plates to move on 4 c

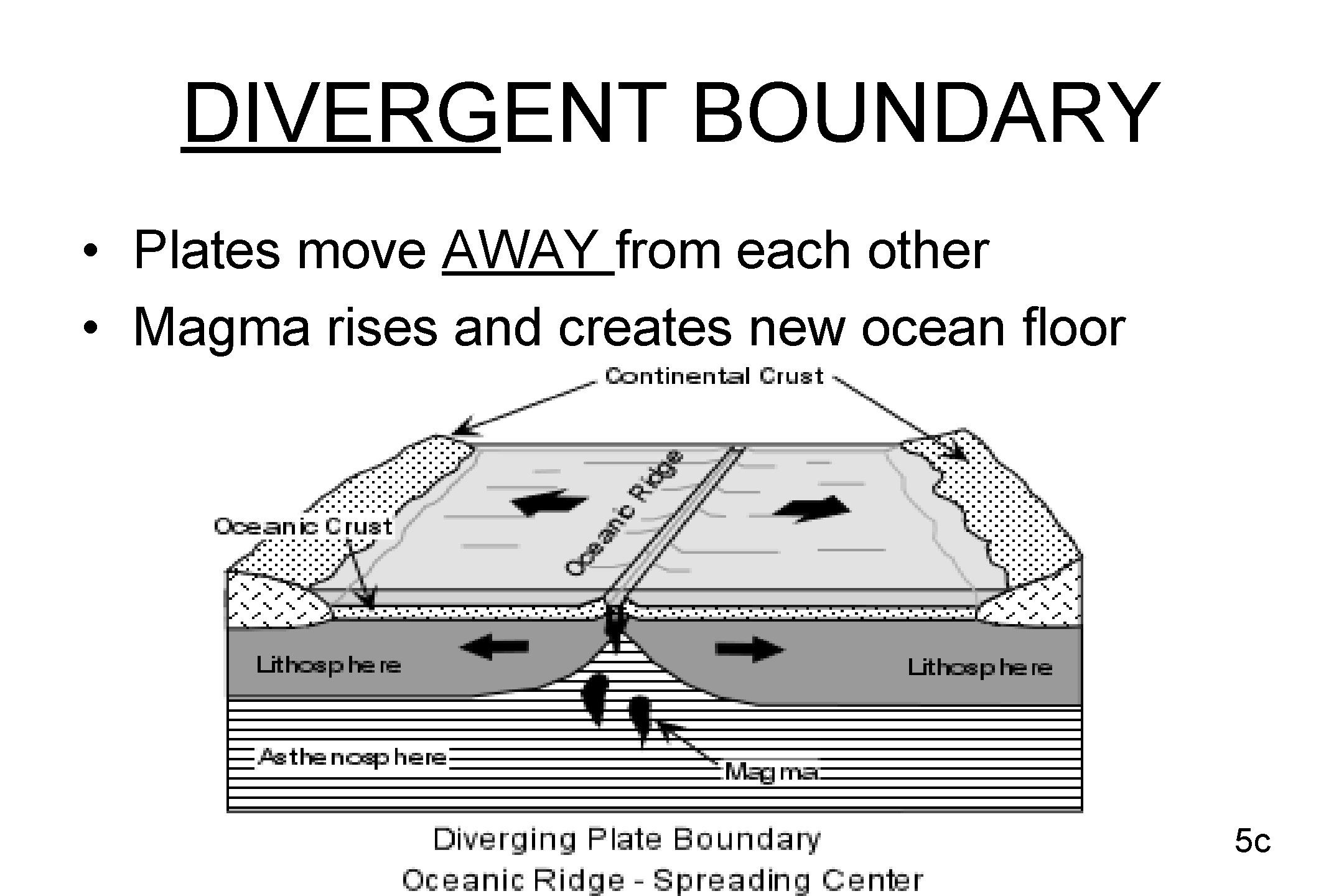
DIVERGENT BOUNDARY • Plates move AWAY from each other • Magma rises and creates new ocean floor 5 c

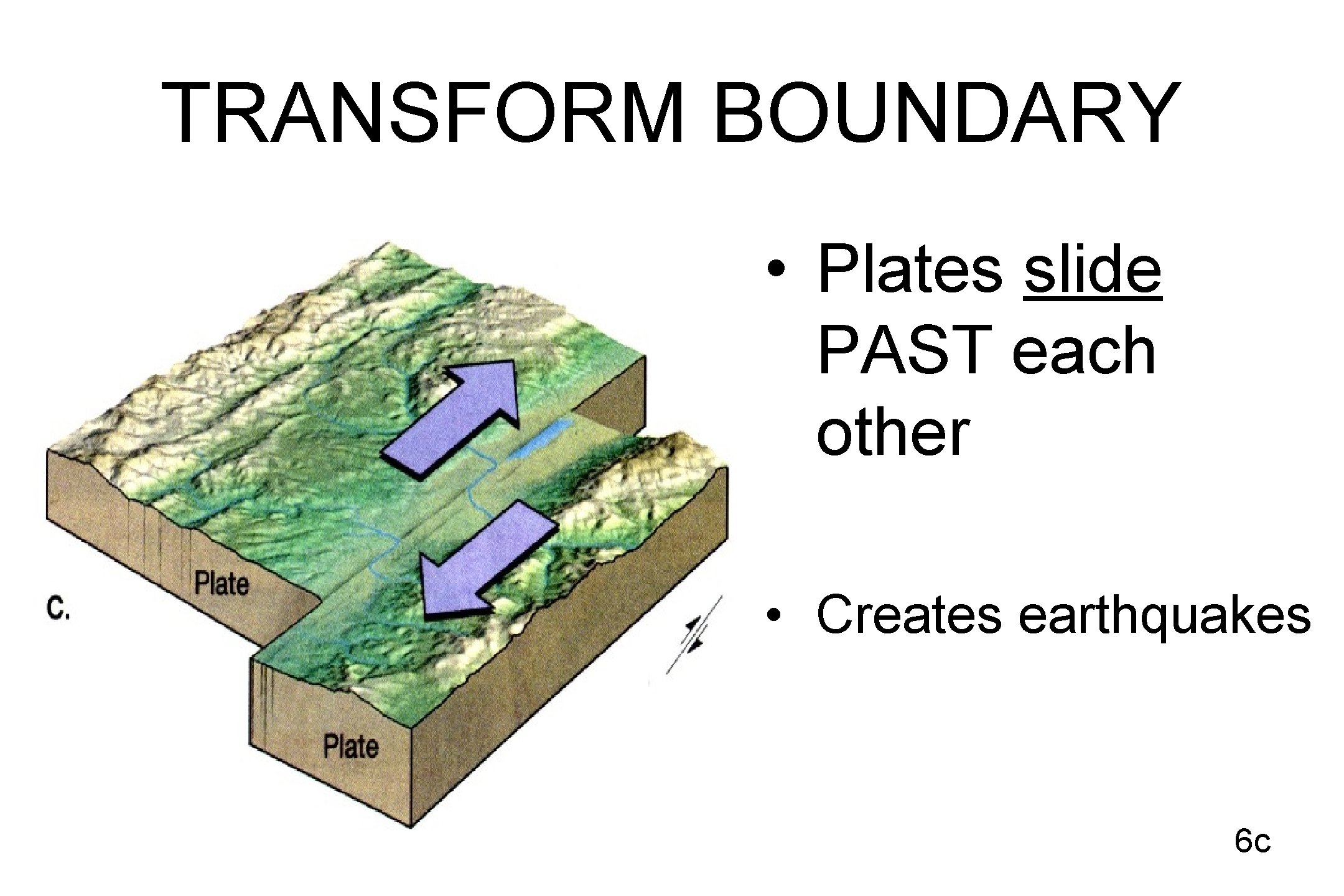
TRANSFORM BOUNDARY • Plates slide PAST each other • Creates earthquakes 6 c

