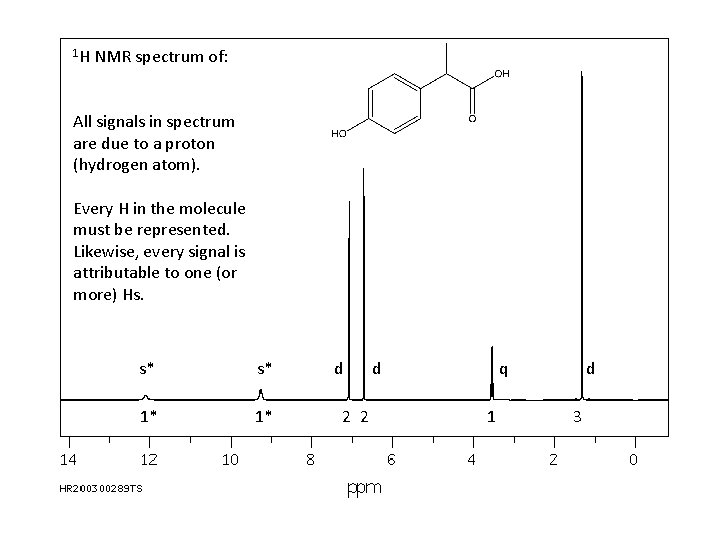
1 H NMR spectrum of All signals in












- Slides: 48

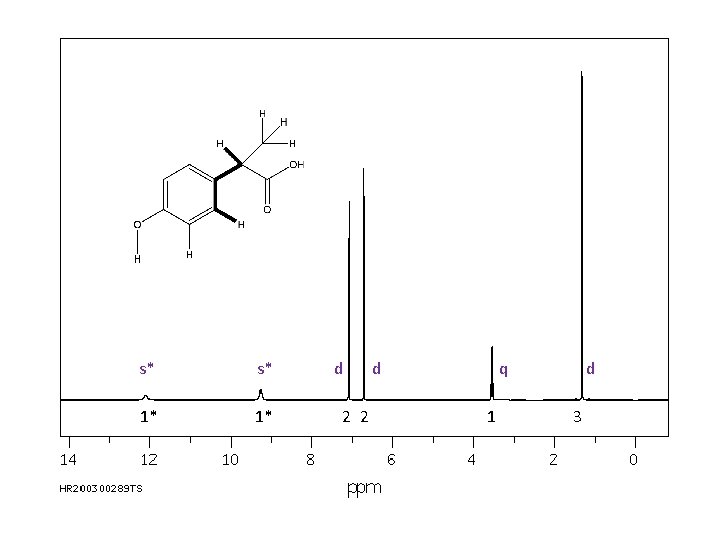
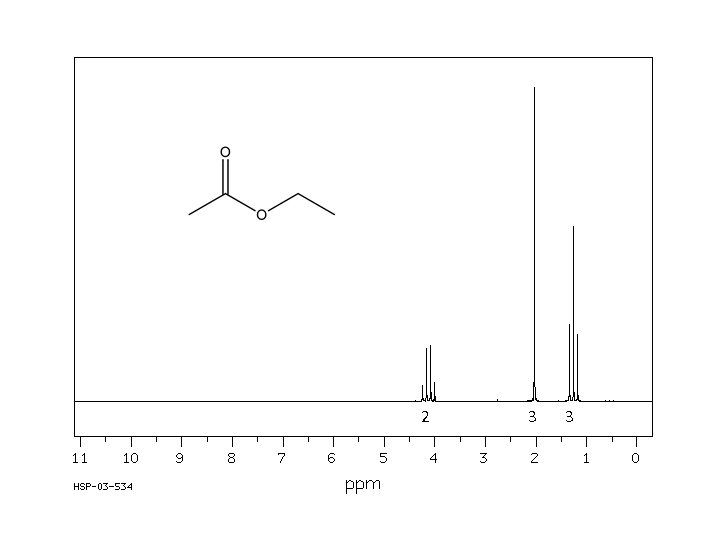
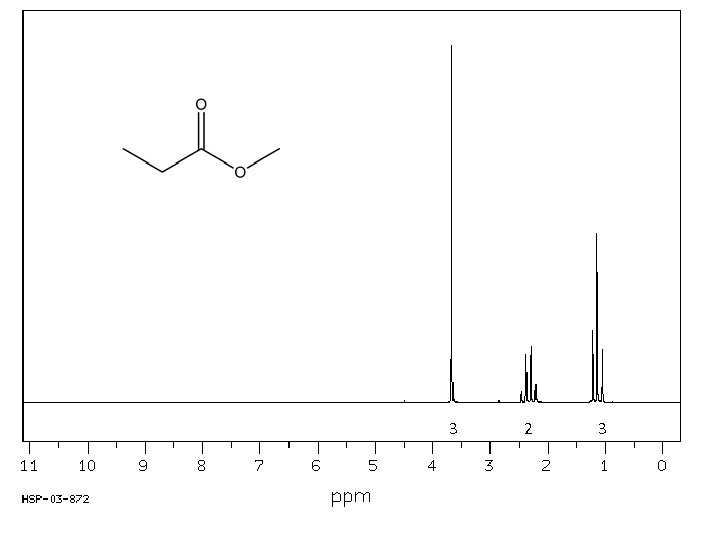
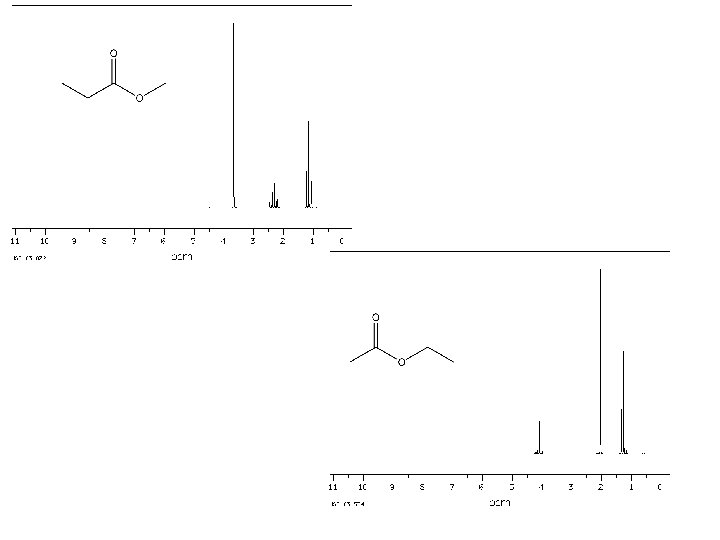
1 H NMR spectrum of: All signals in spectrum are due to a proton (hydrogen atom). Every H in the molecule must be represented. Likewise, every signal is attributable to one (or more) Hs. s* s* 1* 1* d 2 2 d q 1 d 3

