1 H NMR Interpretation Using the NMR Mosaic













- Slides: 56

1 H NMR Interpretation Using the NMR Mosaic

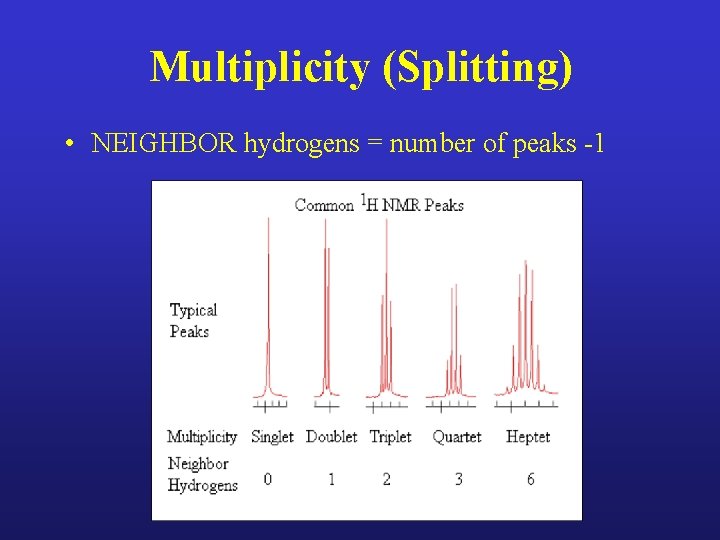
Each peak represents a fragment of the molecule Peak carries three critical pieces of information – Integration: how many hydrogens on that fragment – Chemical shift: functional group on or next to the fragment – Multiplicity: how many next-door hydrogens

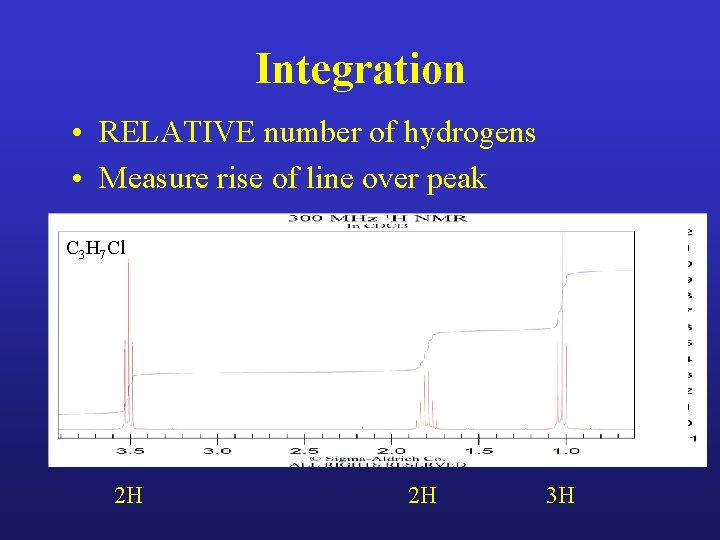
Integration • RELATIVE number of hydrogens • Measure rise of line over peak C 3 H 7 Cl 2 H 2 H 3 H

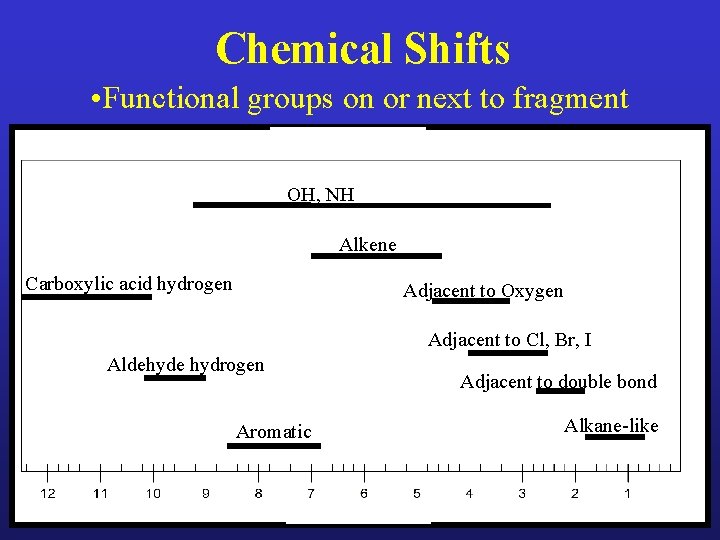
Chemical Shifts • Functional groups on or next to fragment OH, NH Alkene Carboxylic acid hydrogen Adjacent to Oxygen Adjacent to Cl, Br, I Aldehyde hydrogen Aromatic Adjacent to double bond Alkane-like

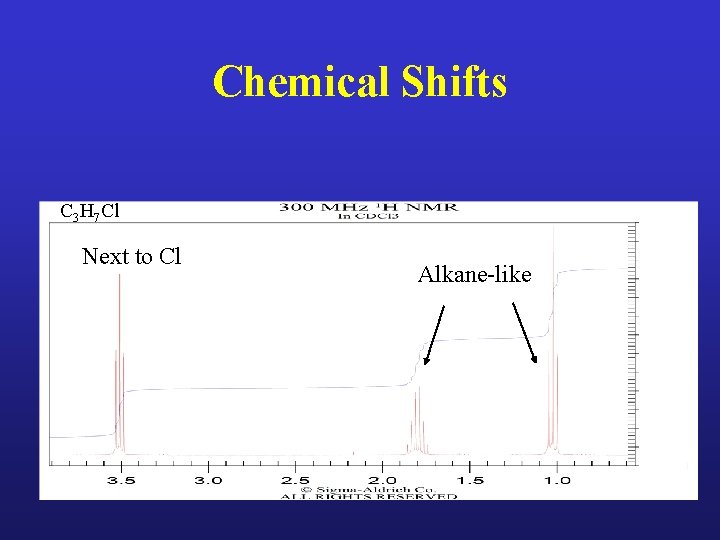
Chemical Shifts C 3 H 7 Cl Next to Cl Alkane-like

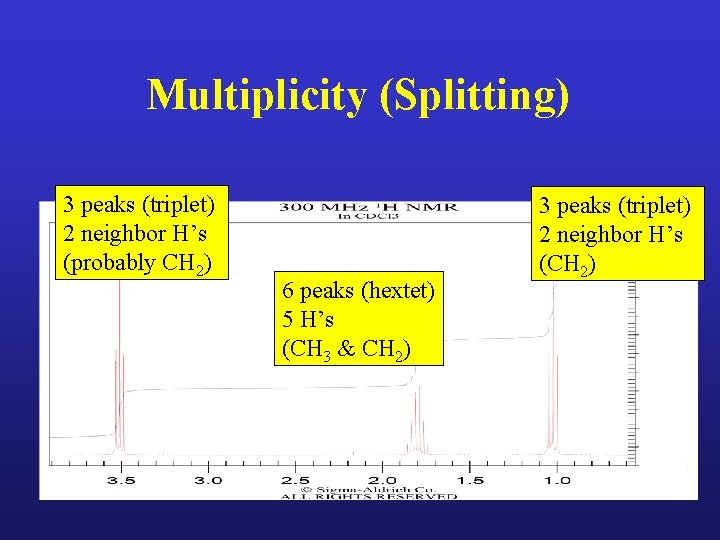
Multiplicity (Splitting) • NEIGHBOR hydrogens = number of peaks -1

