1 General Properties of Lipids Naturallyoccurring organic compounds



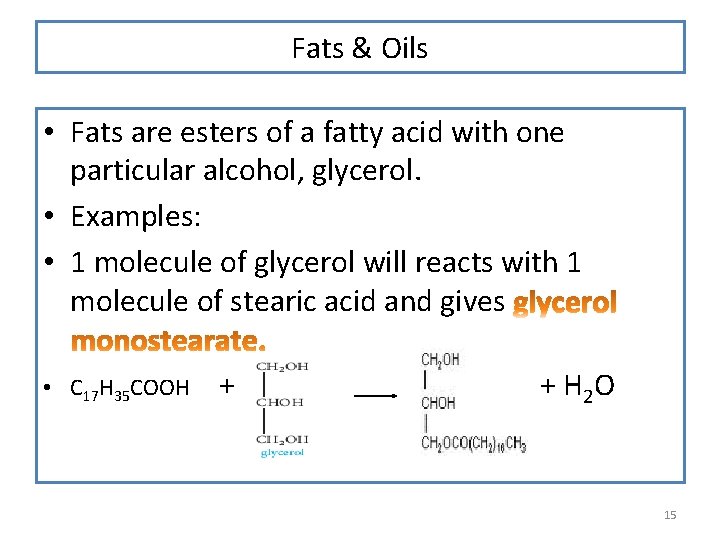








- Slides: 38

1

General Properties of Lipids • Naturally-occurring organic compounds that are: 1 - insoluble in water 2 - soluble in nonpolar organic solvents, such as diethyl ether, acetone, carbon tetrachloride. 3 - Contain carbon, hydrogen, oxygen, sometimes contain nitrogen and phosphorus 2

4 - Lipids can be extracted from cells and tissues by organic solvents. 5 - They yield fatty acids on hydrolysis or combine with fatty acids to form esters. 6 - The solubility property distinguishes lipids from the three other major classes of natural products —carbohydrates, proteins, and nucleic acids—which in general are not soluble in organic solvents. 3

Lipids include. . 1. Fats & Oils (Triglycerides) 2. Waxes: they are simple monoesters (acid & alcohol). 3. Steroids 4. Fat-soluble vitamins (A, D, E, K) 5. Monoglycerides 6. Diglycerides 7. Phospholipids 4

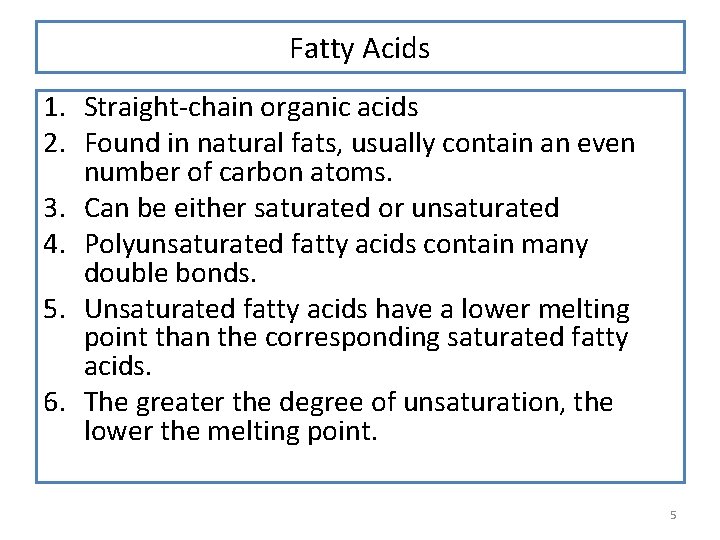
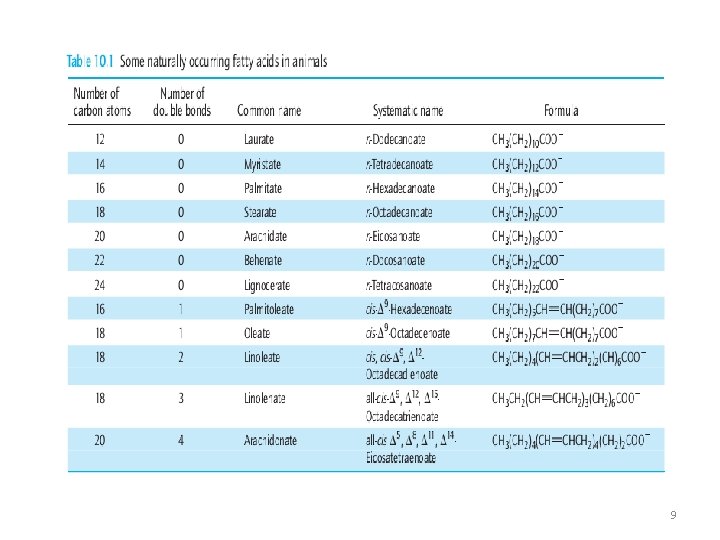
Fatty Acids 1. Straight-chain organic acids 2. Found in natural fats, usually contain an even number of carbon atoms. 3. Can be either saturated or unsaturated 4. Polyunsaturated fatty acids contain many double bonds. 5. Unsaturated fatty acids have a lower melting point than the corresponding saturated fatty acids. 6. The greater the degree of unsaturation, the lower the melting point. 5

