1 Fast Electron Sources A Beta Decay The

1. Fast Electron Sources A. Beta Decay / 衰� The most common source of fast electron in radiation measurement is a radioisotope that decay by betaminus emission. Where X, Y are the initial and final nuclear species. is the anti-neutrino Each species beta decay transition is characterized by a fixed decay energy or Q-value (beta endpoint energy Fig. 1 -1). The beta particle energy varies from decay to decay and can be range from zero to Q-value, see table 1 -1.
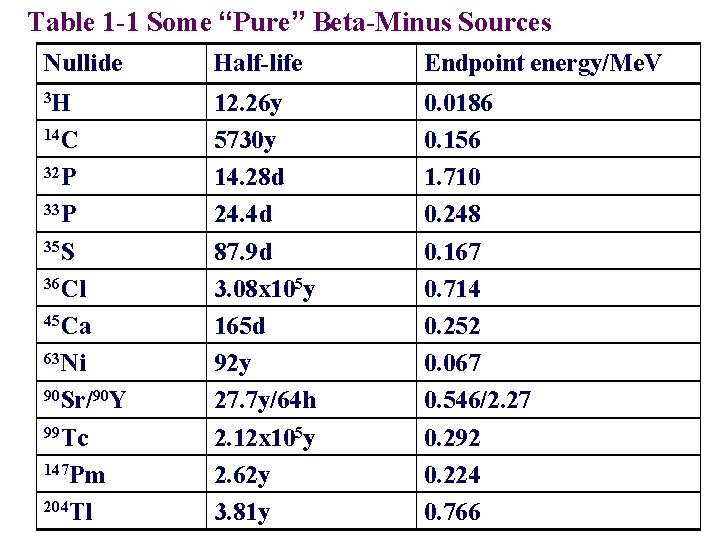
Table 1 -1 Some “Pure” Beta-Minus Sources Nullide Half-life Endpoint energy/Me. V 3 H 12. 26 y 5730 y 14. 28 d 24. 4 d 87. 9 d 3. 08 x 105 y 165 d 92 y 27. 7 y/64 h 2. 12 x 105 y 2. 62 y 3. 81 y 0. 0186 0. 156 1. 710 0. 248 0. 167 0. 714 0. 252 0. 067 0. 546/2. 27 0. 292 0. 224 0. 766 14 C 32 P 33 P 35 S 36 Cl 45 Ca 63 Ni 90 Sr/90 Y 99 Tc 147 Pm 204 Tl
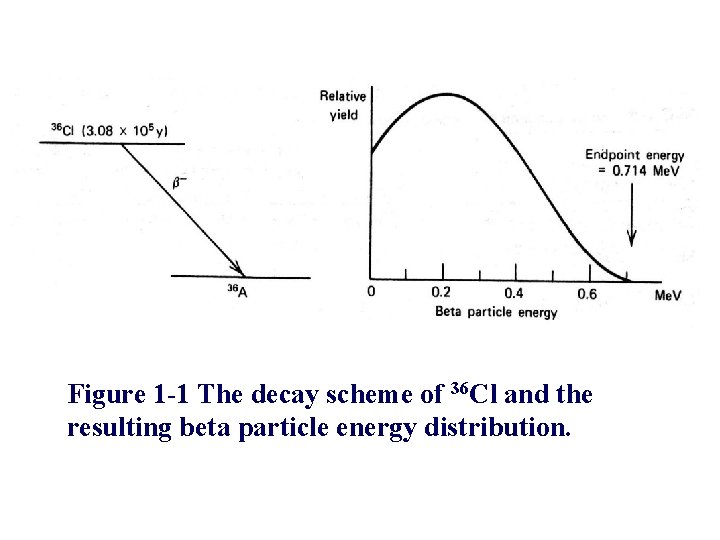
Figure 1 -1 The decay scheme of 36 Cl and the resulting beta particle energy distribution.