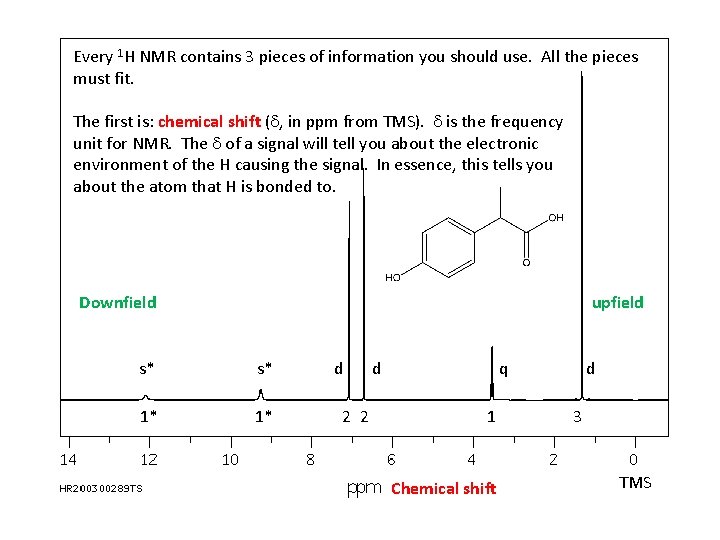
Every 1 H NMR contains 3 pieces of information you should use. All the pieces must fit. The first is: chemical shift (d, in ppm from TMS). d is the frequency unit for NMR. The d of a signal will tell you about the electronic environment of the H causing the signal. In essence, this tells you about the atom that H is bonded to. Downfield upfield s* s* 1* 1* d 2 2 d q 1 Chemical shift d 3 TMS

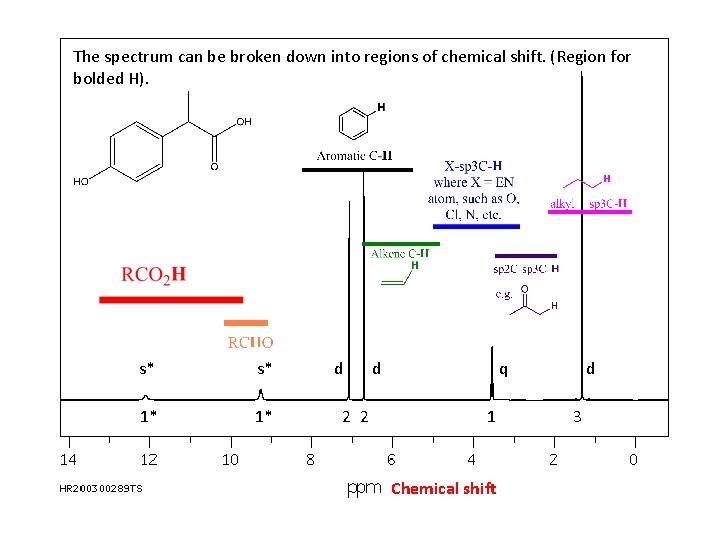
The spectrum can be broken down into regions of chemical shift. (Region for bolded H). s* s* 1* 1* d 2 2 d q 1 Chemical shift d 3