6

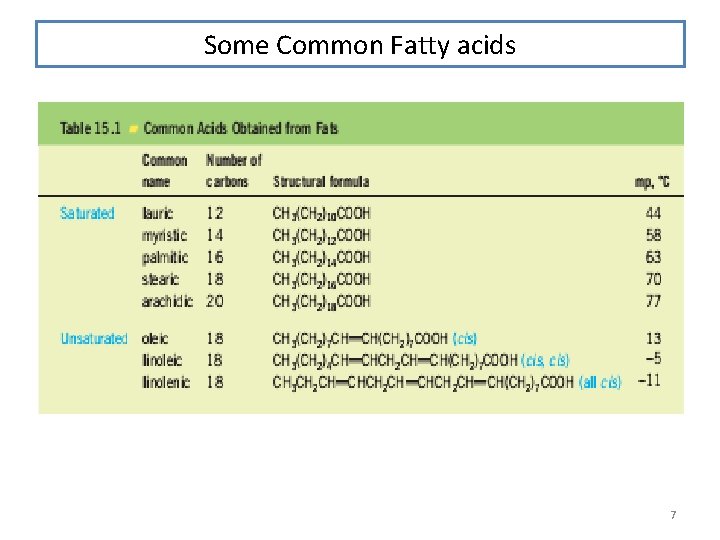
Some Common Fatty acids 7

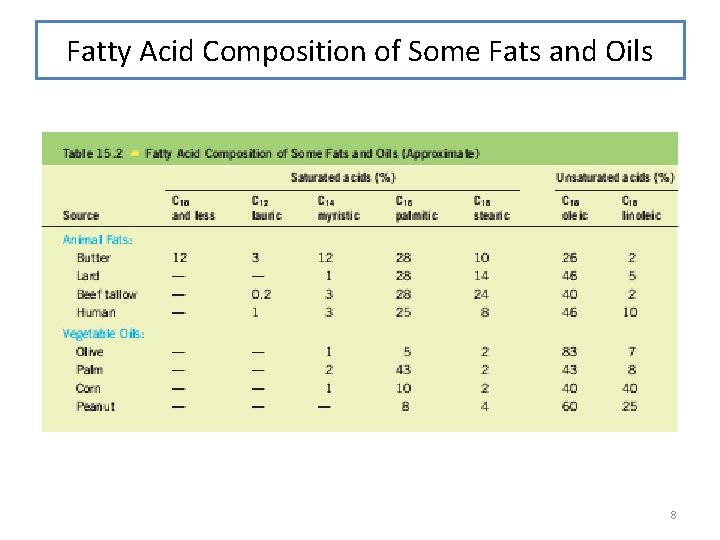
Fatty Acid Composition of Some Fats and Oils 8

9

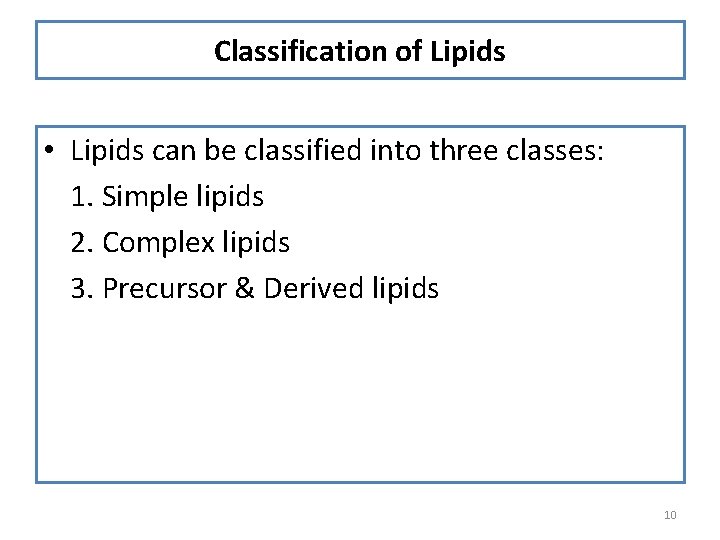
Classification of Lipids • Lipids can be classified into three classes: 1. Simple lipids 2. Complex lipids 3. Precursor & Derived lipids 10

