1 H NMR Interpretation 1 Number of Signals
1 H NMR Interpretation 1. Number of Signals (Resonances) 2. Positions of Signals – Chemical Shift 3. Relative Intensities of Signals – Integrals 4. Splitting Patterns – Spin-Spin Coupling 5. Exchangeable Protons
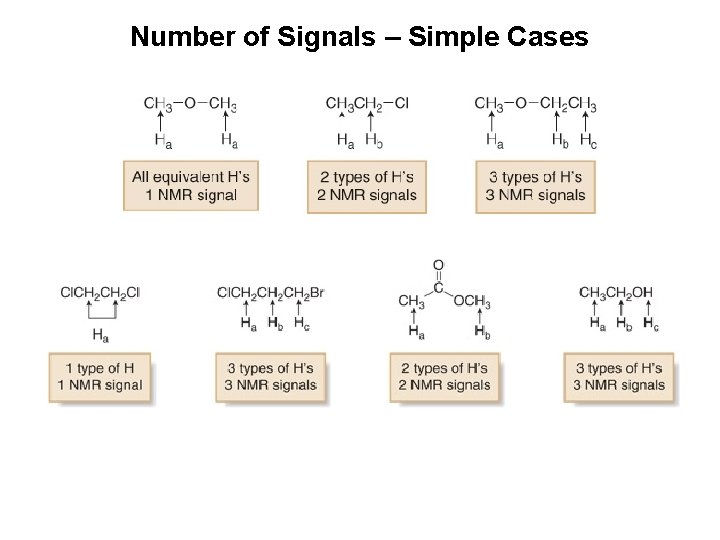
Number of Signals – Simple Cases
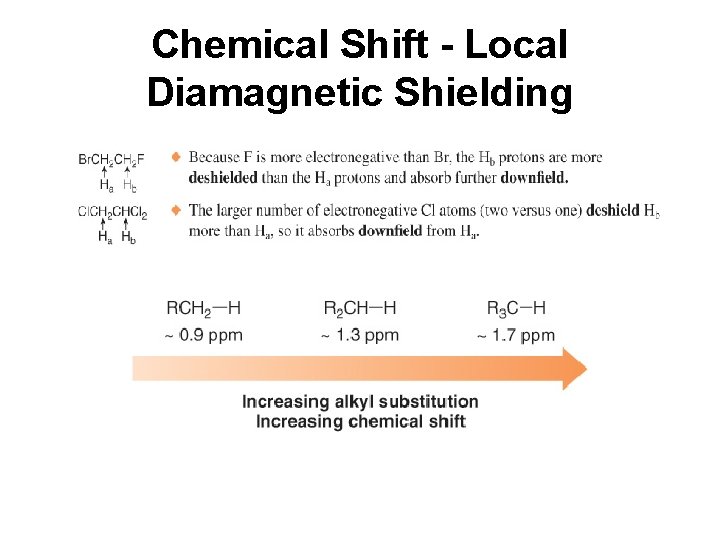
Chemical Shift - Local Diamagnetic Shielding
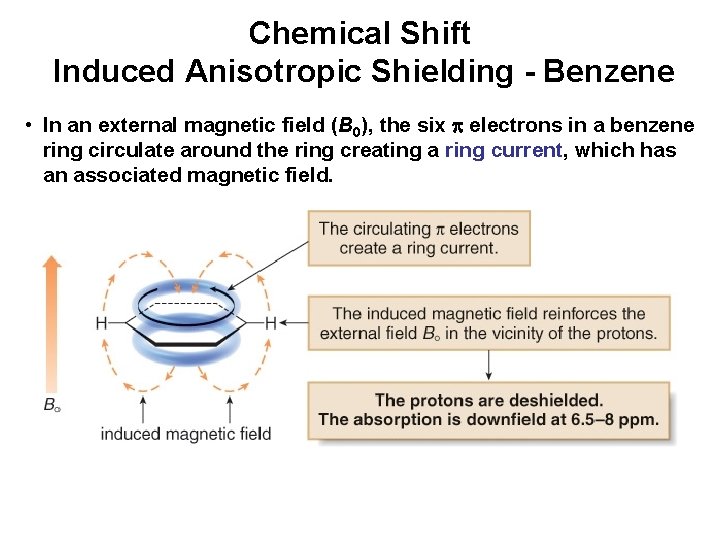
Chemical Shift Induced Anisotropic Shielding - Benzene • In an external magnetic field (B 0), the six electrons in a benzene ring circulate around the ring creating a ring current, which has an associated magnetic field.