Multiplicity (Splitting) 3 peaks (triplet) 2 neighbor H’s (probably CH 2) 6 peaks (hextet) 5 H’s (CH 3 & CH 2) 3 peaks (triplet) 2 neighbor H’s (CH 2)

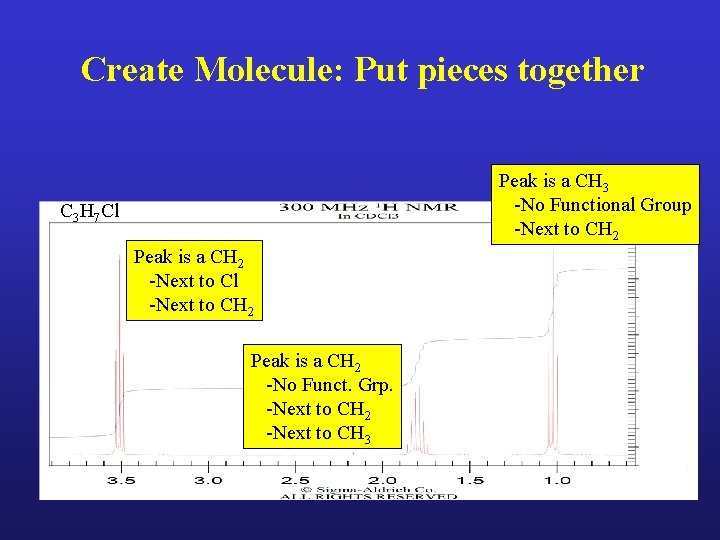
Create Molecule: Put pieces together Peak is a CH 3 -No Functional Group -Next to CH 2 C 3 H 7 Cl Peak is a CH 2 -Next to Cl -Next to CH 2 Peak is a CH 2 -No Funct. Grp. -Next to CH 2 -Next to CH 3

The NMR Mosaic can help • Reminds you to use integration AND chemical shift AND multiplicity • Helps you visualize how the pieces fit together • Helps you recognize mistakes in interpretation • Helps you interpret complex splitting

Steps for Solving 1 H NMR Spectra 1. Calculate integration for each peak a. #H = peak integration x (total # H’s / total integration) b. Smallest peak is often 1 H c. Peaks ~1 d are often 3 H (methyl) 2. Determine number of adjacent hydrogens from splitting - Number of peaks = n+1, where n = number of adjacent hydrogens - Attach appropriate static cling(s) 3. Determine presence of functional group(s) - Attach functional group static cling(s) - Choose functional group Mosaic piece(s)

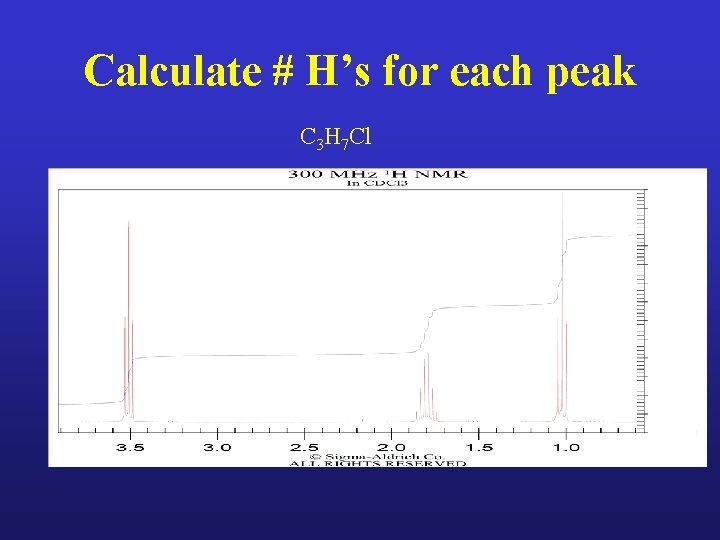
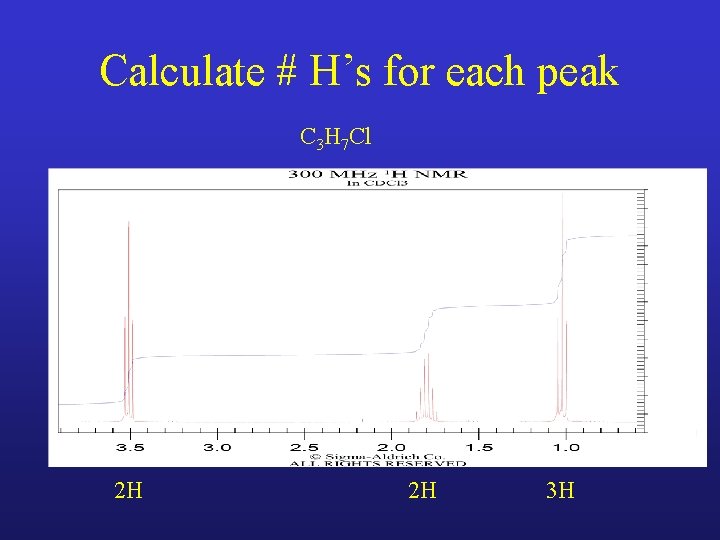
Calculate # H’s for each peak C 3 H 7 Cl

Calculate # H’s for each peak C 3 H 7 Cl 2 H 2 H 3 H

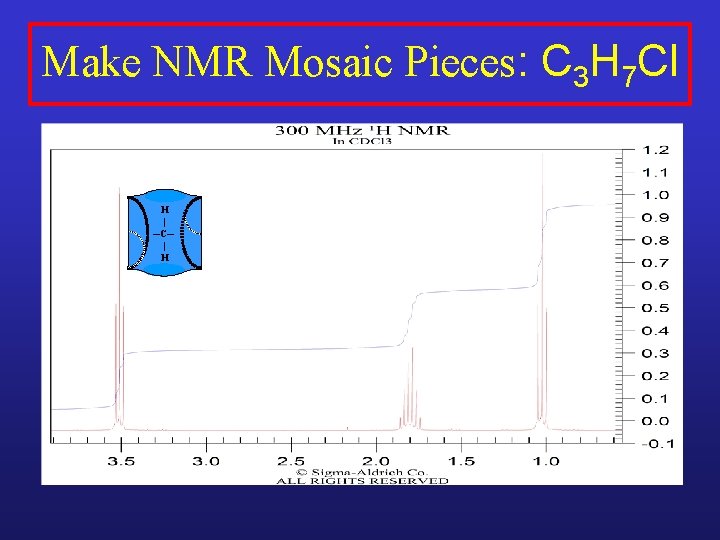
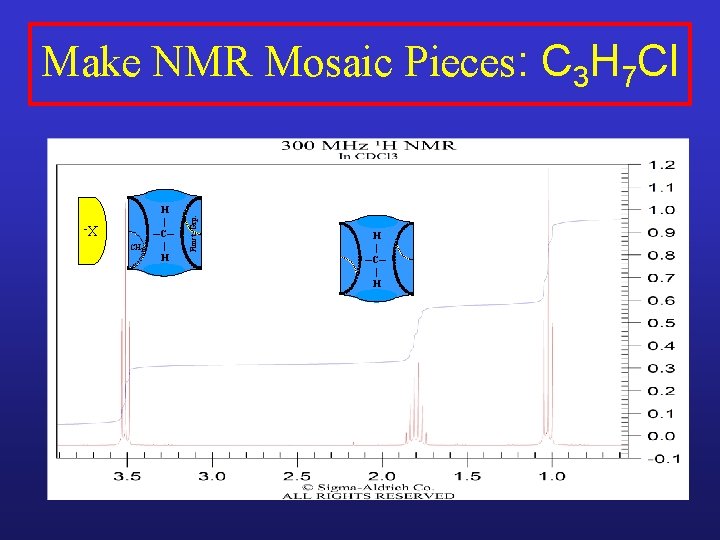
Make NMR Mosaic Pieces: C 3 H 7 Cl H | ─C─ | H