1. Simple Lipids • They are esters of fatty acids, upon hydrolysis they give fatty acids & alcohol: simple lipid hydrolysis Fatty acids + Alcohol • If the result of hydrolysis is: 1. three fatty acids & glycerol (then it is fat or oil) 2. One fatty acid and high molecular mass monohydric alcohol (then it is wax such as carnauba, bees wax) 11

2. Complex Lipids • Are lipids that give one or more fatty acids, an alcohol, and some other type of compounds. Complex lipids hydrolysis fatty acid + alcohol + other compounds • Some examples of complex lipids: 1. Phospholipids 2. Glycolipids (cerebrosides) 3. Sulfolipids 4. Aminolipids 5. Lipoproteins 12

for example will give fatty acids, alcohol, phosphoric acid, and a nitrogenous compound upon hydrolysis • They are subdivided into 2 groups: - Phosphoglycerides (alcohol is glycerol) - Phosphingosides (alcohol is sphingosine which is a nitrogen-containing alcohol) sphingosine (glycosphingolipids) give fatty acids, carbohydrate, and a sphingosine upon hydrolysis. 13

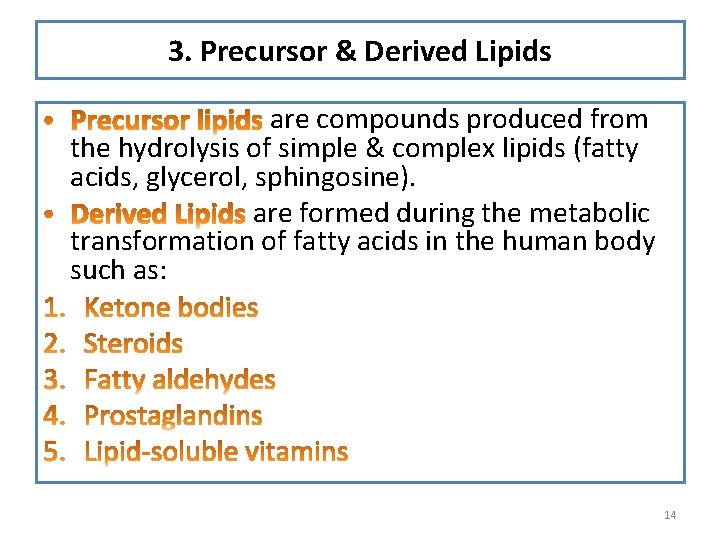
3. Precursor & Derived Lipids are compounds produced from the hydrolysis of simple & complex lipids (fatty acids, glycerol, sphingosine). are formed during the metabolic transformation of fatty acids in the human body such as: 14
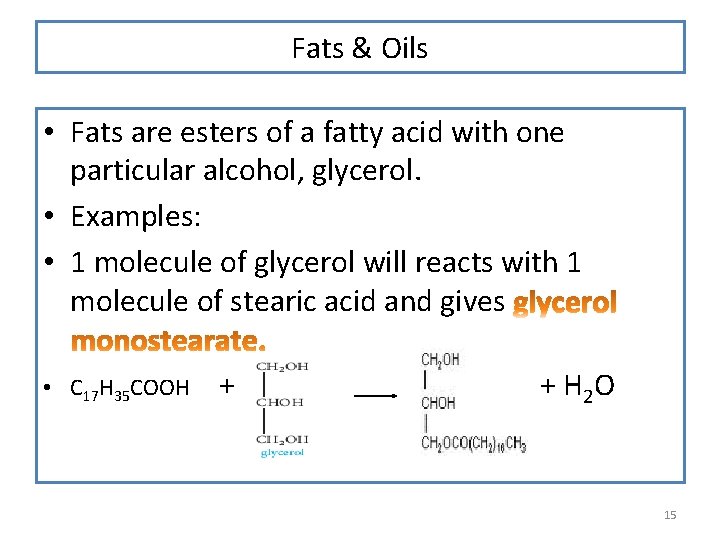
Fats & Oils • Fats are esters of a fatty acid with one particular alcohol, glycerol. • Examples: • 1 molecule of glycerol will reacts with 1 molecule of stearic acid and gives • C 17 H 35 COOH + + + H 2 O 15