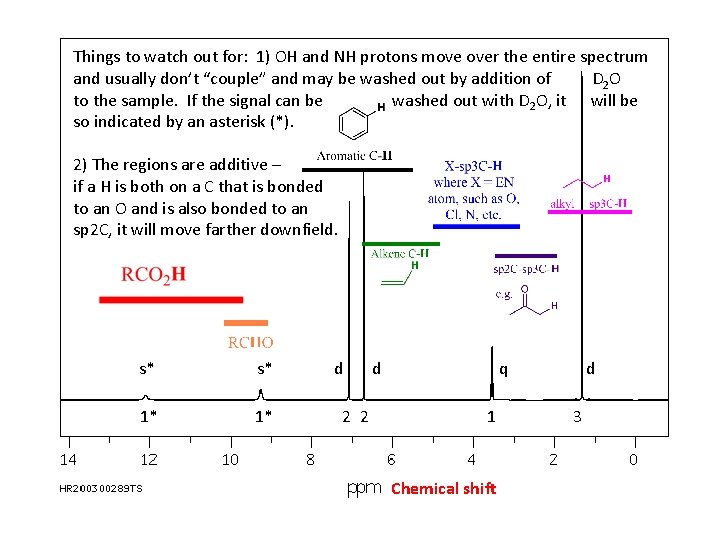
Things to watch out for: 1) OH and NH protons move over the entire spectrum and usually don’t “couple” and may be washed out by addition of D 2 O to the sample. If the signal can be washed out with D 2 O, it will be so indicated by an asterisk (*). 2) The regions are additive – if a H is both on a C that is bonded to an O and is also bonded to an sp 2 C, it will move farther downfield. s* s* 1* 1* d 2 2 d q 1 Chemical shift d 3

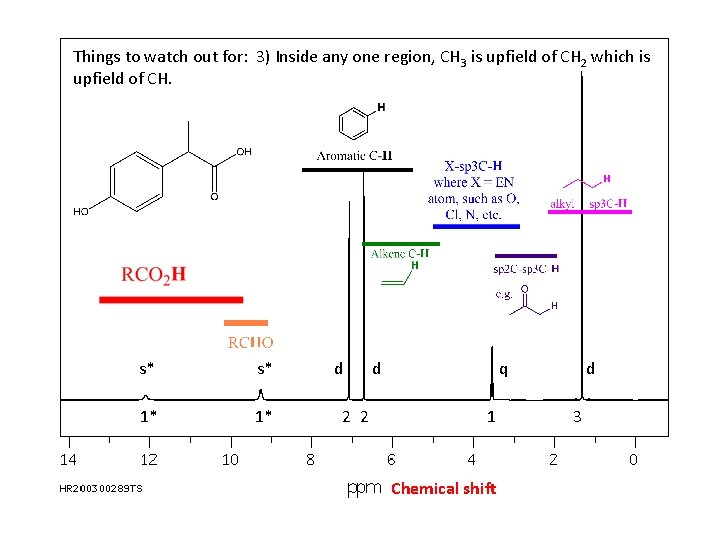
Things to watch out for: 3) Inside any one region, CH 3 is upfield of CH 2 which is upfield of CH. s* s* 1* 1* d 2 2 d q 1 Chemical shift d 3

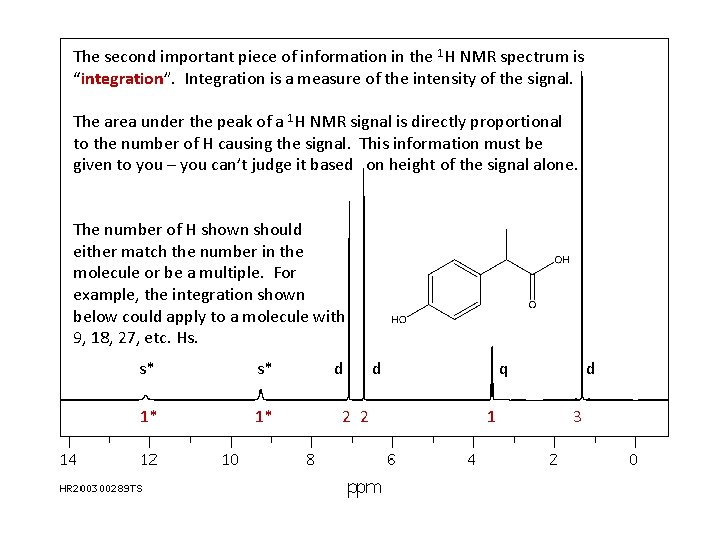
The second important piece of information in the 1 H NMR spectrum is “integration”. Integration is a measure of the intensity of the signal. The area under the peak of a 1 H NMR signal is directly proportional to the number of H causing the signal. This information must be given to you – you can’t judge it based on height of the signal alone. The number of H shown should either match the number in the molecule or be a multiple. For example, the integration shown below could apply to a molecule with 9, 18, 27, etc. Hs. s* s* 1* 1* d 2 2 d q 1 d 3