CONVERGENT BOUNDARY MOUNTAINS EARTHQUAKES VOLCANOES TRENCHES 7 c

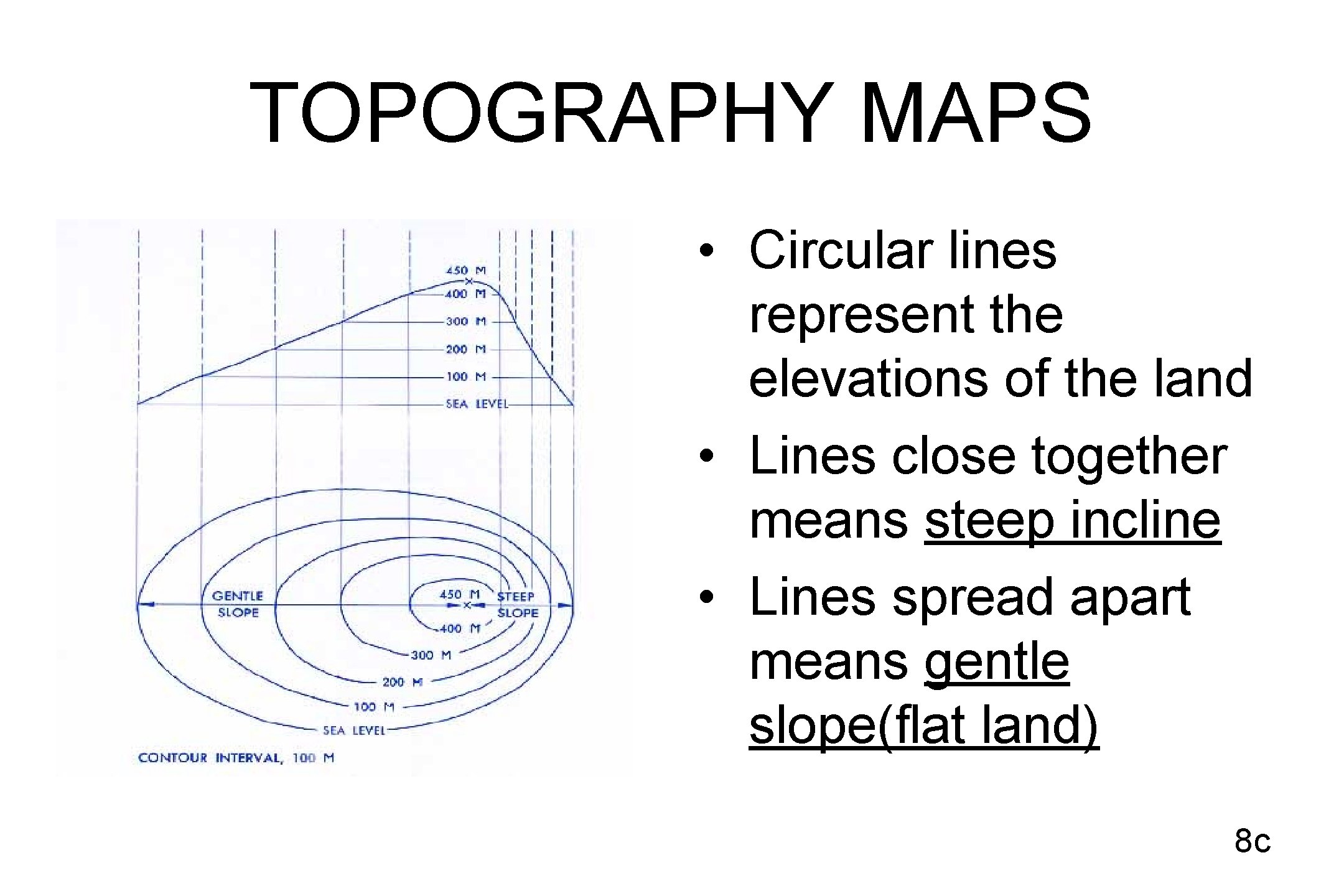
TOPOGRAPHY MAPS • Circular lines represent the elevations of the land • Lines close together means steep incline • Lines spread apart means gentle slope(flat land) 8 c

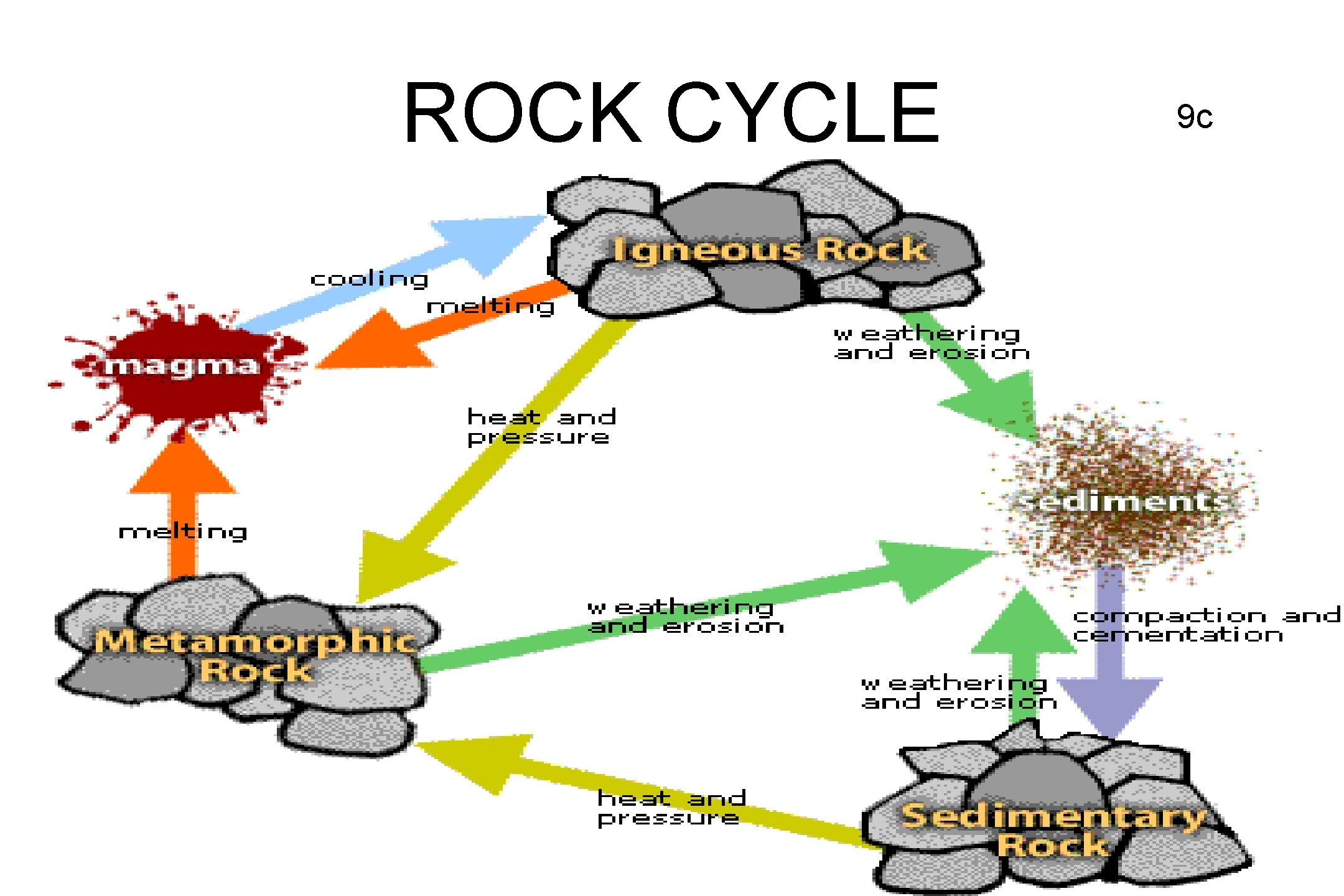
ROCK CYCLE • NOTES ON BACK 9 c

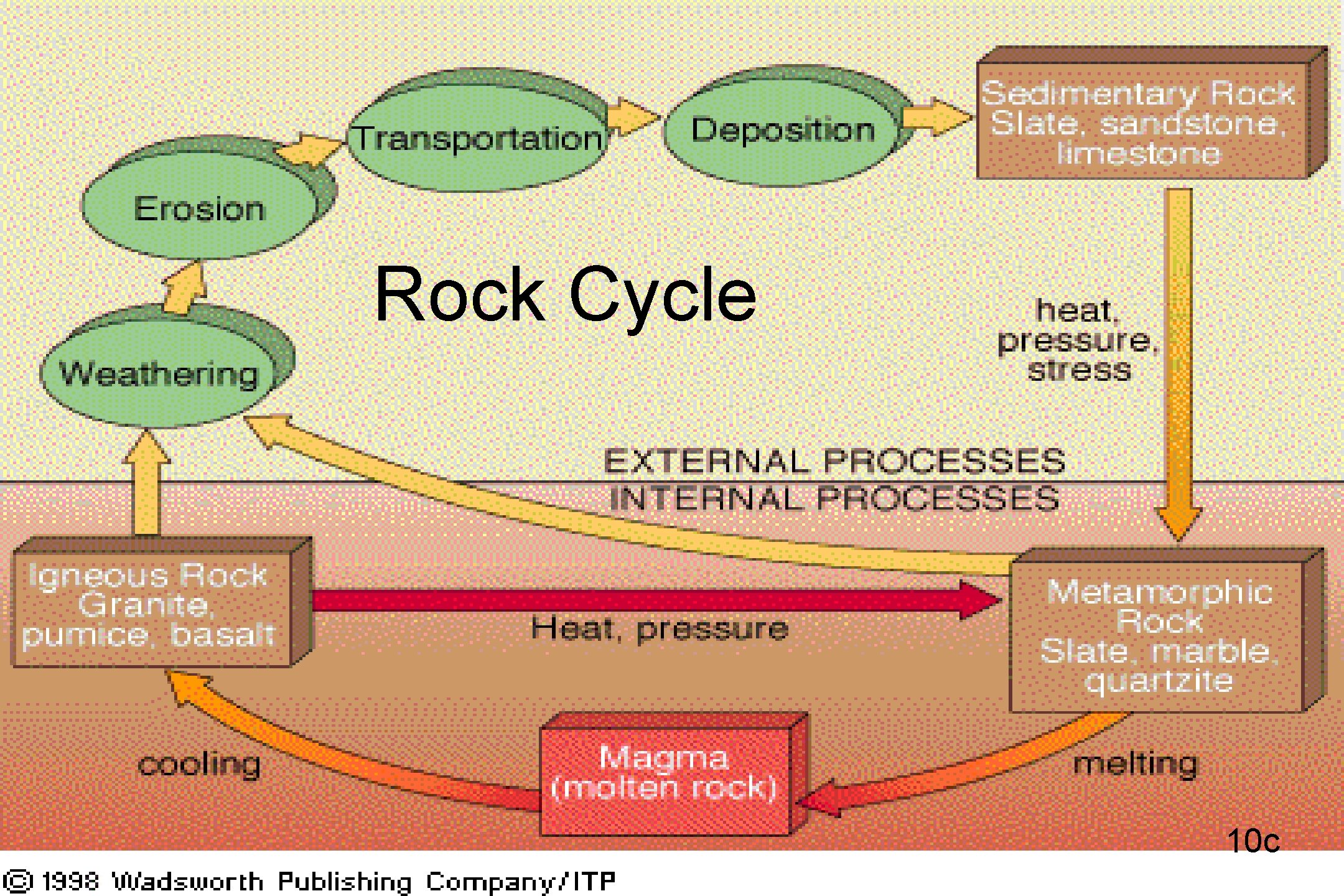
Rock Cycle 10 c

ROCK CYCLE • HEAT AND PRESSURE MAKE METAMORPHIC ROCK • MELTING AND COOLING MAKE IGNEOUS ROCK • CEMENTATION AND COMPACTION MAKE SEDIMENTARY ROCK • WEATHERING AND EROSION MAKE SEDIMENTS 11 c