• Reacting with a second molecule of stearic acid, glycerol will give • While reacting with a third molecule of stearic acid, glycerol will give (also known as ). • Note: Fats & oils can contain same three fatty acids or different ones which can be saturated, unsaturated or some combination of these. 16

Iodine Number • The number of grams of iodine that will react with double bonds present in a 100 g of fat or oil. • Unsaturated fats & oils will react with iodine, while saturated fats & oils will not react with iodine readily. • The more unsaturated the fat or oil is, the more iodine it will combine • Conclusion: have a lower iodine number (more saturated) have a higher iodine number (more unsaturated). 17

• Fats have iodine number below 70, while oils have iodine number above 70. oils should not be confused with mineral oils, which is a mixture of saturated hydrocarbons. : are volatile aromatic liquids used as flavors and perfumes. 18

Used of Fats in the body , producing more energy/g than either carbohydrate or protein produce. , are produced from protein or carbohydrate are produced by fats. than carbohydrate. in storing energy in the body 19

for the organs (by surrounding them for stabilizing). helping to keep body warm in cold weather. which are found in cell membranes, and in mitochondria. 20

Physical Properties of Fats & Oils • Pure fats are white, solid • Pure oils are yellow, liquid. • Odorless & tasteless, but over a period of time they become rancid. • Insoluble in water • Soluble in organic solvents such as benzene, acetone, ether. • Fats do not diffuse through a membrane. • Fats have a greasy feeling. 21

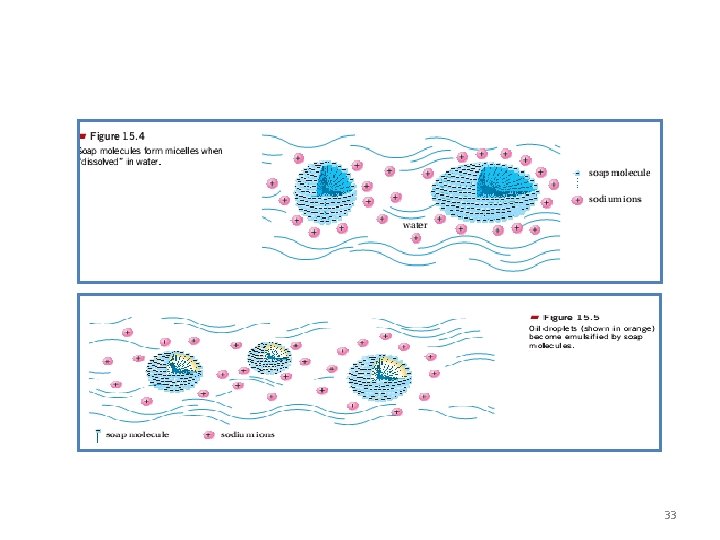
• Fats form a when shaken with water. - The emulsion can be made permanent by the addition of an such as soap. - Fats & oils must be emulsified by the bile before they can be digested. 22

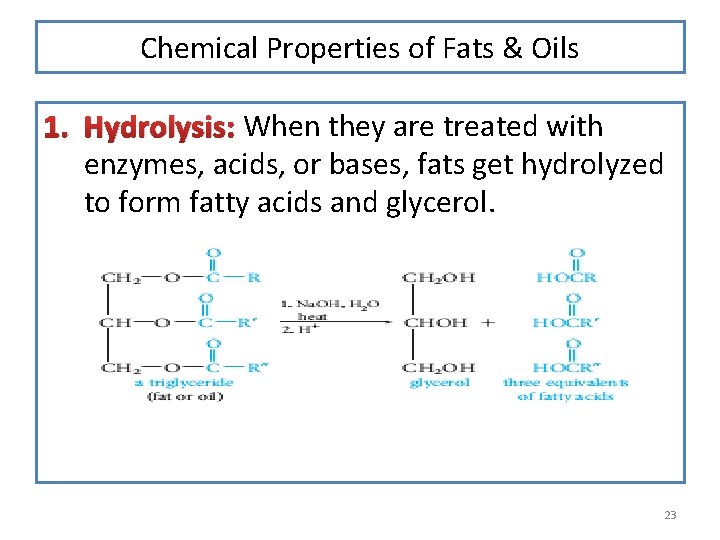
Chemical Properties of Fats & Oils 1. Hydrolysis: When they are treated with enzymes, acids, or bases, fats get hydrolyzed to form fatty acids and glycerol. 23