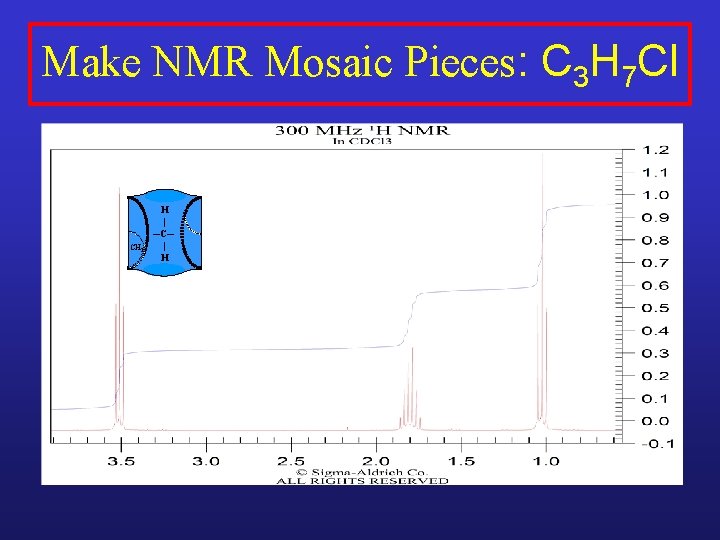
Make NMR Mosaic Pieces: C 3 H 7 Cl CH 2 H | ─C─ | H

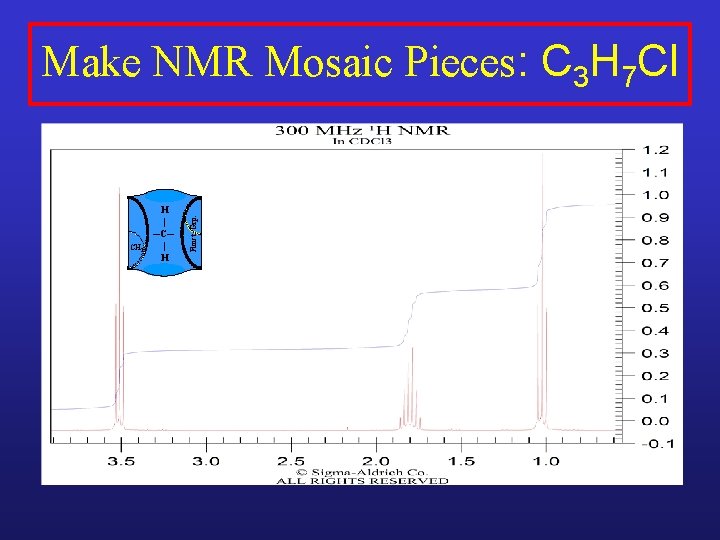
CH 2 H | ─C─ | H Funct. Grp. Make NMR Mosaic Pieces: C 3 H 7 Cl

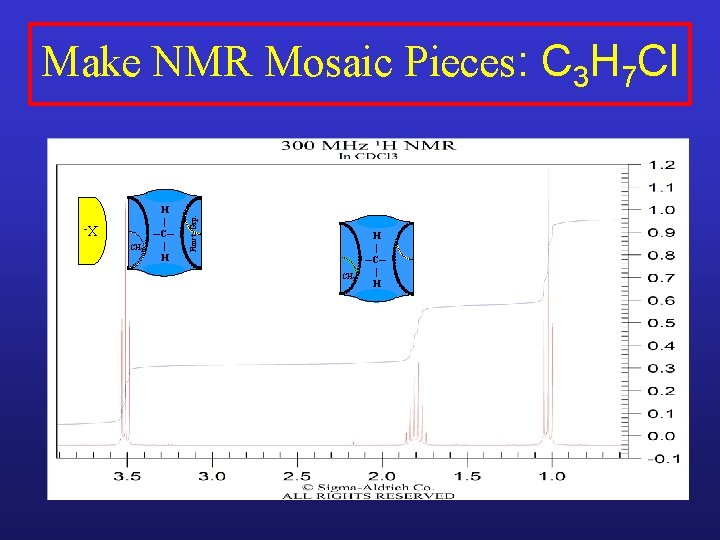
XCH 2 H | ─C─ | H Funct. Grp. Make NMR Mosaic Pieces: C 3 H 7 Cl

XCH 2 H | ─C─ | H Funct. Grp. Make NMR Mosaic Pieces: C 3 H 7 Cl H | ─C─ | H

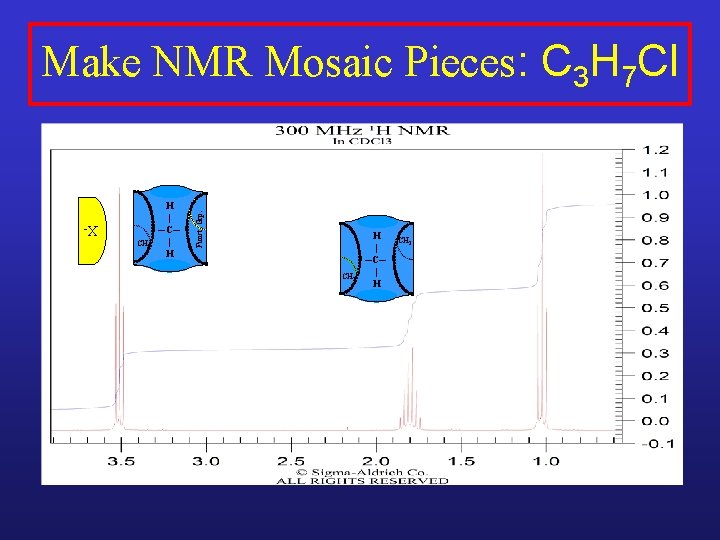
XCH 2 H | ─C─ | H Funct. Grp. Make NMR Mosaic Pieces: C 3 H 7 Cl CH 3 H | ─C─ | H

XCH 2 H | ─C─ | H Funct. Grp. Make NMR Mosaic Pieces: C 3 H 7 Cl CH 3 H | ─C─ | H CH 2

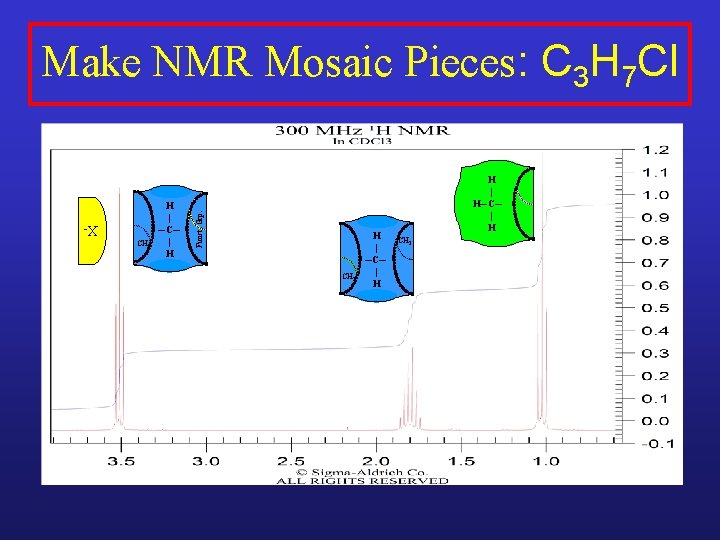
XCH 2 H | ─C─ | H Funct. Grp. Make NMR Mosaic Pieces: C 3 H 7 Cl CH 3 H | ─C─ | H H | H─C─ | H CH 2

XCH 2 H | ─C─ | H Funct. Grp. Make NMR Mosaic Pieces: C 3 H 7 Cl CH 3 H | ─C─ | H H | H─C─ | H CH 2

Fit pieces together Great! Errors. Error.