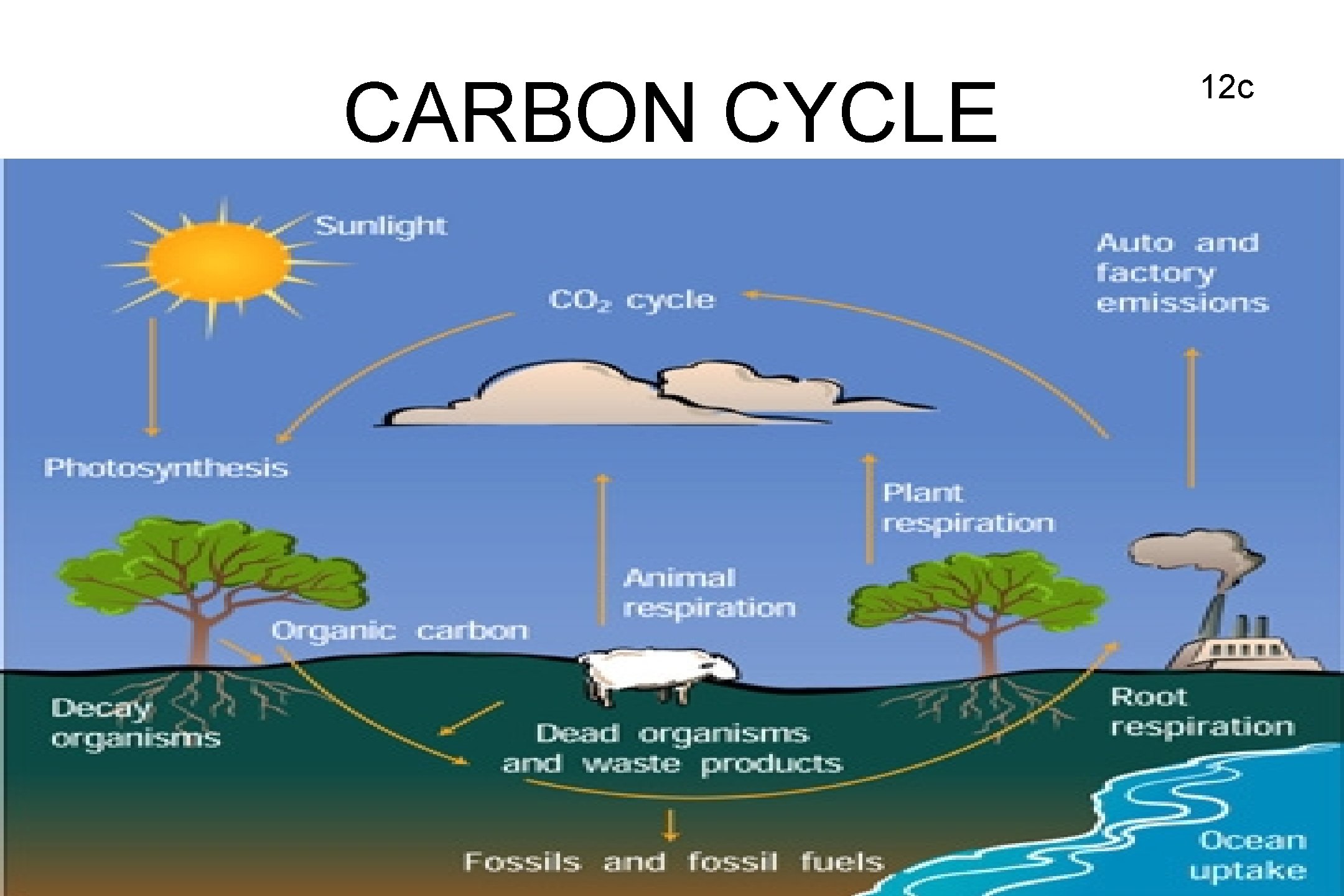
CARBON CYCLE 12 c

CARBON CYCLE • Photosynthesis is the only process that removes CO 2 from the atmosphere. • Respiration and decomposition put CO 2 back into atmosphere naturally. • Burning of fossil fuels puts excess CO 2 into atmosphere causing global warming. 13 c

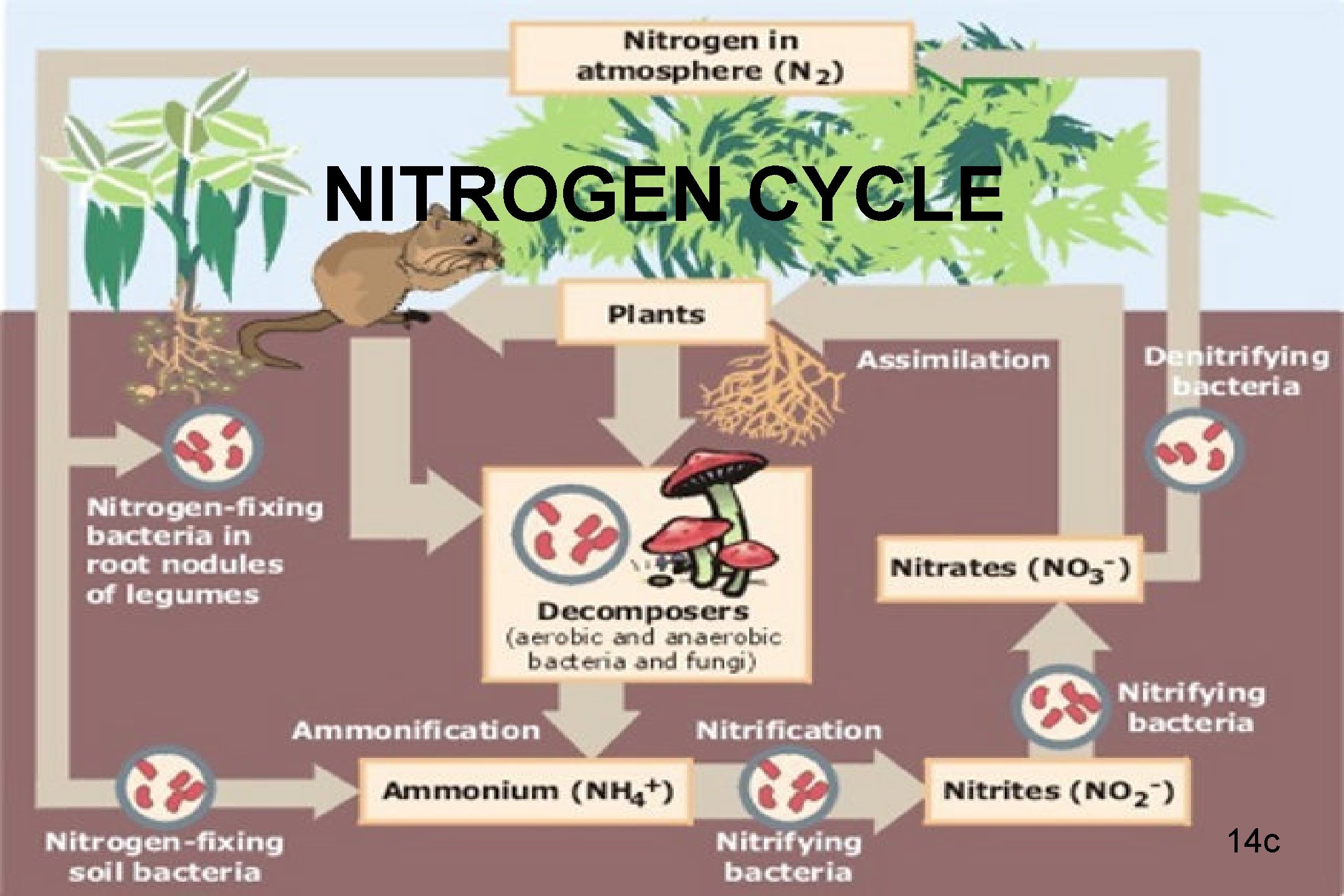
NITROGEN CYCLE 14 c

NITROGEN CYCLE • 80% of air is nitrogen • Nitrogen in atmosphere not in form to be used by living organisms • Lightning and nitrogen-fixing bacteria change nitrogen into useable form • Plants absorb nitrogen through roots • Consumer gets nitrogen when eating • Decomposition puts nitrogen back into atmosphere 15 c

HUMAN IMPACT ON NITROGEN • Nitrogen-rich fertilizers damage nearby waterways • Waste from livestock put too much nitrogen in soil; plants overgrow rapidly; use up nitrogen and die • Herbivore population increases with producer increase • When plant die off, herbivores starve 16 c