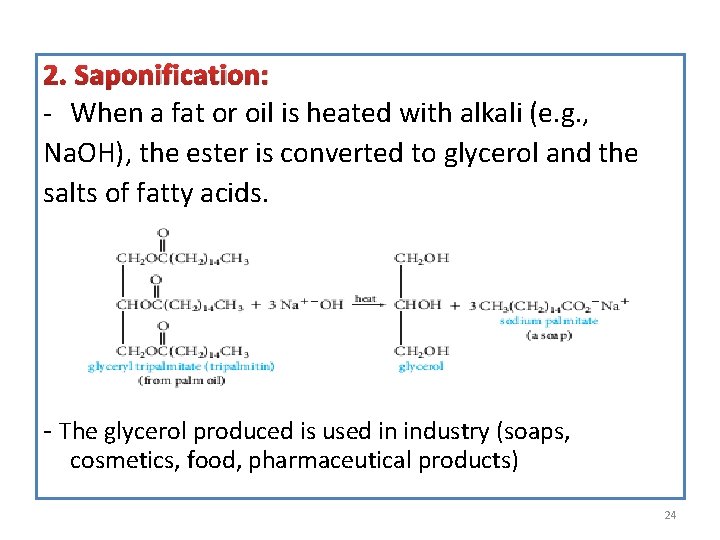
2. Saponification: - When a fat or oil is heated with alkali (e. g. , Na. OH), the ester is converted to glycerol and the salts of fatty acids. - The glycerol produced is used in industry (soaps, cosmetics, food, pharmaceutical products) 24

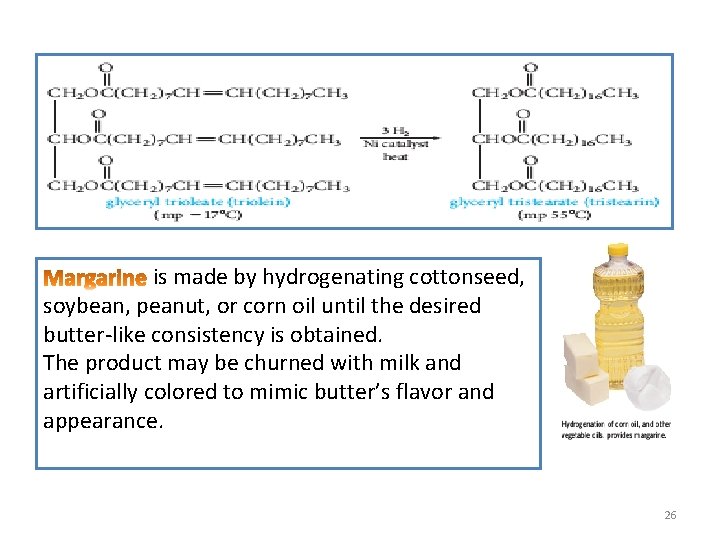
3. Hydrogenation of vegetable oils: • Vegetable oils, which are highly unsaturated, are converted into solid vegetable fats, by catalytically hydrogenating some or all of the double bonds. • This process, called , is illustrated by the hydrogenation of glyceryl trioleate to glyceryl tristearate. 25

is made by hydrogenating cottonseed, soybean, peanut, or corn oil until the desired butter-like consistency is obtained. The product may be churned with milk and artificially colored to mimic butter’s flavor and appearance. 26