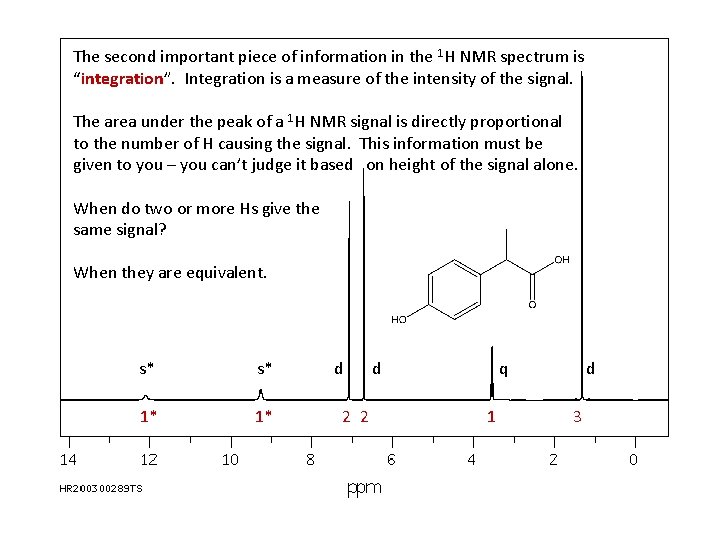
The second important piece of information in the 1 H NMR spectrum is “integration”. Integration is a measure of the intensity of the signal. The area under the peak of a 1 H NMR signal is directly proportional to the number of H causing the signal. This information must be given to you – you can’t judge it based on height of the signal alone. When do two or more Hs give the same signal? When they are equivalent. s* s* 1* 1* d 2 2 d q 1 d 3

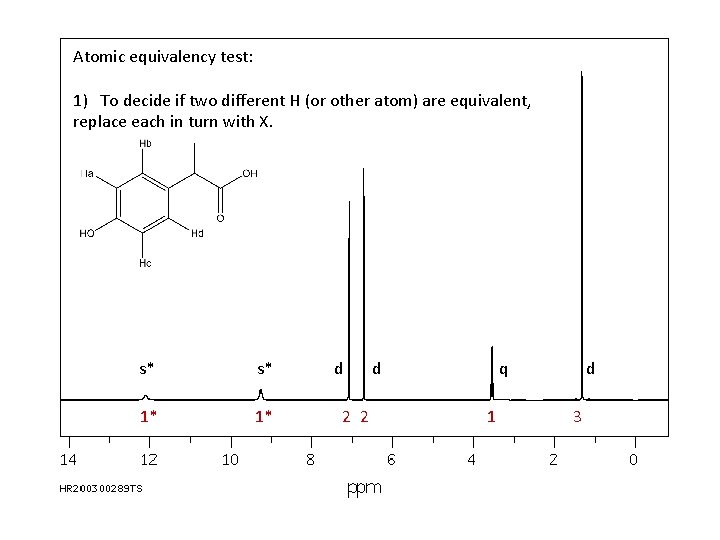
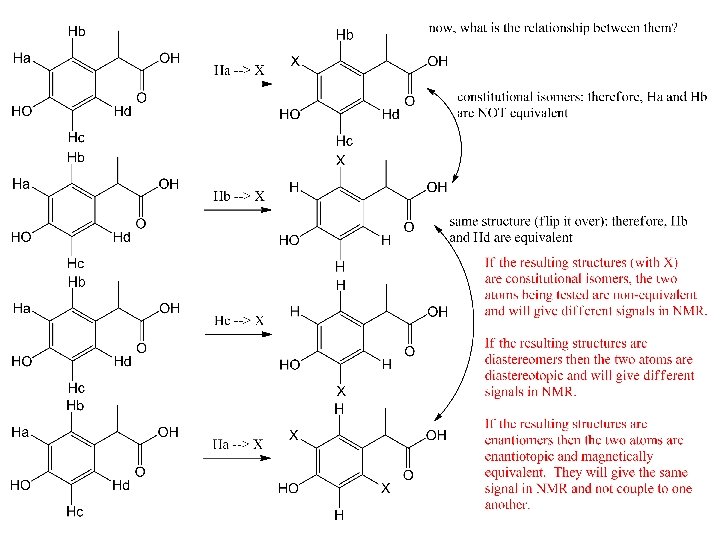
Atomic equivalency test: 1) To decide if two different H (or other atom) are equivalent, replace each in turn with X. s* s* 1* 1* d 2 2 d q 1 d 3