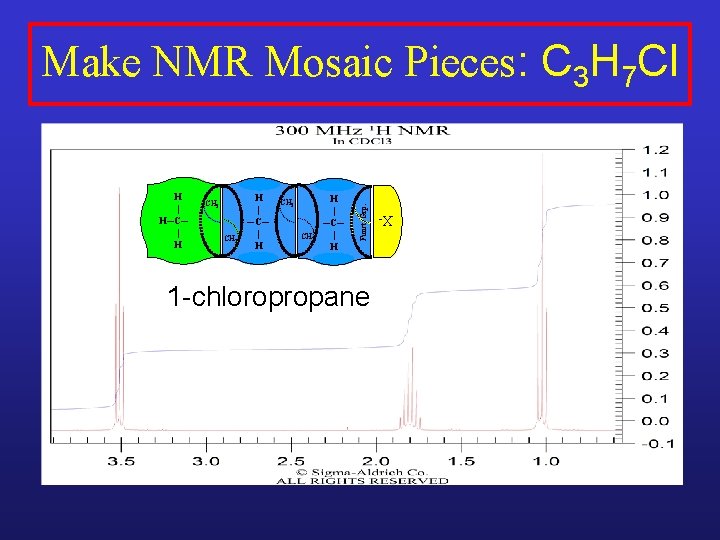
CH 2 CH 3 H | ─C─ | H CH 2 H | ─C─ | H 1 -chloropropane X- H | H─C─ | H Funct. Grp. Make NMR Mosaic Pieces: C 3 H 7 Cl

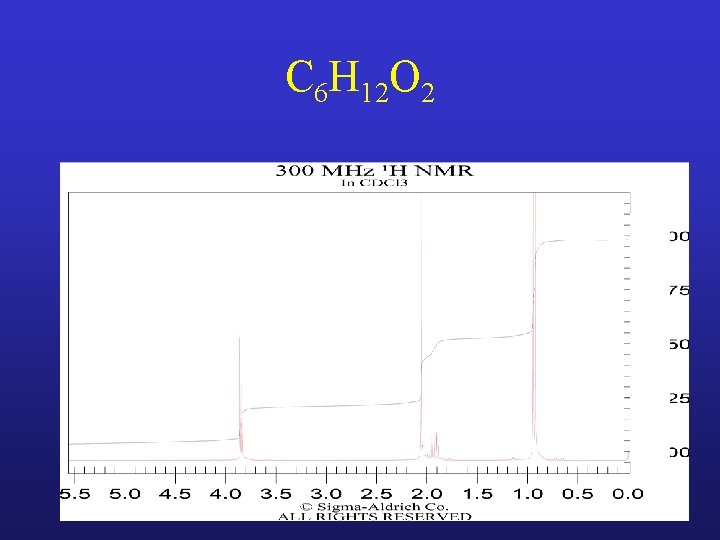
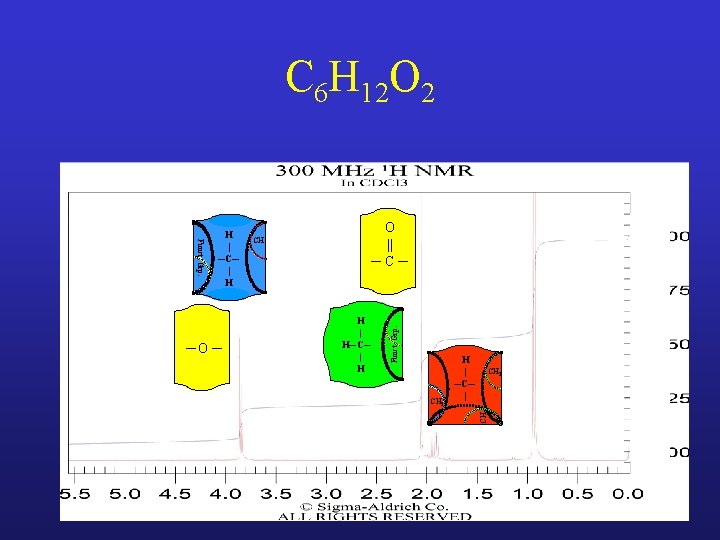
C 6 H 12 O 2

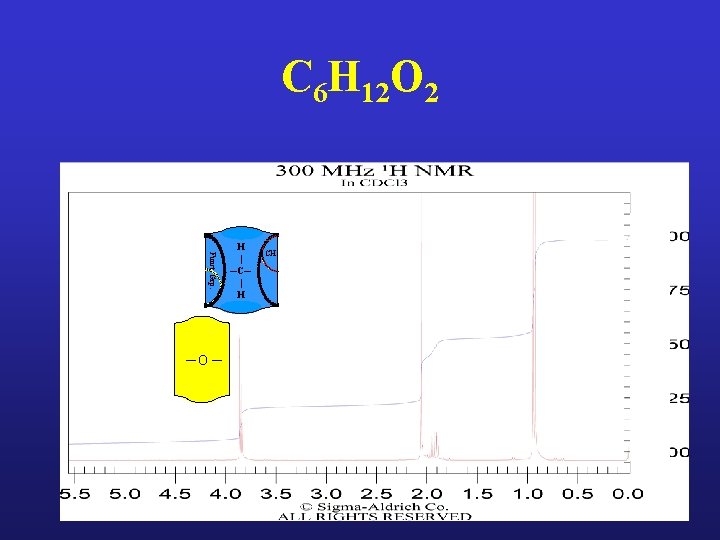
C 6 H 12 O 2 Funct. Grp. ─O ─ H | ─C─ | H CH

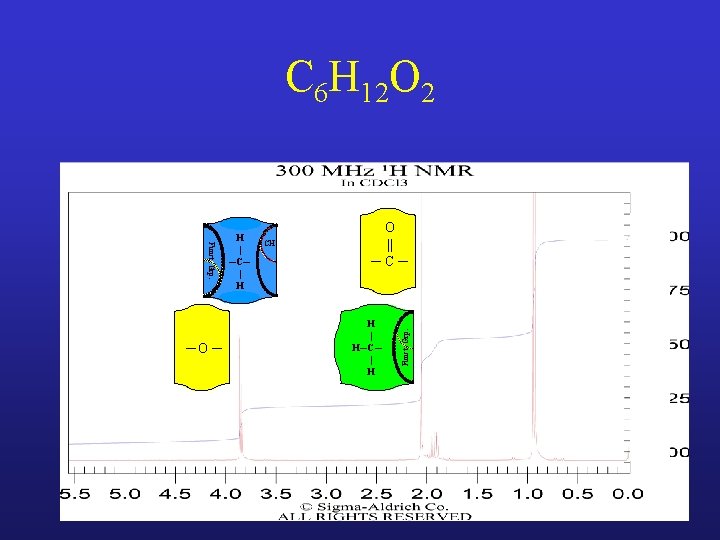
C 6 H 12 O 2 CH O || ─C─ H | H─C─ | H Funct. Grp. ─O ─ H | ─C─ | H