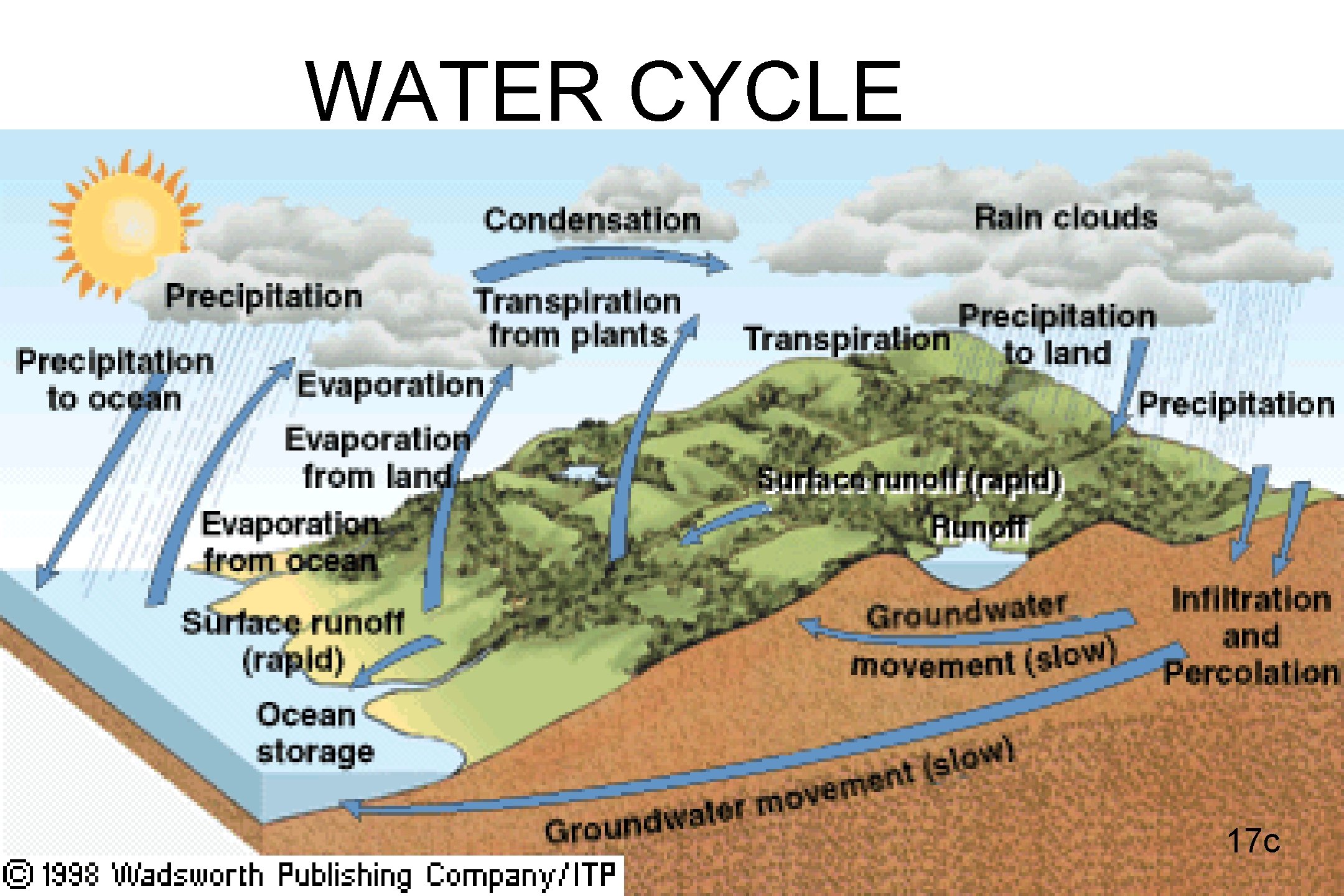
WATER CYCLE 17 c

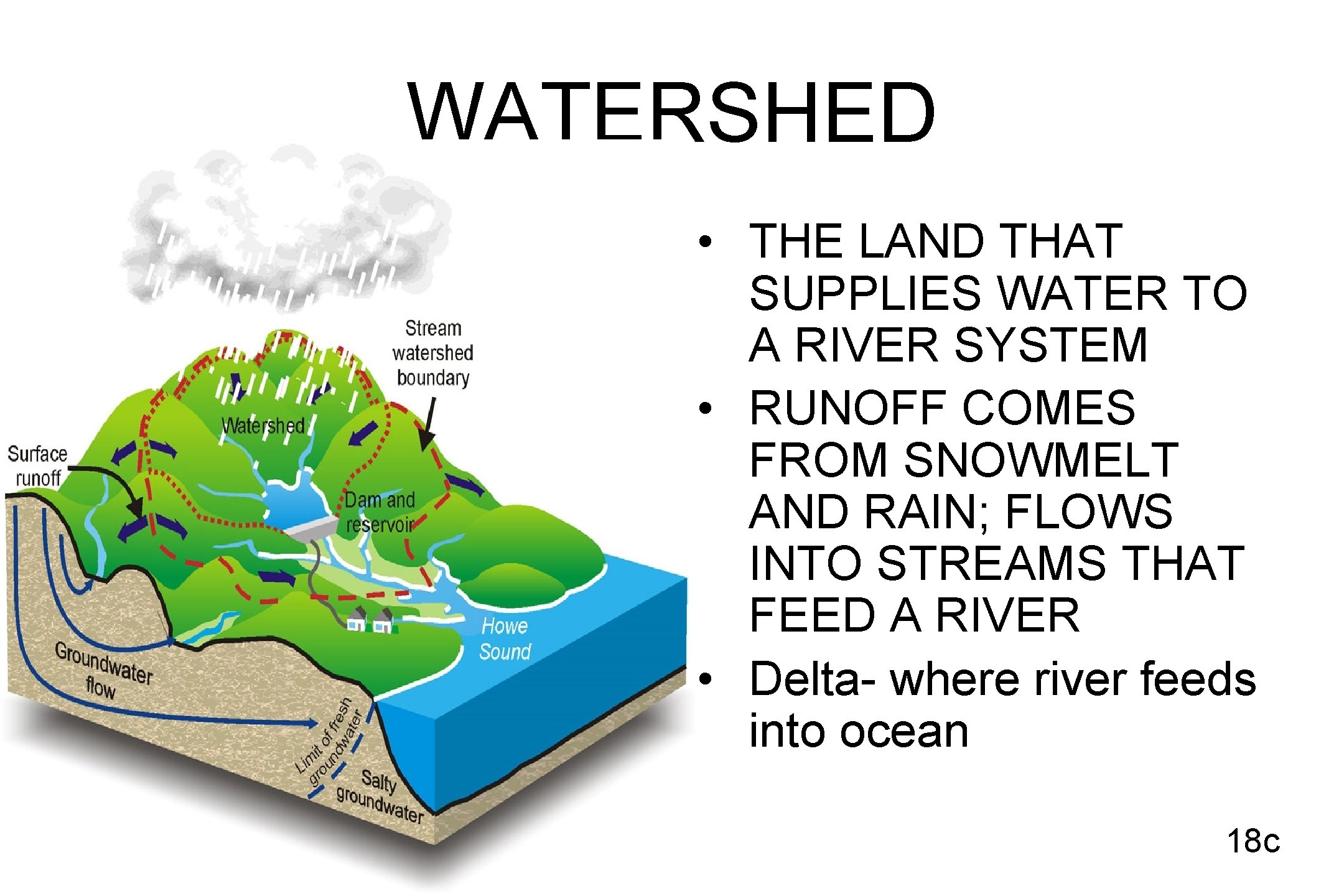
WATERSHED • THE LAND THAT SUPPLIES WATER TO A RIVER SYSTEM • RUNOFF COMES FROM SNOWMELT AND RAIN; FLOWS INTO STREAMS THAT FEED A RIVER • Delta- where river feeds into ocean 18 c

• GROUNDWATER – water that fills the cracks and spaces in underground soil and rock layers (aquifers). This is where most fresh water is found. • SURFACE WATER – water found on the surface such as in lakes, rivers, and oceans 19 c

PHYSICAL WEATHERING • FROST WEDGING – rocks break when water freezes and thaws in the cracks • ABRASION – rocks break as they rub or bounce off each other • EXFOLIATION – peeling away of large sheets of loosened material at the surface of a rock • Roots growing in cracks of rocks • Humans and animals walking in nature 20 c

CHEMICAL WEATHERING • Changes the composition of the rock • OXIDATION – oxygen interacts chemically with minerals in rock • HYDRATION – water interacts chemically with minerals in rock • CARBONATION – carbon dioxide interacts chemically with minerals in the rock • Plant roots can also chemically react with rocks 21 c