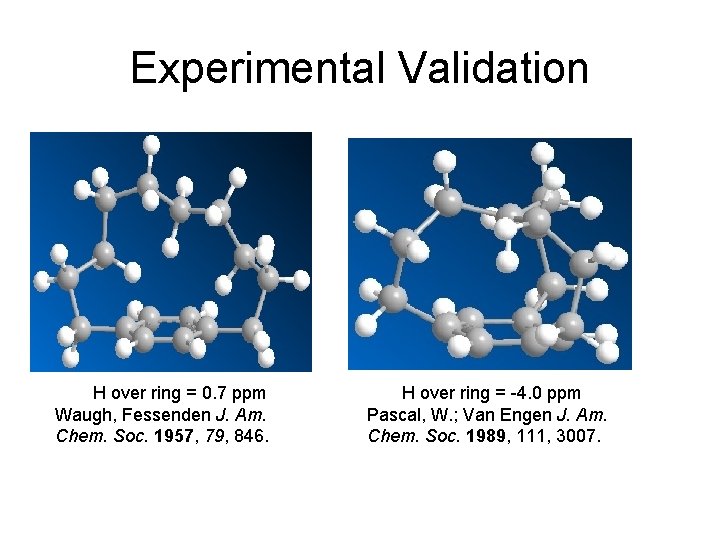
Experimental Validation H over ring = 0. 7 ppm Waugh, Fessenden J. Am. Chem. Soc. 1957, 79, 846. H over ring = -4. 0 ppm Pascal, W. ; Van Engen J. Am. Chem. Soc. 1989, 111, 3007.
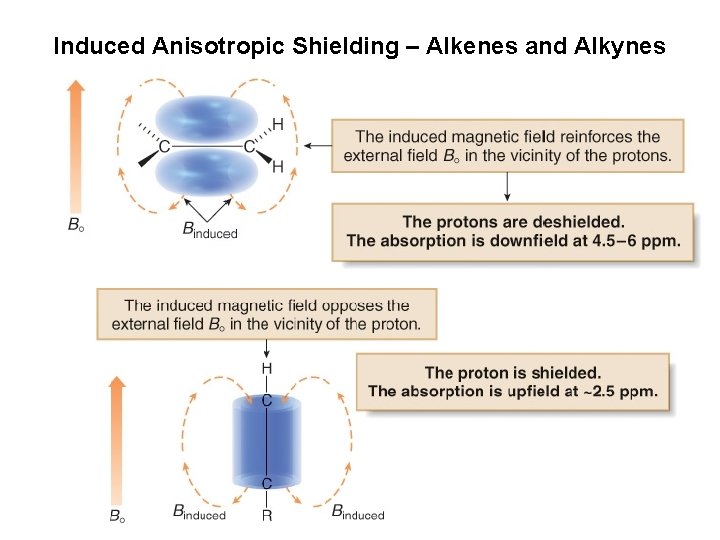
Induced Anisotropic Shielding – Alkenes and Alkynes
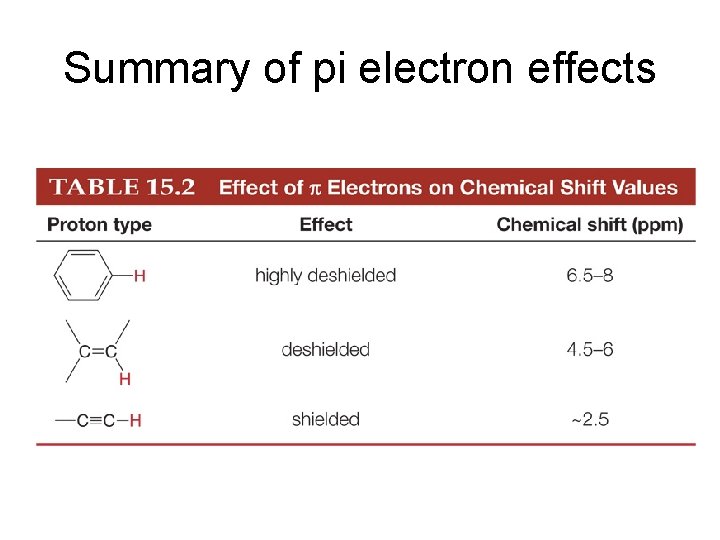
Summary of pi electron effects
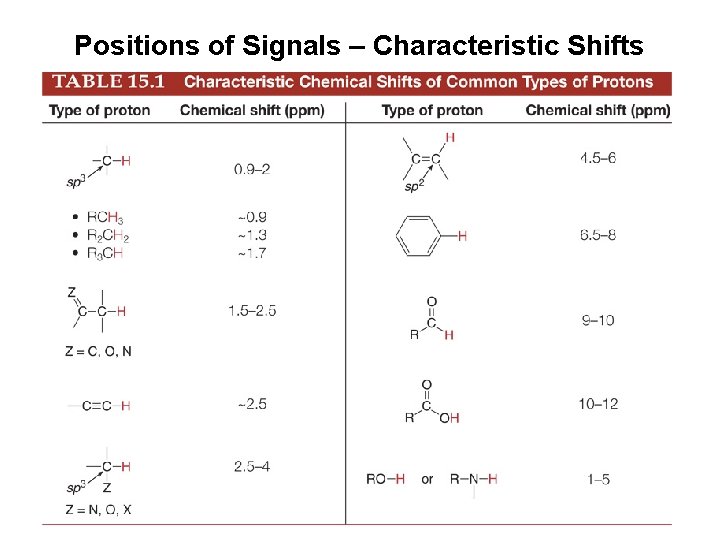
Positions of Signals – Characteristic Shifts
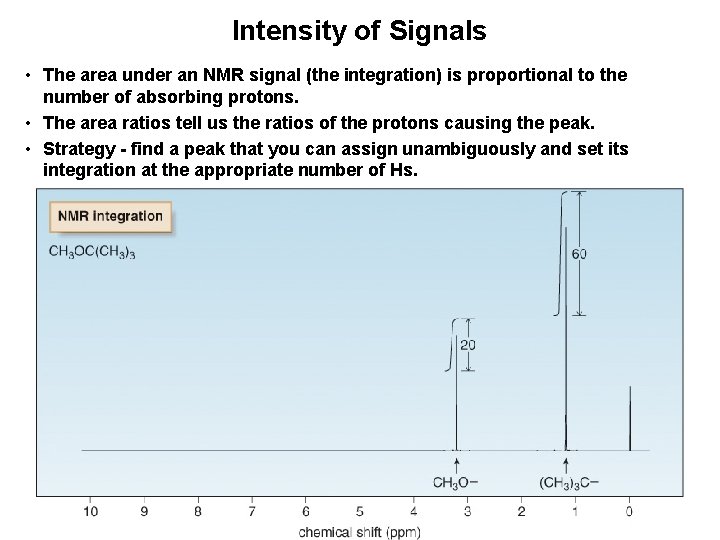
Intensity of Signals • The area under an NMR signal (the integration) is proportional to the number of absorbing protons. • The area ratios