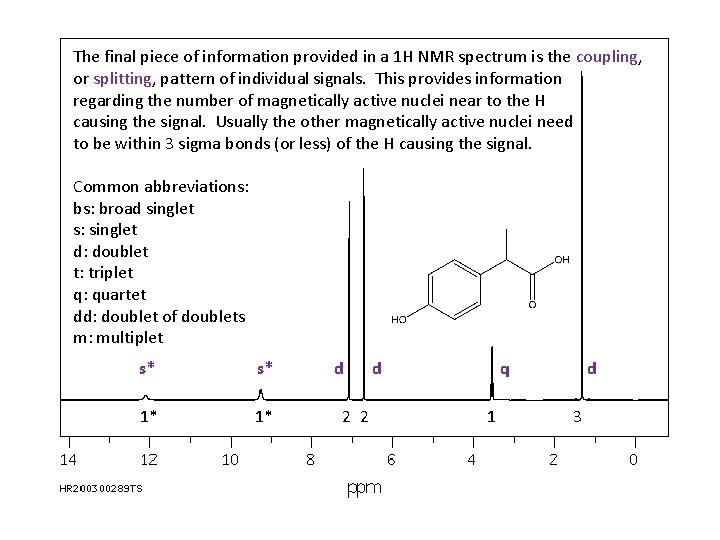
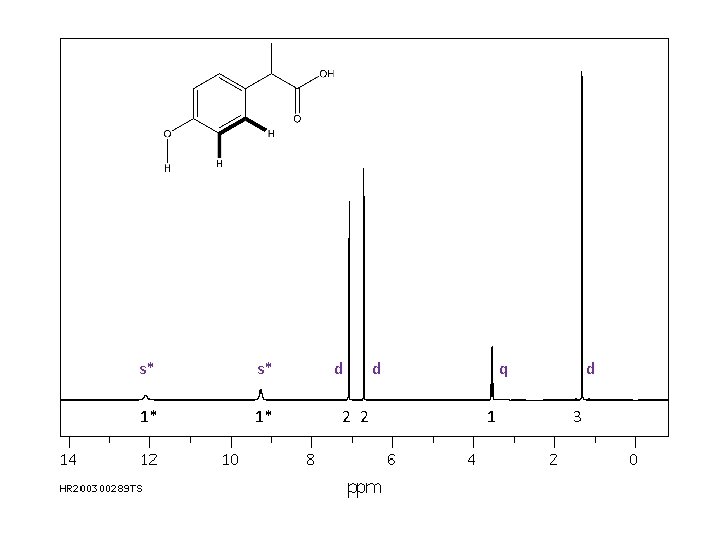
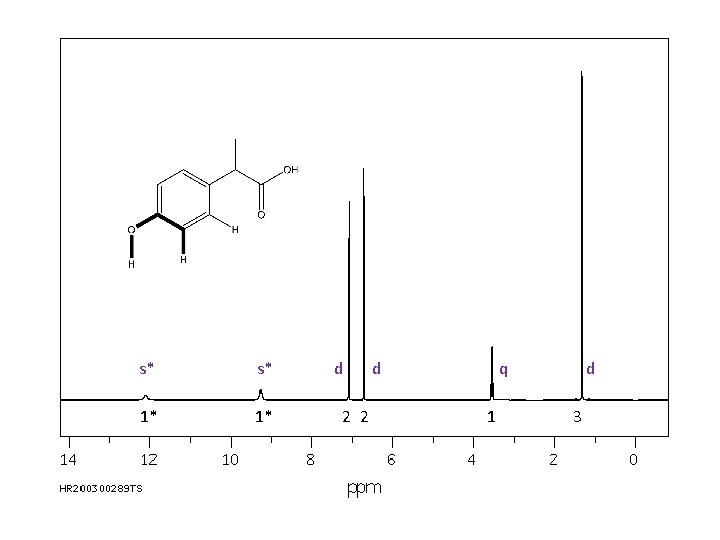
The final piece of information provided in a 1 H NMR spectrum is the coupling, or splitting, pattern of individual signals. This provides information regarding the number of magnetically active nuclei near to the H causing the signal. Usually the other magnetically active nuclei need to be within 3 sigma bonds (or less) of the H causing the signal. Common abbreviations: broad singlet s: singlet d: doublet t: triplet q: quartet dd: doublet of doublets m: multiplet s* s* 1* 1* d 2 2 d q 1 d 3

s* s* 1* 1* d 2 2 d q 1 d 3

s* s* 1* 1* d 2 2 d q 1 d 3

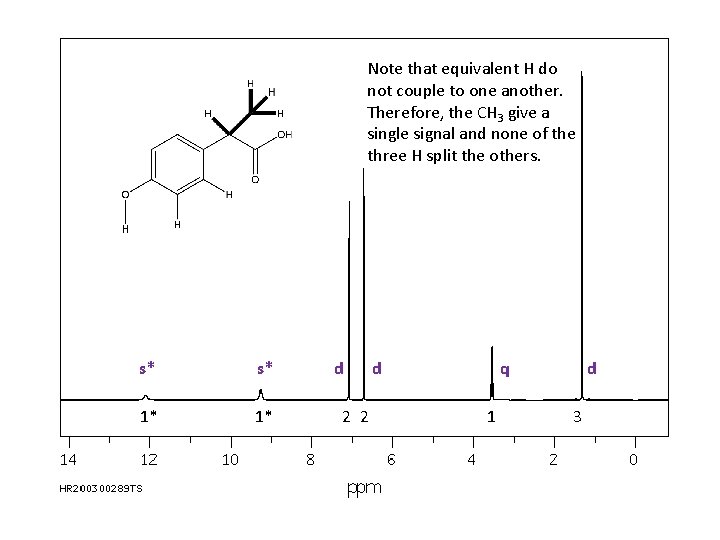
Note that equivalent H do not couple to one another. Therefore, the CH 3 give a single signal and none of the three H split the others. s* s* 1* 1* d 2 2 d q 1 d 3