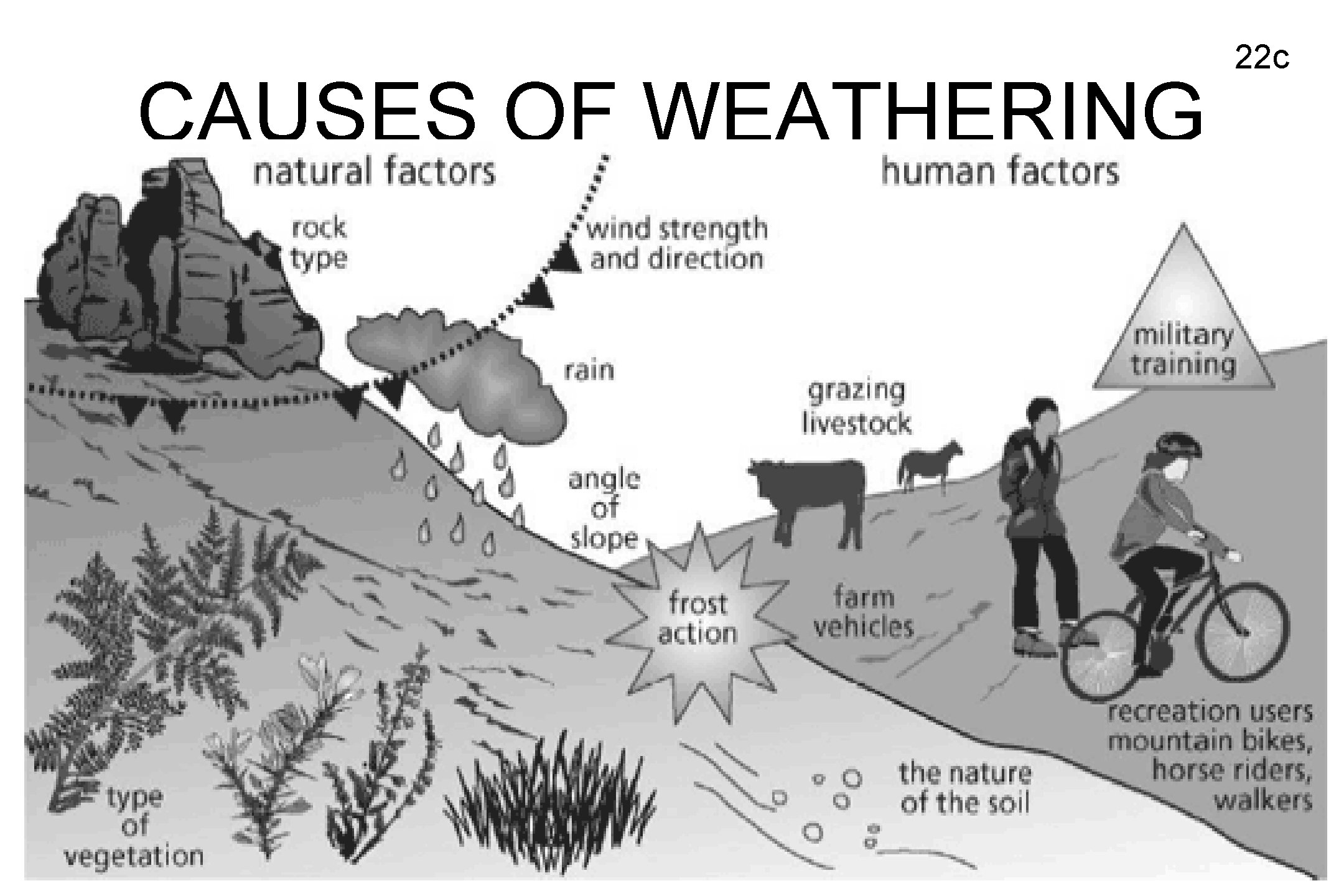
CAUSES OF WEATHERING 22 c

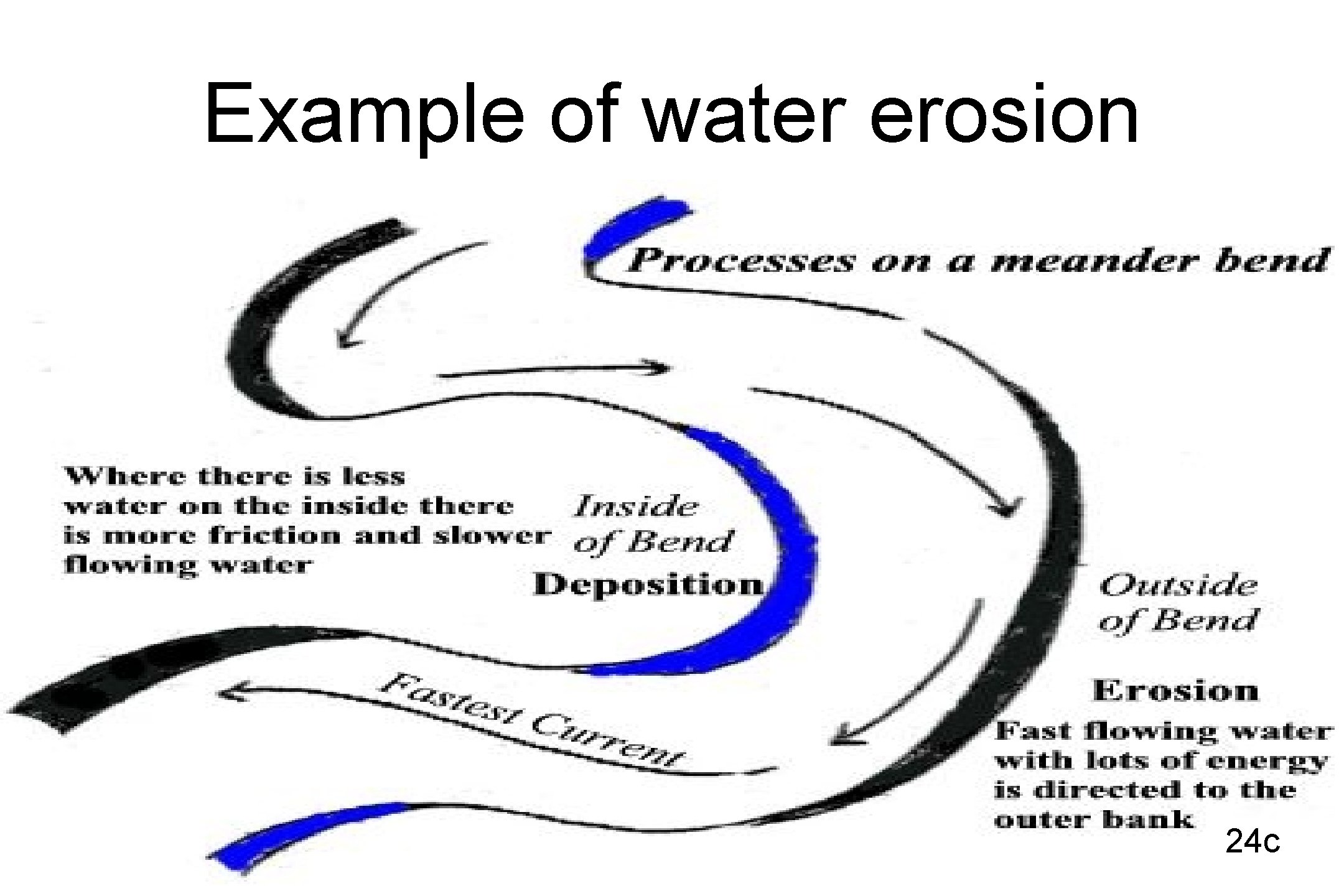
EROSION • WATER EROSION – Raindrops – splash erosion moves small particles – Streams and runoff moving water moves objects • The faster the water moves the larger the particles that are moved 23 c

Example of water erosion 24 c

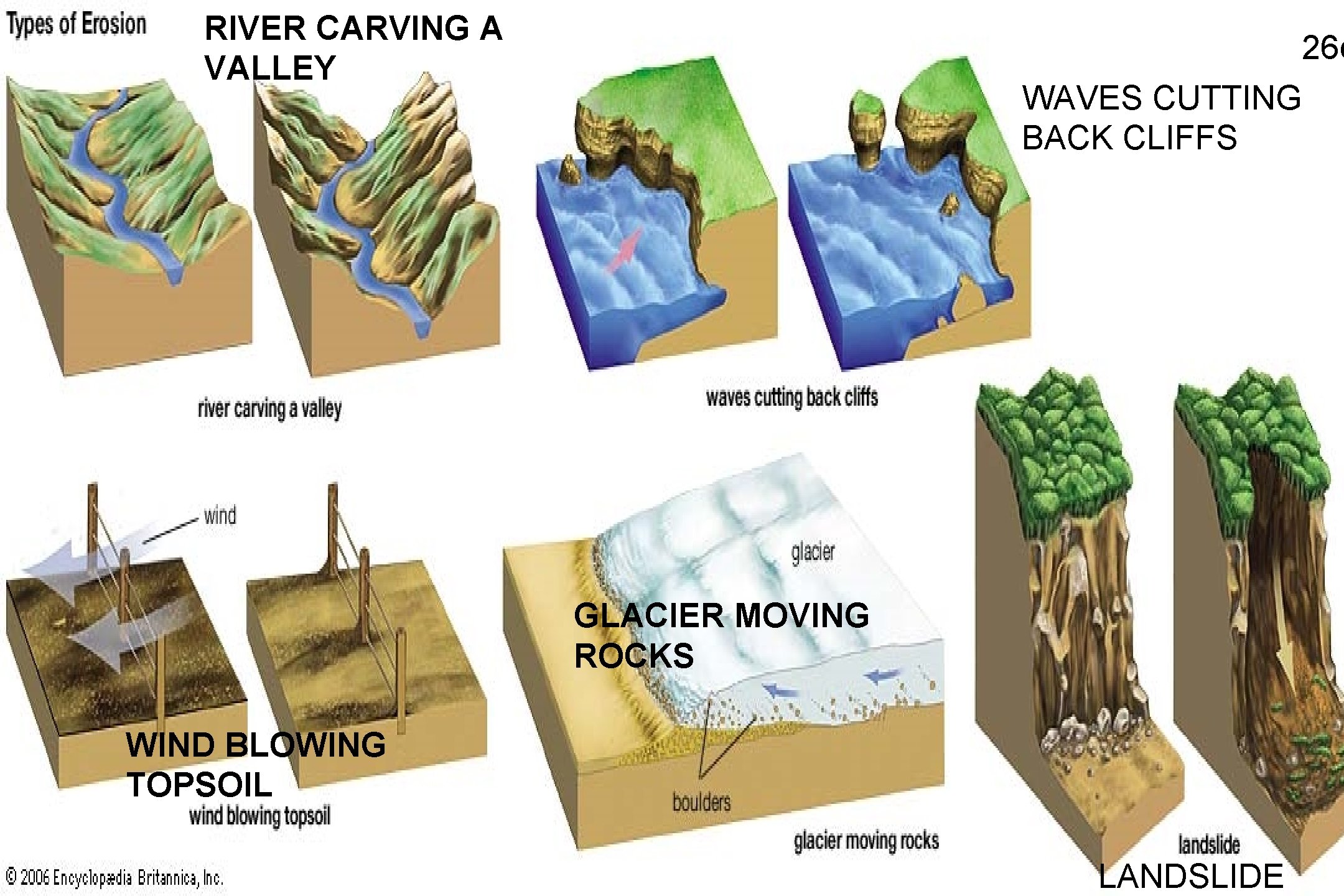
EROSION • WIND – faster the wind, the larger the particles that can move – Causes sand dunes • ICE – abrasion – glacier cuts into the rock underneath and breaks it • WAVE – incoming wave deposits sand; 25 c outgoing wave removes sand