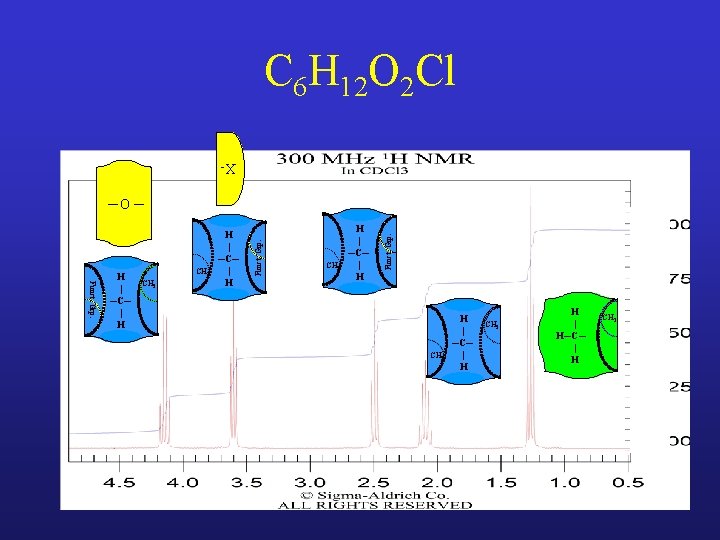
C 6 H 12 O 2 H | H─C─ | H CH 2 H | ─C─ | CH 3 ─O ─ O || ─C─ CH Funct. Grp. H | ─C─ | H

C 6 H 12 O 2 H | H─C─ | H CH 2 H | ─C─ | CH 3 ─O ─ O || ─C─ CH Funct. Grp. H | ─C─ | H H | H─C─ | H CH CH

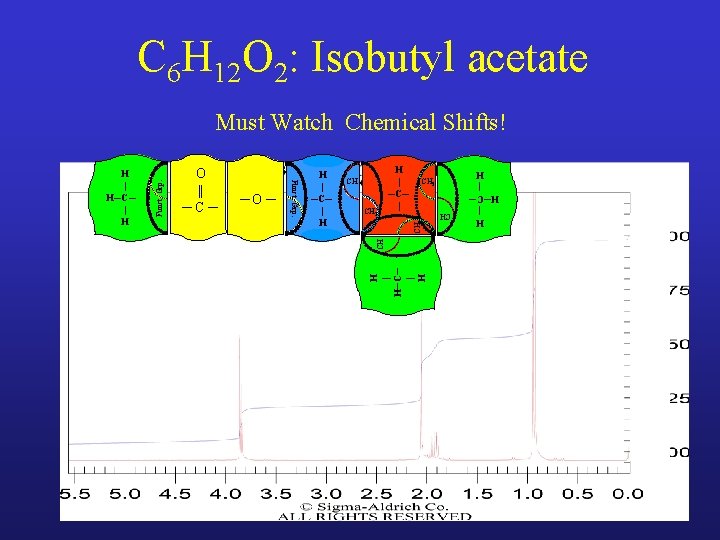
C 6 H 12 O 2: Isobutyl acetate CH CH 3 H | H─C─ | H CH CH 3 CH 2 H | ─C─ | CH ─O ─ H | ─C─ | H H | H─C─ | H O || ─C─ Funct. Grp. H | H─C─ | H Funct. Grp. Must Watch Chemical Shifts!

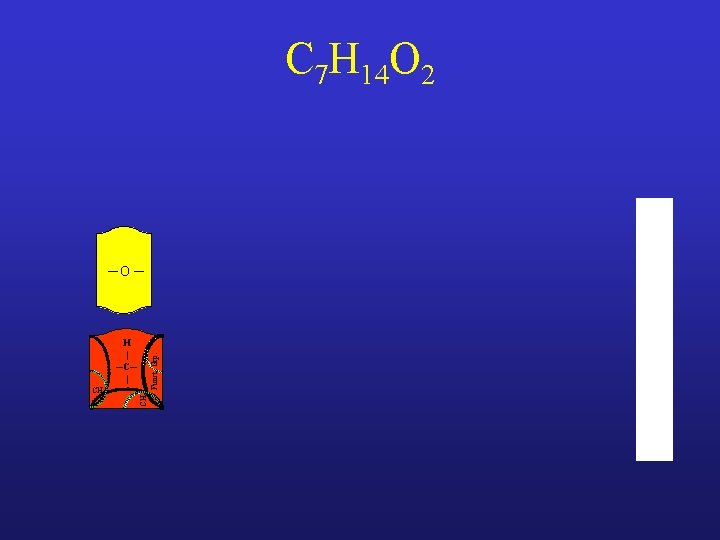
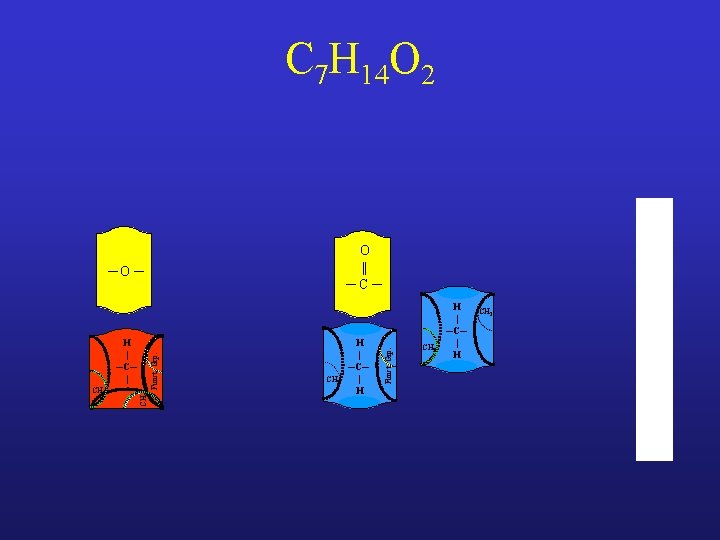
C 7 H 14 O 2

C 7 H 14 O 2 ─O ─ CH 3 Funct. Grp. H | ─C─ |

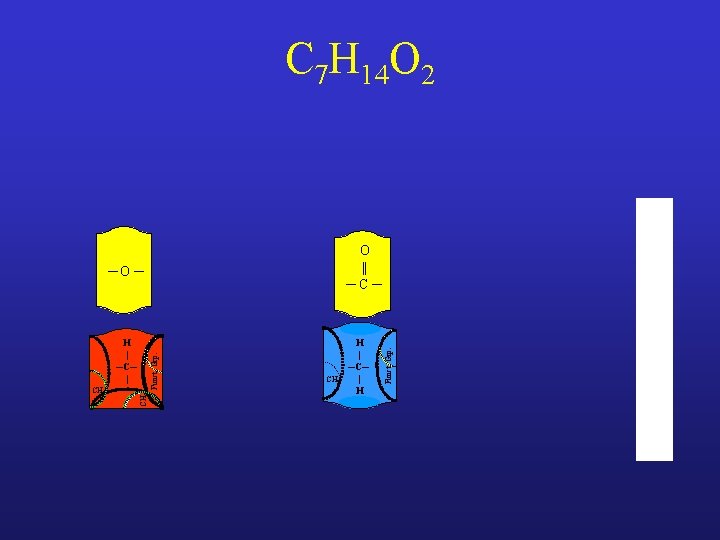
C 7 H 14 O 2 ─O ─ CH 3 Funct. Grp. H | ─C─ | CH 2 H | ─C─ | H Funct. Grp. O || ─C─