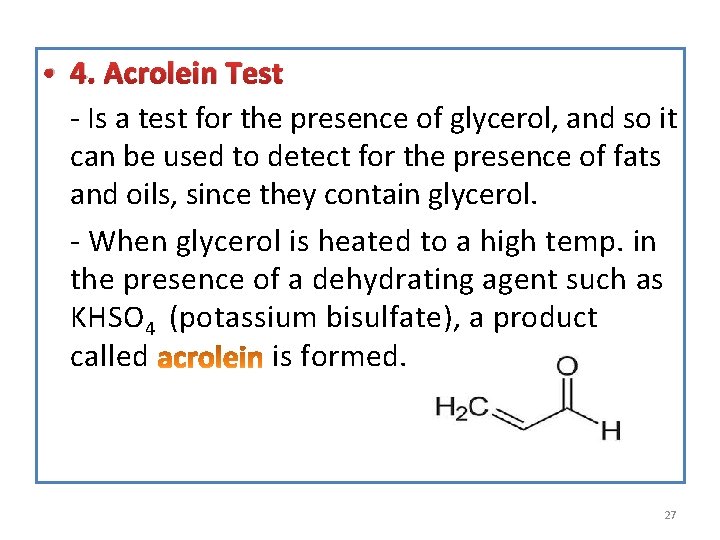
• 4. Acrolein Test - Is a test for the presence of glycerol, and so it can be used to detect for the presence of fats and oils, since they contain glycerol. - When glycerol is heated to a high temp. in the presence of a dehydrating agent such as KHSO 4 (potassium bisulfate), a product called is formed. 27

5. Rancidity Fats develop an unpleasant odor and taste when allowed to stand at room temp. for a short time, so they become rancid. Rancidity is due to 2 types of reactions: 1. Hydrolysis 2. Oxidation 28

Hydrolysis: Ø When butter is let to stand at room temp. hydrolysis takes place between the fats and the water present in the butter. Ø The result of this hydrolysis is fatty acid and glycerol. Ø One of the products of the hydrolysis is butyric acid, responsible for the disagreeable odor. Ø Note: to avoid rancidity keep the butter covered in a cool place. 29

Oxidation: Ø Oxygen present in air oxidizes some unsaturated fatty acids of fats & oils. Ø If this oxidation produces a short chain acids or aldehydes, the fat turns rancid (as evidenced by a disagreeable odor and taste). Ø Oxidation of fats can be inhibited by the addition of antioxidants such as vitamin C & E. 30

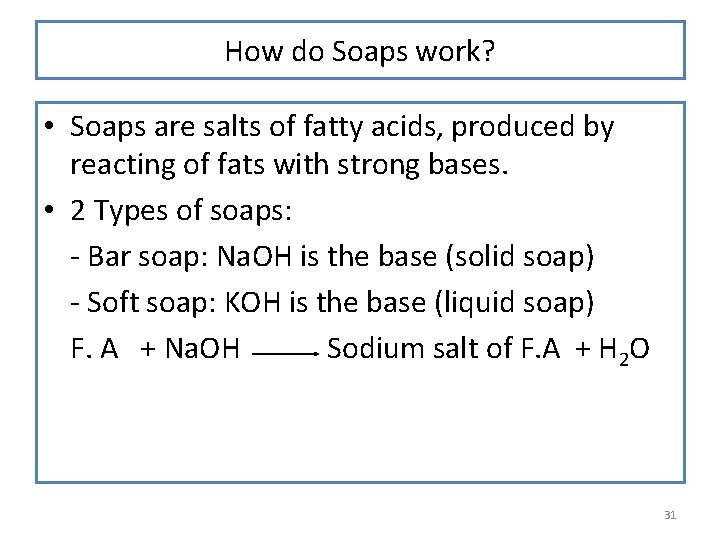
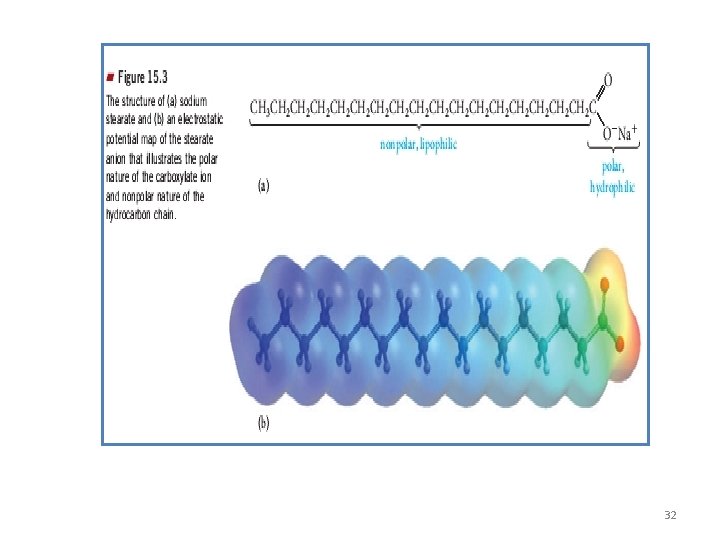
How do Soaps work? • Soaps are salts of fatty acids, produced by reacting of fats with strong bases. • 2 Types of soaps: - Bar soap: Na. OH is the base (solid soap) - Soft soap: KOH is the base (liquid soap) F. A + Na. OH Sodium salt of F. A + H 2 O 31