RIVER CARVING A VALLEY 26 c WAVES CUTTING BACK CLIFFS GLACIER MOVING ROCKS WIND BLOWING TOPSOIL LANDSLIDE

OCEAN AFFECTS CLIMATE • Water takes longer to heat than land • Ocean helps cool the land in the summer • Ocean helps keep the land warm in the winter 27 c

OCEAN AFFECTS COASTAL CLIMATE • Warm land heats up the air causing the air to rise • Cool air from ocean comes on shore (sea breeze) to fill in space left by warm air • Opposite happens at night 28 c

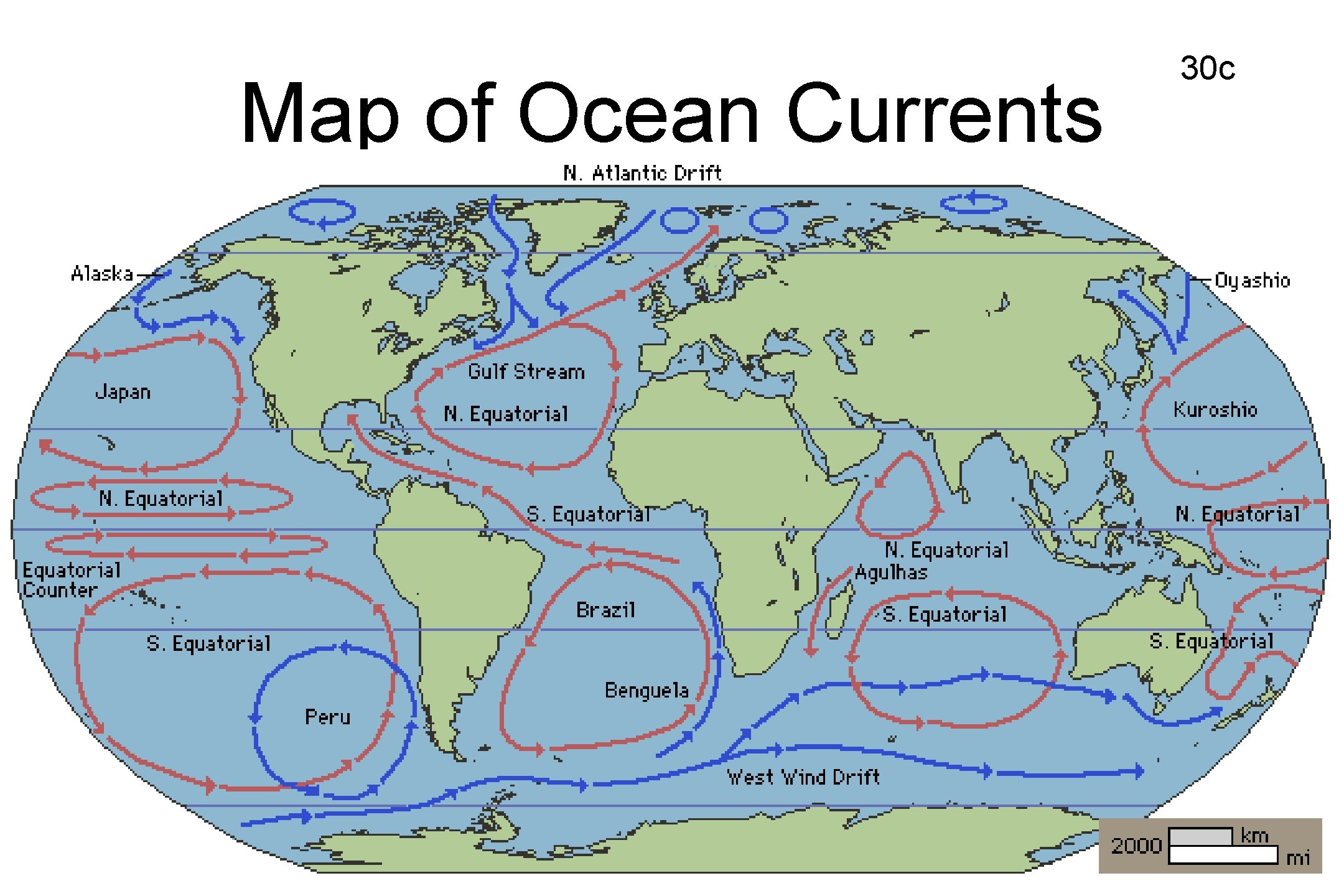
OCEAN CURRENTS AFFECT CLIMATE • Currents formed from solar energy, gravity, and Earth’s rotation • Surface currents affected by wind. • Warm water from equator goes up toward north pole and south towards south pole • Cold water from the poles pushes up toward equator 29 c

Map of Ocean Currents 30 c

IMPACT OF HUMANS ON EARTH’S SYSTEMS • Global warming – burning of fossil fuels • Ozone layer – destroyed by CFSs; ozone absorbs sun’s UV rays • Pesticides – poison air, water, and food • Acid rain – nitrogen in air turns rain acidic; increases chemical weathering • Loss of non-renewable resources 31 c • Destruction of natural habitat

Hurricanes • Need WARM WATER from the tropics to form 32 c

SPIRAL GALAXY • BULGE IN CENTER • SPIRAL ARMS • MILKY WAY – name of our galaxy 33 c