C 7 H 14 O 2 ─O ─ CH 3 Funct. Grp. H | ─C─ | CH 2 H | ─C─ | H Funct. Grp. O || ─C─ CH 3 H | ─C─ | H CH 2

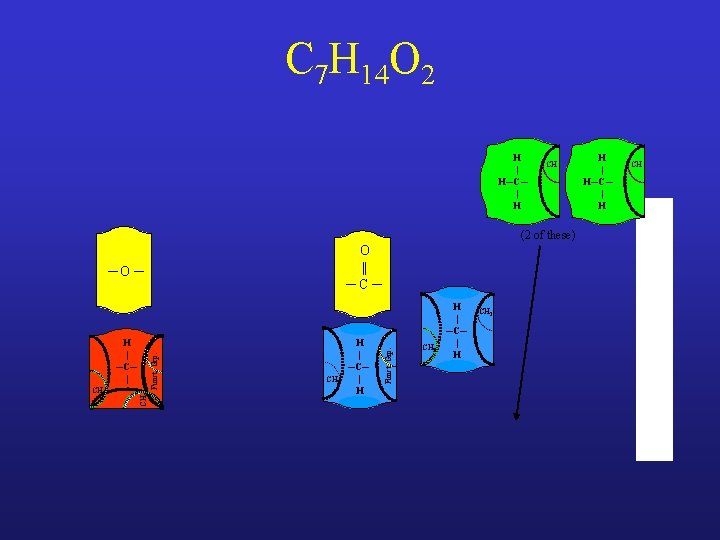
C 7 H 14 O 2 H | H─C─ | H CH (2 of these) ─O ─ CH 3 Funct. Grp. H | ─C─ | CH 2 H | ─C─ | H Funct. Grp. O || ─C─ CH 3 H | ─C─ | H CH 2 H | H─C─ | H CH

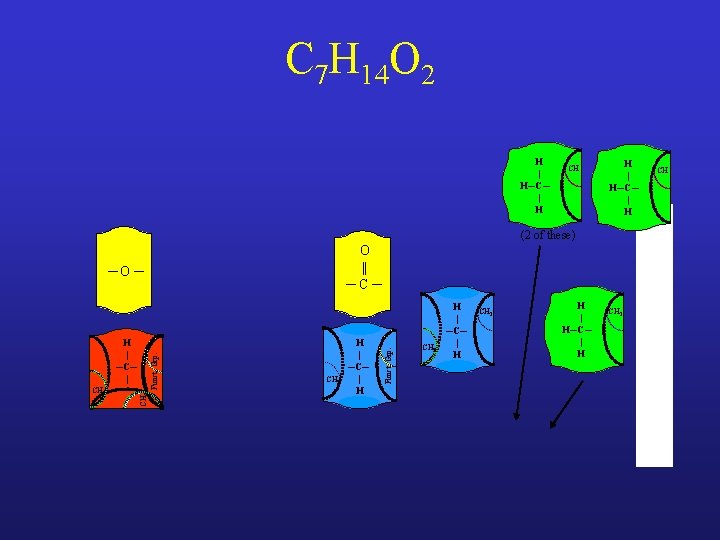
C 7 H 14 O 2 H | H─C─ | H CH H | H─C─ | H (2 of these) ─O ─ CH 3 Funct. Grp. H | ─C─ | CH 2 H | ─C─ | H Funct. Grp. O || ─C─ CH 3 H | ─C─ | H CH 2 H | H─C─ | H CH 2 CH

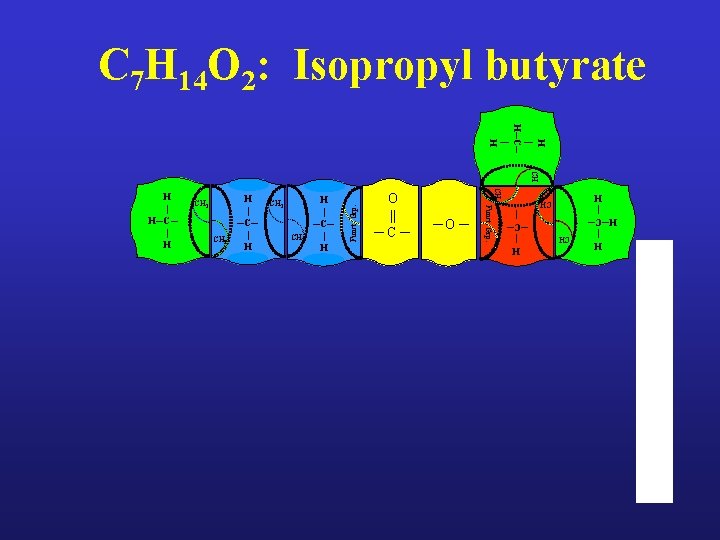
C 7 H 14 O 2: Isopropyl butyrate H | H─C─ | H CH ─O ─ H | ─C─ | O || ─C─ CH 3 CH 2 H | ─C─ | H H | H─C─ | H CH 2 Funct. Grp. CH CH 3 H | ─C─ | H Funct. Grp. CH 2 CH 3 H | H─C─ | H

C 6 H 11 O 2 Cl

C 6 H 12 O 2 Cl ─O ─ Funct. Grp. H | ─C─ | H CH 3

C 6 H 12 O 2 Cl XFunct. Grp. H | ─C─ | H CH 2 CH 3 H | ─C─ | H Funct. Grp. ─O ─

C 6 H 12 O 2 Cl XFunct. Grp. H | ─C─ | H CH 3 CH 2 H | ─C─ | H Funct. Grp. ─O ─

C 6 H 12 O 2 Cl XFunct. Grp. H | ─C─ | H CH 3 CH 2 H | ─C─ | H Funct. Grp. ─O ─ CH 2 H | ─C─ | H CH 2

C 6 H 12 O 2 Cl XFunct. Grp. H | ─C─ | H CH 3 CH 2 H | ─C─ | H Funct. Grp. ─O ─ CH 2 H | ─C─ | H CH 2 H | H─C─ | H CH 2