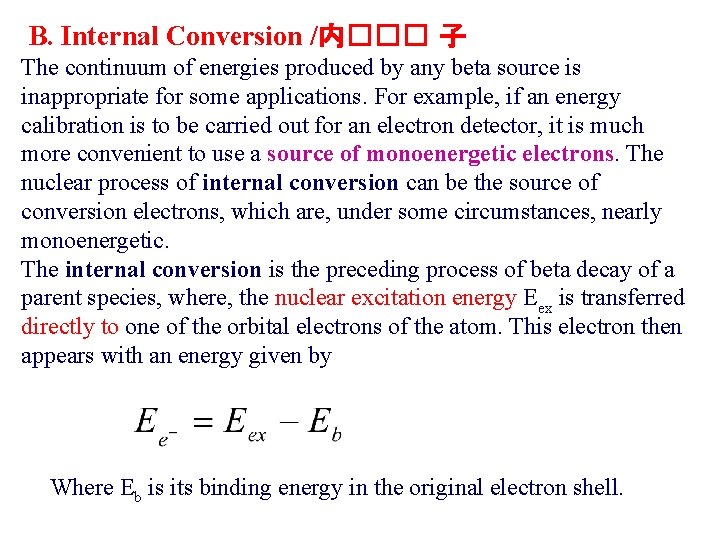
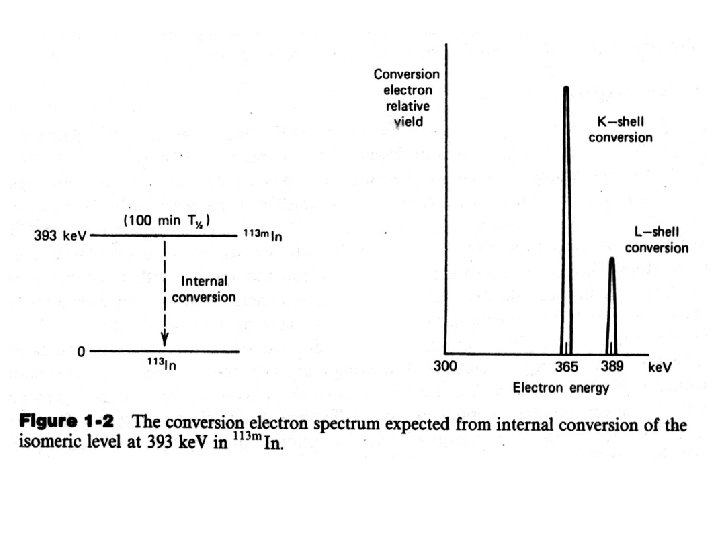
B. Internal Conversion /内��� 子 The continuum of energies produced by any beta source is inappropriate for some applications. For example, if an energy calibration is to be carried out for an electron detector, it is much more convenient to use a source of monoenergetic electrons. The nuclear process of internal conversion can be the source of conversion electrons, which are, under some circumstances, nearly monoenergetic. The internal conversion is the preceding process of beta decay of a parent species, where, the nuclear excitation energy Eex is transferred directly to one of the orbital electrons of the atom. This electron then appears with an energy given by Where Eb is its binding energy in the original electron shell.