ELIPTICAL GALAXY • MASSIVE BLOB OF STARS • ONLY CONTAINS OLD STARS • OLDEST TYPE OF GALAXY 34 c

IRREGULAR GALAXY • LOOKS LIKE A CLOUD OF STARS OR A NEBULA 35 c

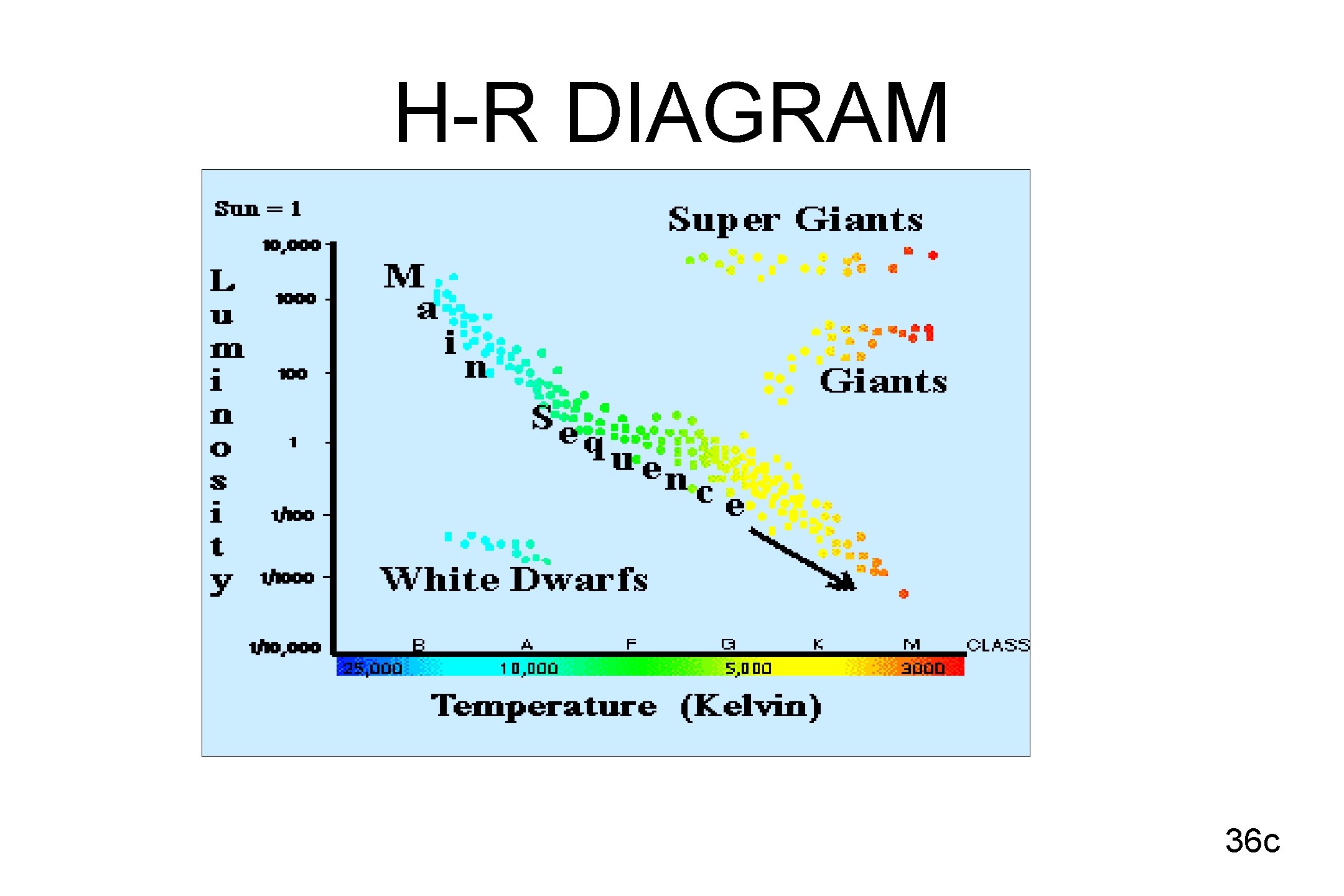
H-R DIAGRAM 36 c

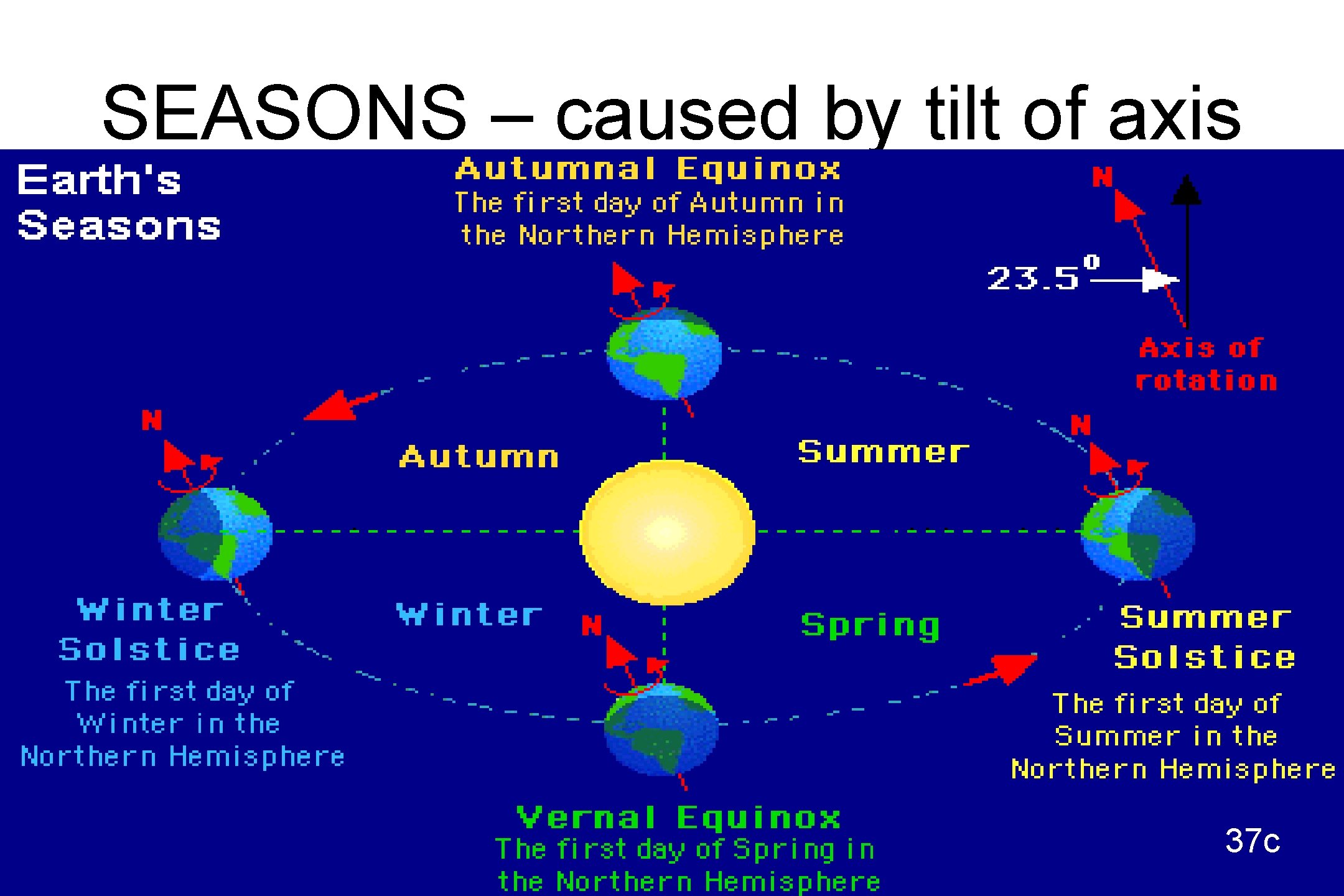
SEASONS – caused by tilt of axis 37 c

LUNAR CYCLE • Lasts 28. 5 days • Every 3. 5 days is a new phase sun Waxing – (to grow) increasing amount of light. Waning – (to shrink) decreasing amount of light. 38 c

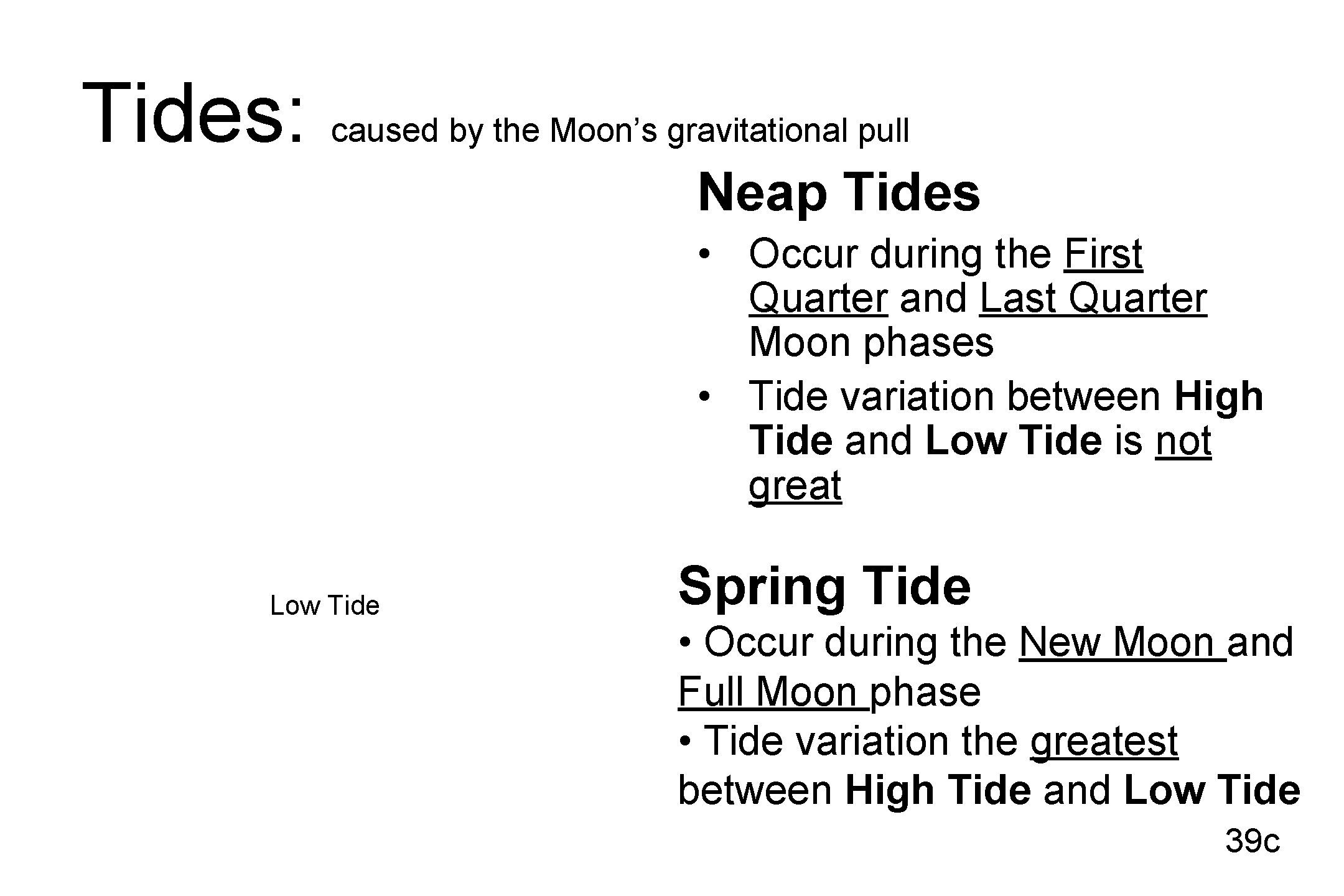
Tides: caused by the Moon’s gravitational pull Neap Tides • Occur during the First Quarter and Last Quarter Moon phases • Tide variation between High Tide and Low Tide is not great Low Tide Spring Tide • Occur during the New Moon and Full Moon phase • Tide variation the greatest between High Tide and Low Tide 39 c

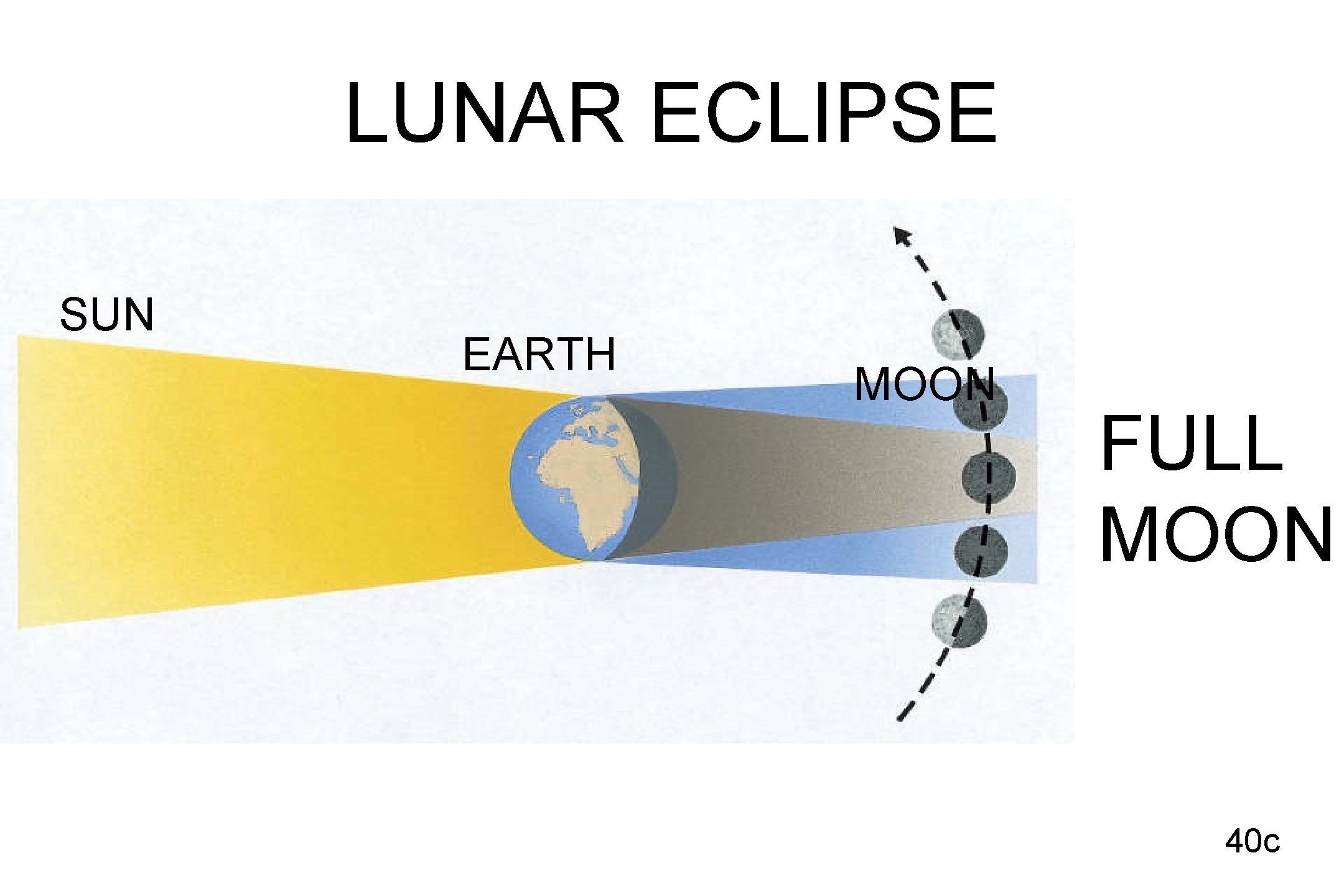
LUNAR ECLIPSE SUN EARTH MOON FULL MOON 40 c