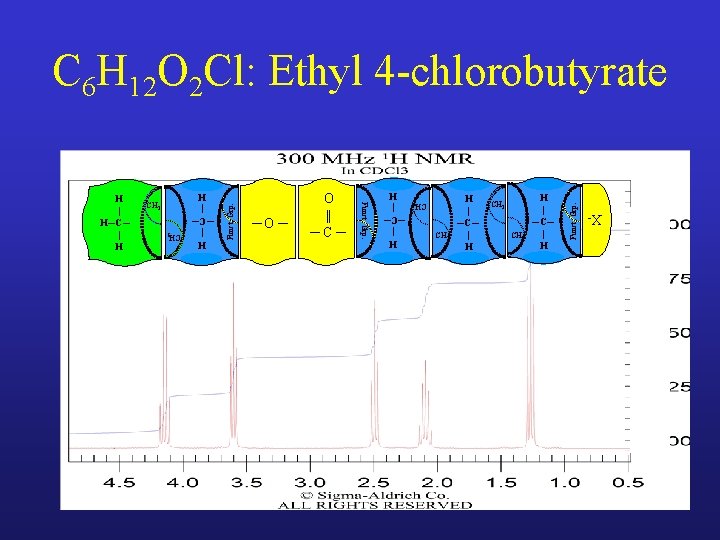
CH 2 H | ─C─ | H Funct. Grp. CH 2 H | ─C─ | H X- H | ─C─ | H ─O ─ O || ─C─ CH 2 H | ─C─ | H CH 2 Funct. Grp. CH 3 H | H─C─ | H Funct. Grp. C 6 H 12 O 2 Cl: Ethyl 4 -chlorobutyrate

NMR Mosaic Simplifies Interpretation of Complex Multiplets • Adjoining pieces specify connectivity • Interpretation is no longer “impossible”

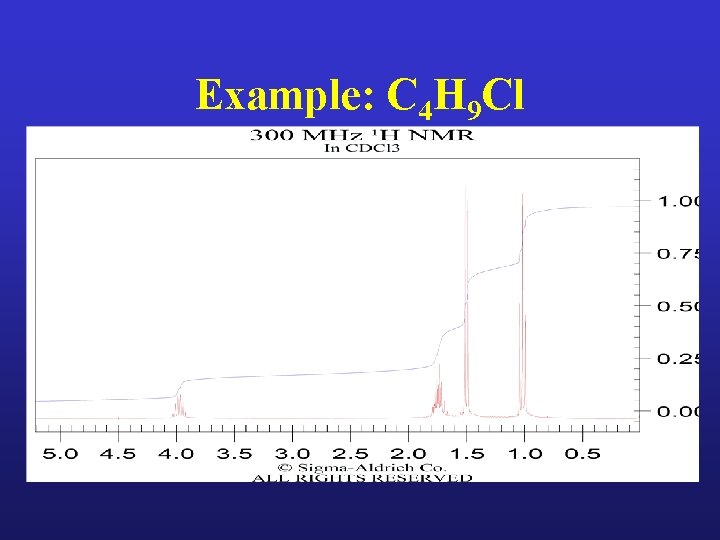
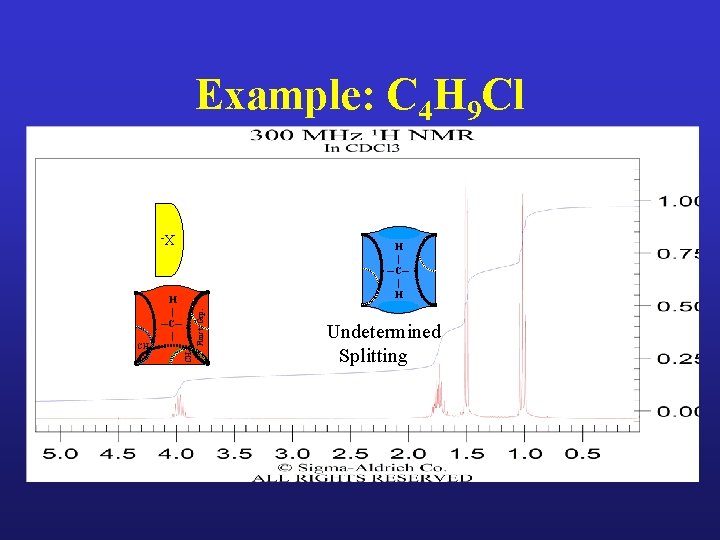
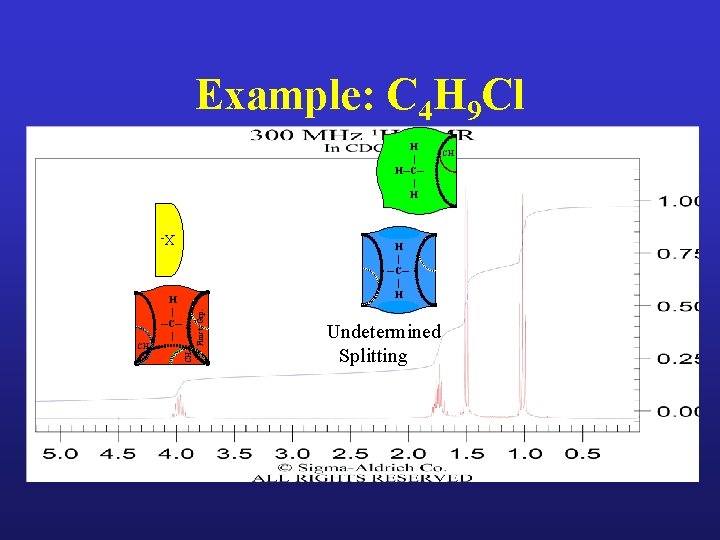
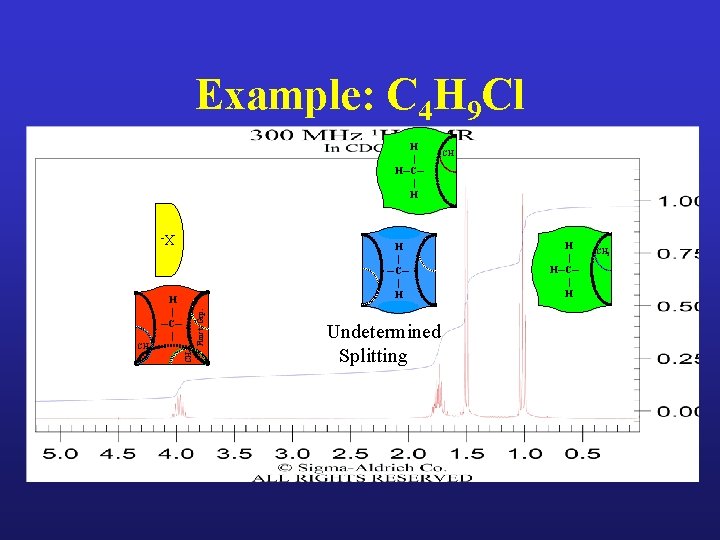
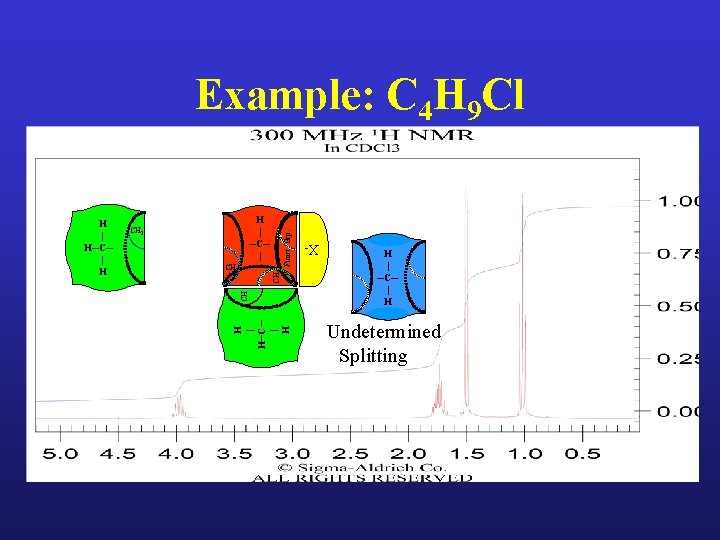
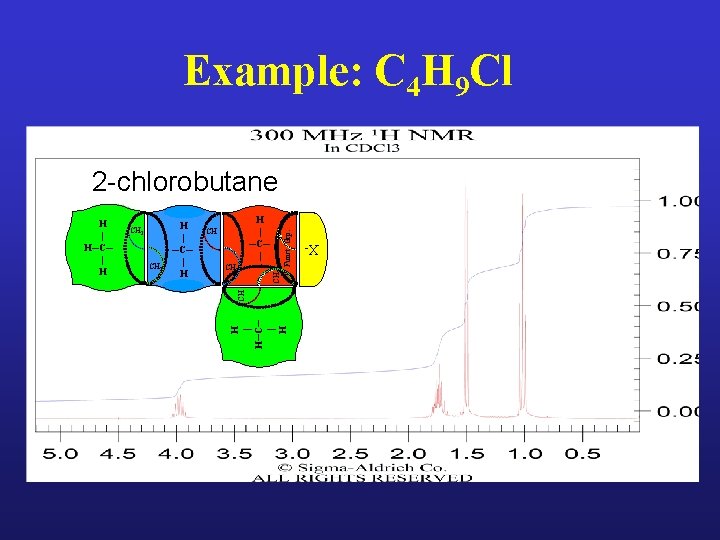
Example: C 4 H 9 Cl