32

33

Prostaglandins, Leukotrienes, and Lipoxins • Prostaglandins are a group of compounds related to the unsaturated fatty acids. • On the assumption that these substances came from the prostate gland, they were named prostaglandins. • We now know that prostaglandins are widely • distributed in almost all human tissues, that they are biologically active in minute concentration, and that they have various effects on: - fat metabolism, - heart rate, - blood pressure. 34

• Prostaglandins have excited much interest in the medical community, where they are used in the treatment of: - inflammatory diseases, such as asthma and rheumatoid arthritis; - treatment of peptic ulcers; - control of hypertension; - regulation of blood pressure - metabolism; - inducing of labor - therapeutic abortions. 35

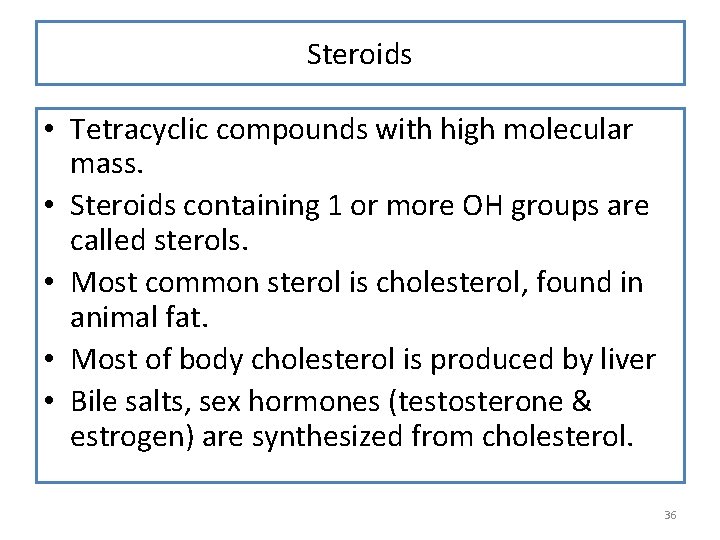
Steroids • Tetracyclic compounds with high molecular mass. • Steroids containing 1 or more OH groups are called sterols. • Most common sterol is cholesterol, found in animal fat. • Most of body cholesterol is produced by liver • Bile salts, sex hormones (testosterone & estrogen) are synthesized from cholesterol. 36

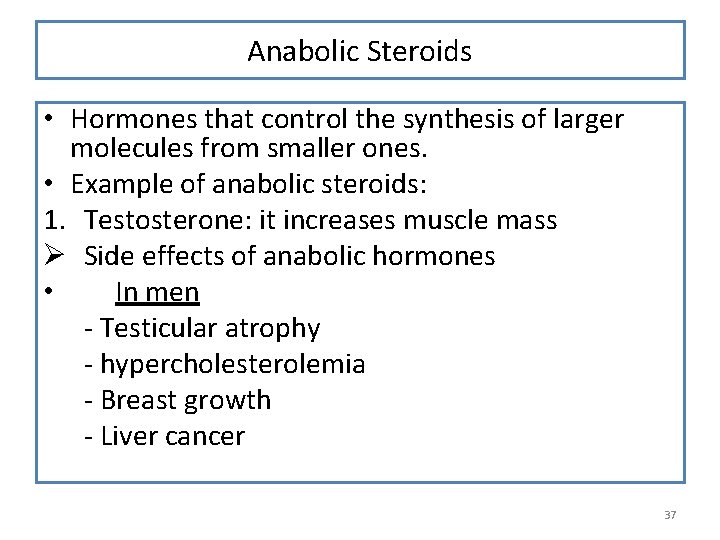
Anabolic Steroids • Hormones that control the synthesis of larger molecules from smaller ones. • Example of anabolic steroids: 1. Testosterone: it increases muscle mass Ø Side effects of anabolic hormones • In men - Testicular atrophy - hypercholesterolemia - Breast growth - Liver cancer 37

• In women - Increased masculinity - Formation of a greater body hair - Deepening of the voice - menstrual irregularities 38