s* s* 1* 1* d 2 2 d q 1 d 3

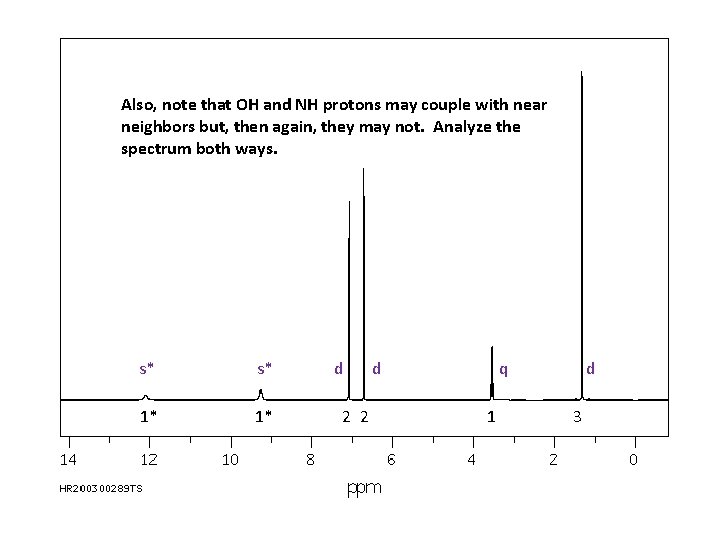
Also, note that OH and NH protons may couple with near neighbors but, then again, they may not. Analyze the spectrum both ways. s* s* 1* 1* d 2 2 d q 1 d 3