tell us the ratios of the protons causing the peak. • Strategy - find a peak that you can assign unambiguously and set its integration at the appropriate number of Hs.
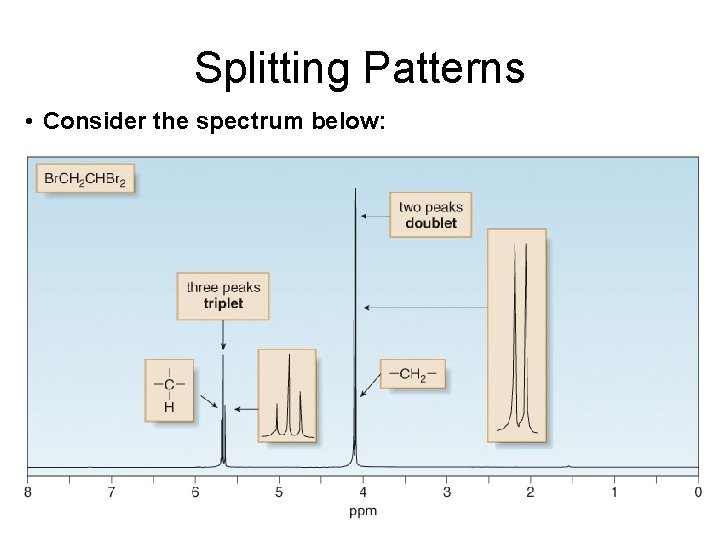
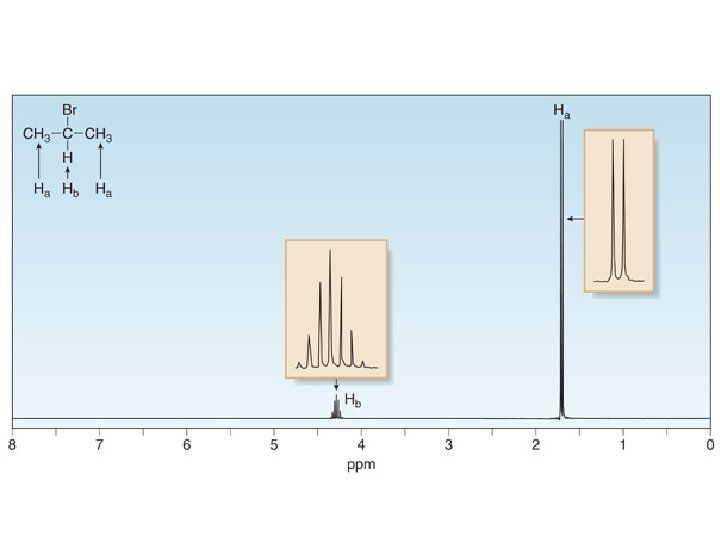
Splitting Patterns • Consider the spectrum below:

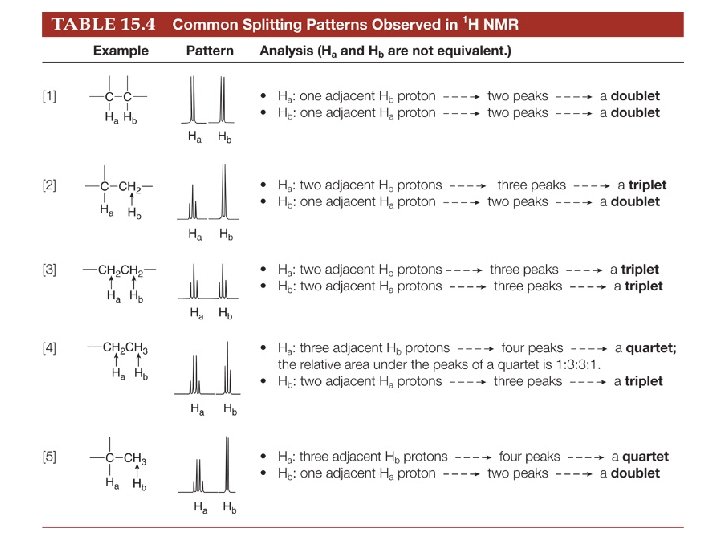
Peak ratios in a multiplet. • Doublet – The two spin states of the proton causing splitting are nearly equally populated (because the energy difference is so small). Therefore a doublet is has a peak ratio of 1: 1. • Triplet - Because there are two different ways to align one proton with B 0, and one proton