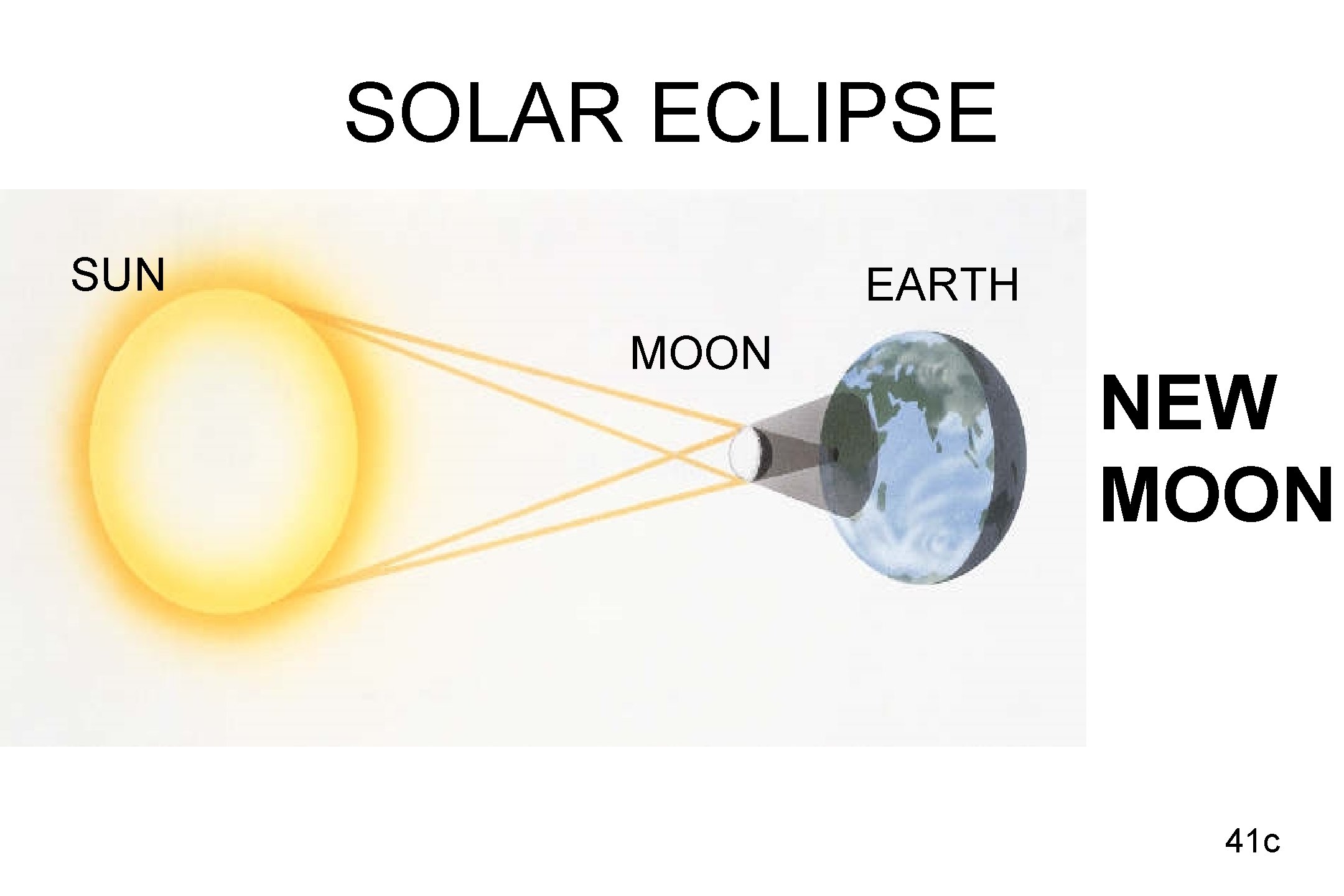
SOLAR ECLIPSE SUN EARTH MOON NEW MOON 41 c

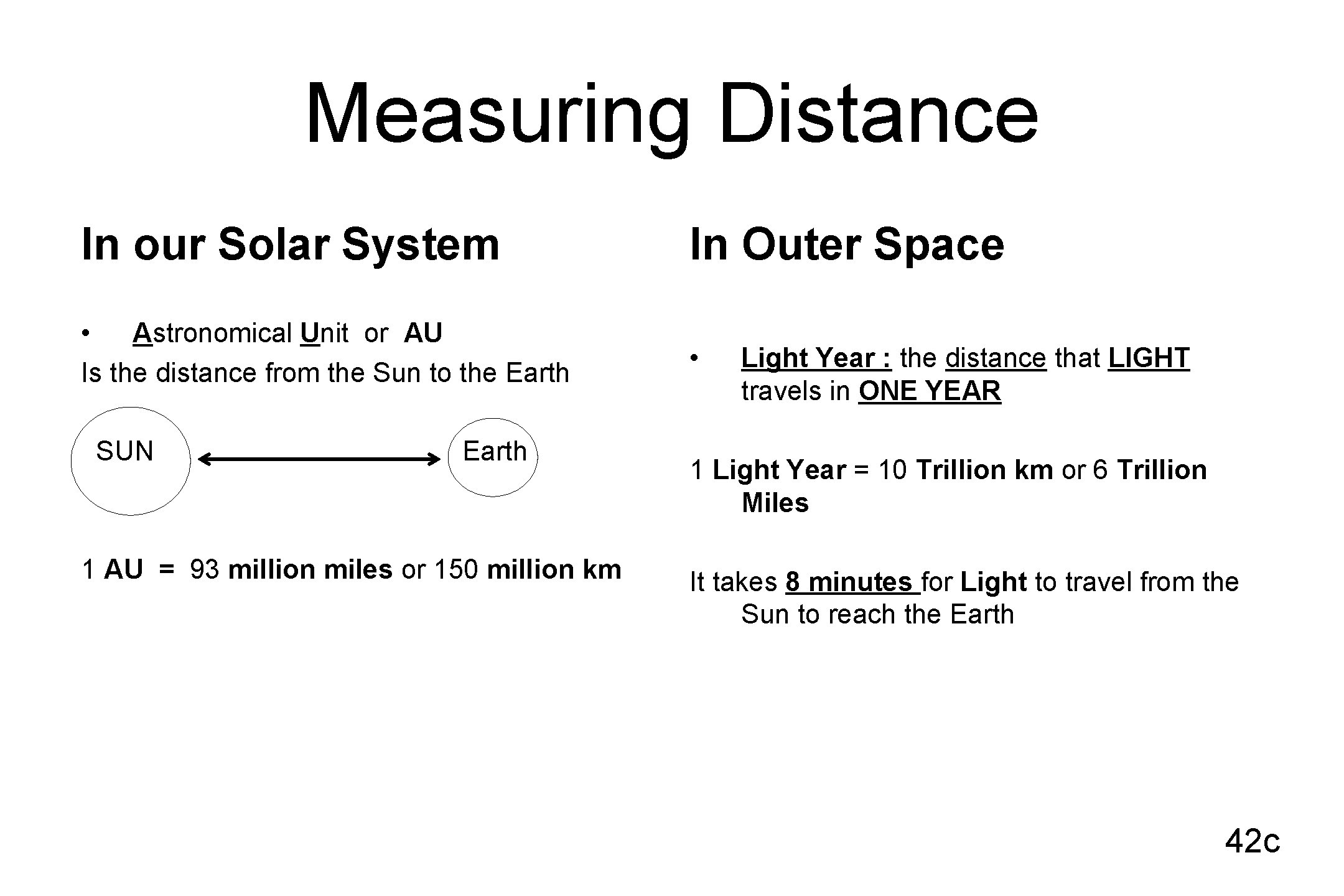
Measuring Distance In our Solar System In Outer Space • Astronomical Unit or AU Is the distance from the Sun to the Earth • SUN Earth 1 AU = 93 million miles or 150 million km Light Year : the distance that LIGHT travels in ONE YEAR 1 Light Year = 10 Trillion km or 6 Trillion Miles It takes 8 minutes for Light to travel from the Sun to reach the Earth 42 c