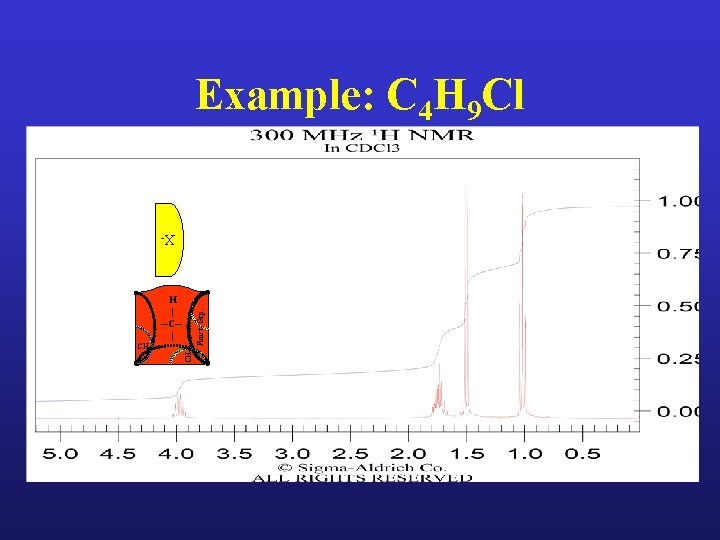
Example: C 4 H 9 Cl XCH 3 CH 2 Funct. Grp. H | ─C─ |

Example: C 4 H 9 Cl X- H | ─C─ | H CH 3 CH 2 Funct. Grp. H | ─C─ | Undetermined Splitting

Example: C 4 H 9 Cl H | H─C─ | H X- H | ─C─ | H CH 3 CH 2 Funct. Grp. H | ─C─ | Undetermined Splitting CH

Example: C 4 H 9 Cl H | H─C─ | H X- H | ─C─ | H CH 3 CH 2 Funct. Grp. H | ─C─ | Undetermined Splitting CH H | H─C─ | H CH 2

Example: C 4 H 9 Cl H | H─C─ | H CH CH 3 CH 2 Funct. Grp. H | ─C─ | CH 2 X- H | H─C─ | H H | ─C─ | H Undetermined Splitting

Example: C 4 H 9 Cl 2 -chlorobutane CH 3 CH 2 Funct. Grp. H | ─C─ | CH CH CH 3 H | ─C─ | H H | H─C─ | H CH 2 X- H | H─C─ | H

NMR Mosaic Helps You Catch Mistakes • Mosaic pieces no longer match • Focus students on incorrectly interpreted peaks

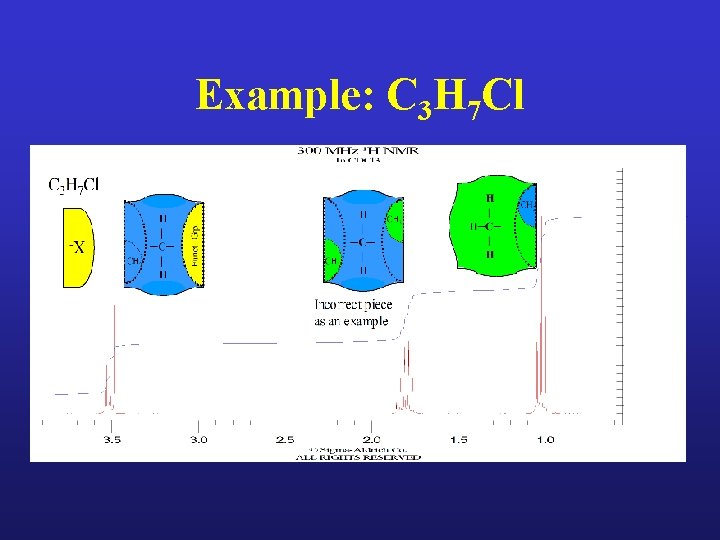
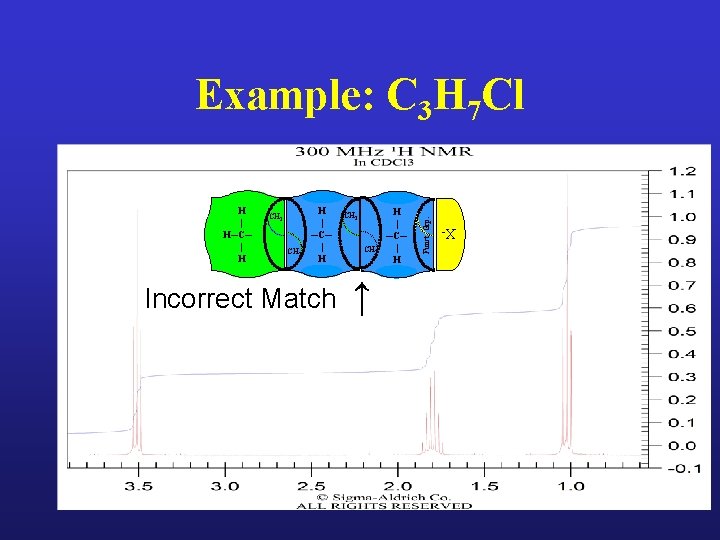
Example: C 3 H 7 Cl

CH 2 CH 3 H | ─C─ | H Incorrect Match CH 3 CH 2 ↑ H | ─C─ | H X- H | H─C─ | H Funct. Grp. Example: C 3 H 7 Cl

CH 2 CH 3 H | ─C─ | H CH 2 H | ─C─ | H Corrected Piece Matches X- H | H─C─ | H Funct. Grp. Example: C 3 H 7 Cl

Steps for Solving 1 H NMR Spectra 1. Calculate integration for each peak 2. For each peak: • Choose correct base Mosaic piece based on integration - Determine number of adjacent hydrogens from splitting and add static cling tab(s) to base piece - Determine presence of functional group(s) - Add functional group static cling(s) - Choose appropriate functional group Mosaic piece(s) 3. Put Mosaic pieces together, making sure all colors match and all functional group chemical shifts match.