against B 0—that is, a b and a b—the middle peak of the triplet is twice as intense as the two outer peaks, making the ratio of the areas under the three peaks 1: 2: 1. • Higher – use Pascals triangle
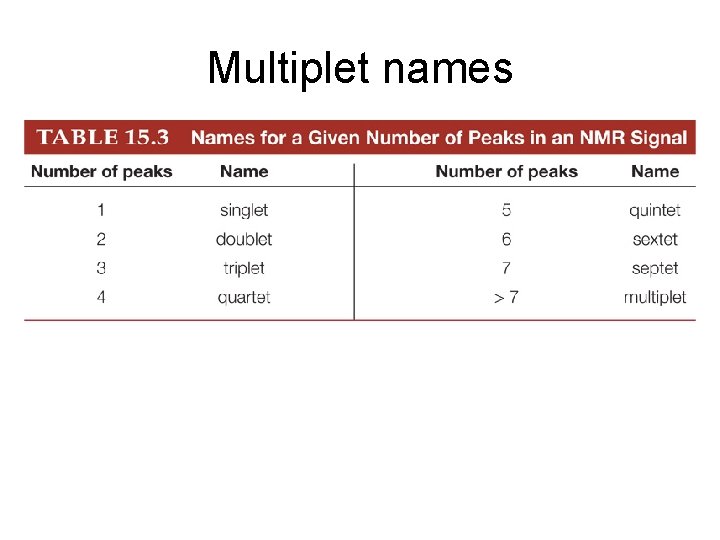
Multiplet names
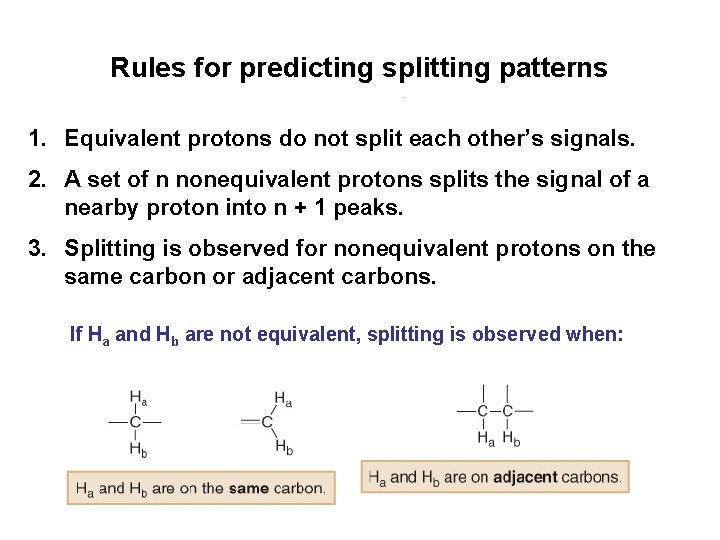
Rules for predicting splitting patterns 1. Equivalent protons do not split each other’s signals. 2. A set of n nonequivalent protons splits the signal of a nearby proton into n + 1 peaks. 3. Splitting is observed for nonequivalent protons on the same carbon or adjacent carbons. If Ha and Hb are not equivalent, splitting is observed when:
- Slides: 15