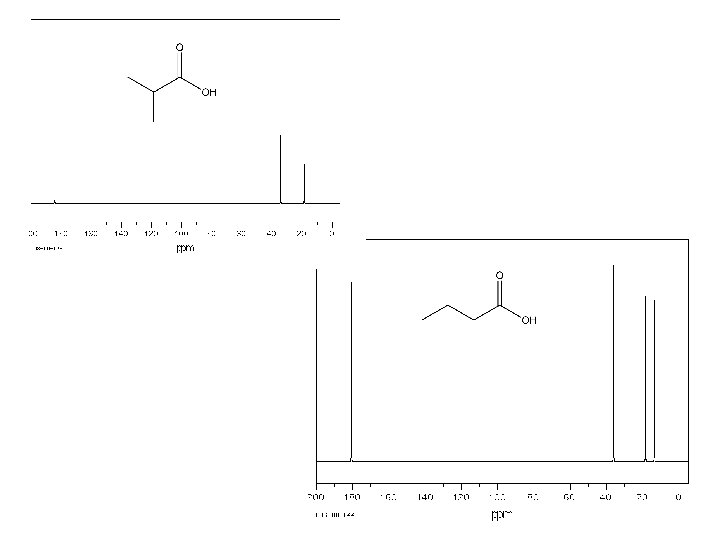
2 3 3




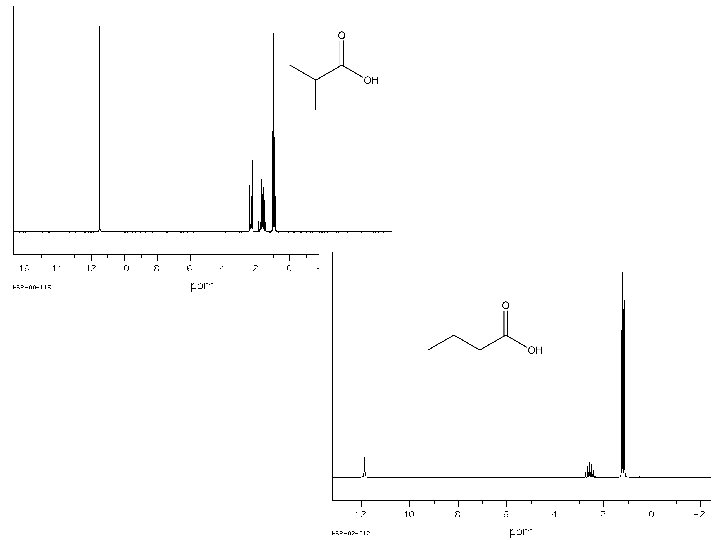
3 2 3





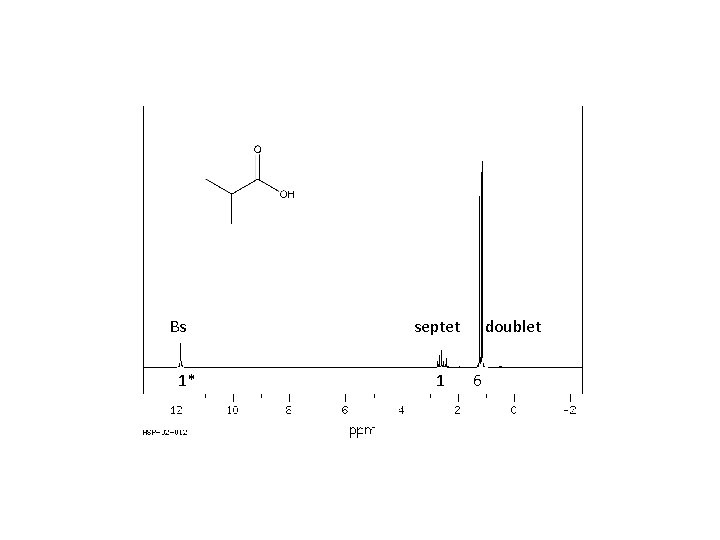
Bs 1* septet 1 doublet 6




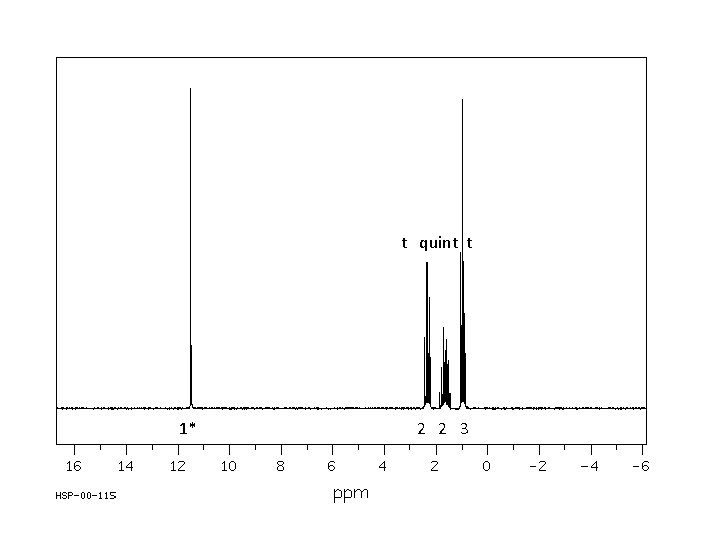
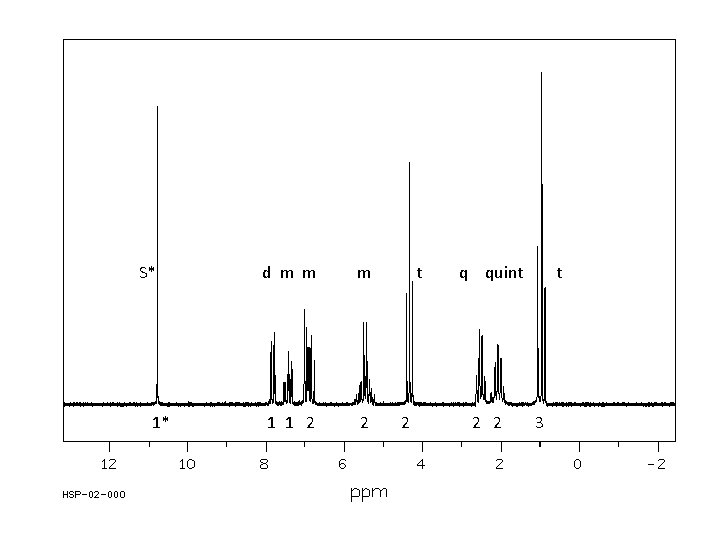
t quint t 1* 2 2 3





S* 1* d m m m 1 1 2 2 t 2 q quint 2 2 t 3

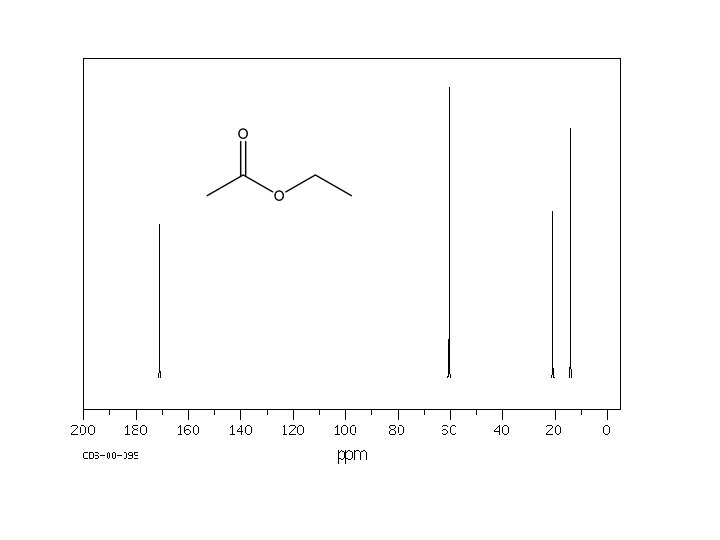
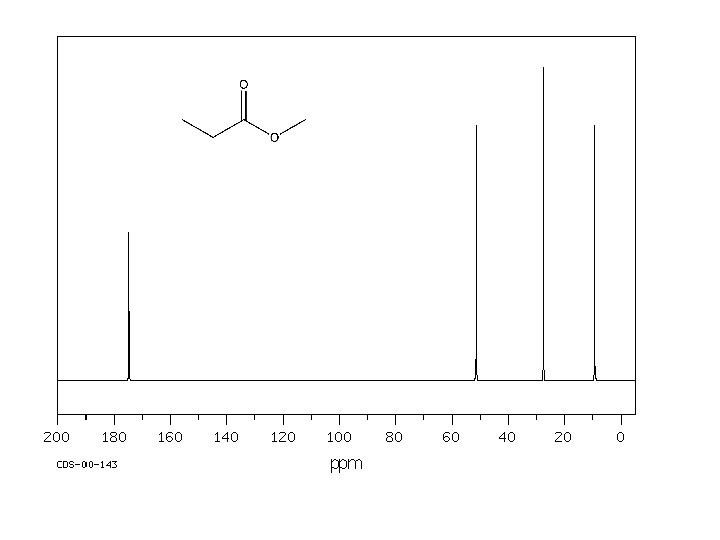
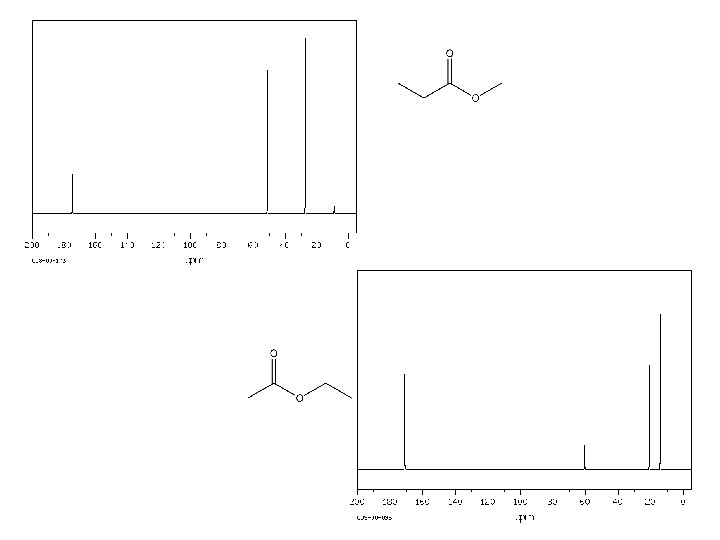
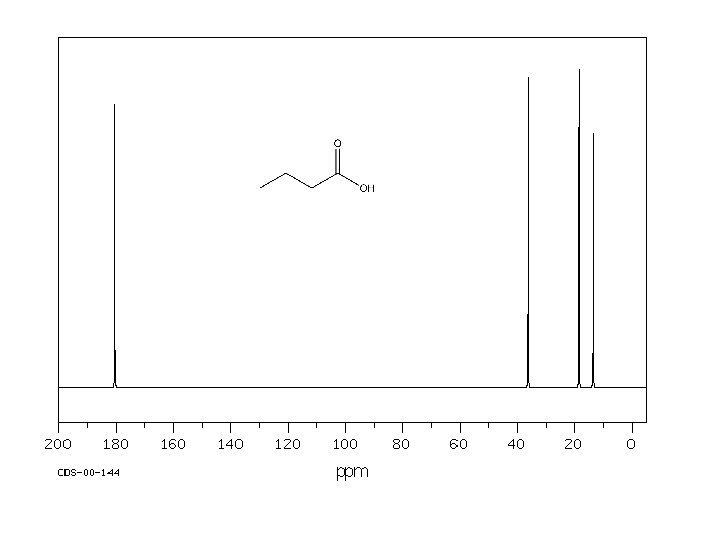
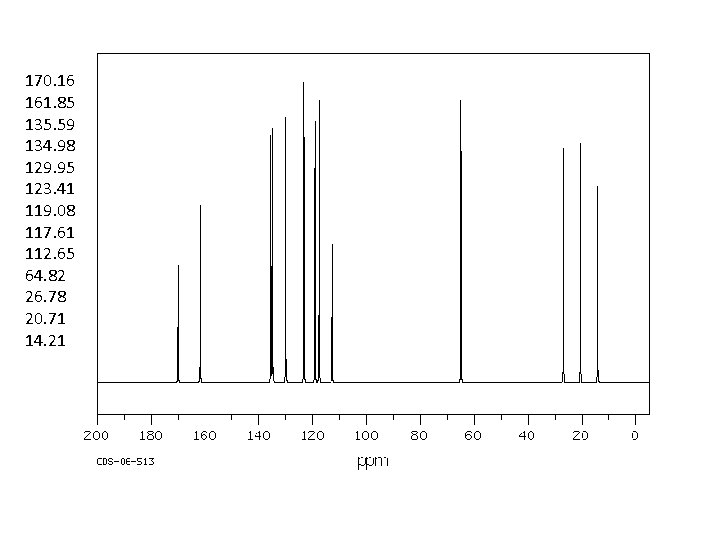
170. 16 161. 85 135. 59 134. 98 129. 95 123. 41 119. 08 117. 61 112. 65 64. 82 26. 78 20. 71 14. 21