C. Auger Electron /俄歇� 子 Auger electrons are roughly the analogue of internal conversion electrons when the excitation energy originates in the atom rather than in the nucleus. A preceding process (such as EC) may leave the atom with a vacancy in a normally complete electron shell. This vacancy is often filled by electrons from the outer shell of the atom with the emission of a characteristic X-ray photon. Alternatively, the excitation energy of the atom may be transferred directly to one of the outer electron, causing it to be ejected from the atom. This electron is called Auger electron and appears with an energy given by the difference between the original atomic excitation energy and the binding energy of the shell from which the electron was ejected. Auger electrons produce a discrete energy spectrum, with different groups corresponding to different initial and final states.
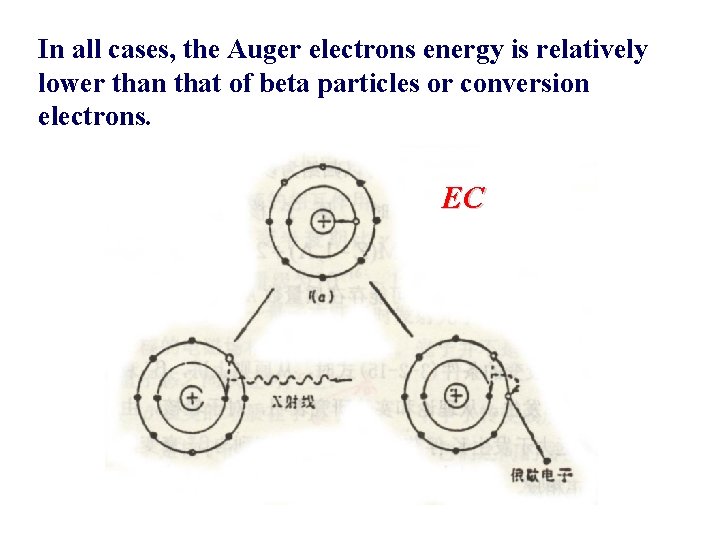
In all cases, the Auger electrons energy is relatively lower than that of beta particles or conversion electrons. EC


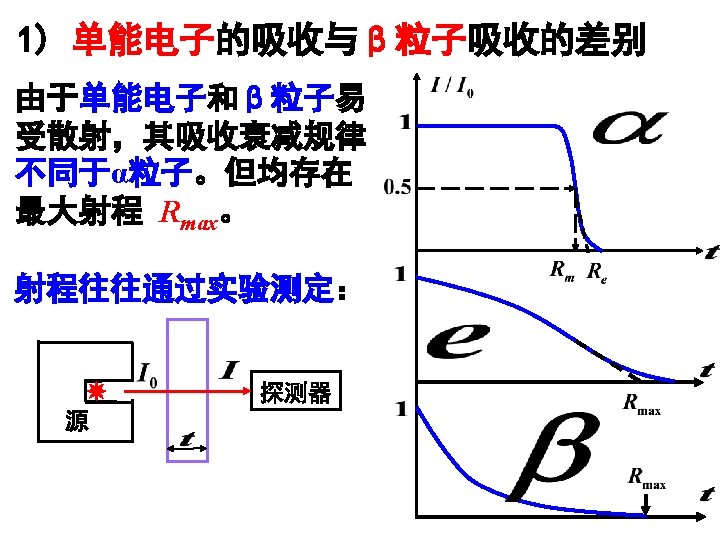

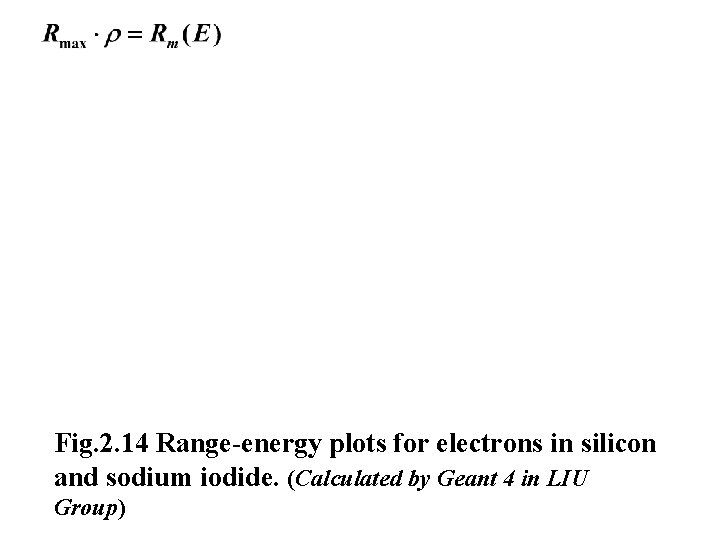
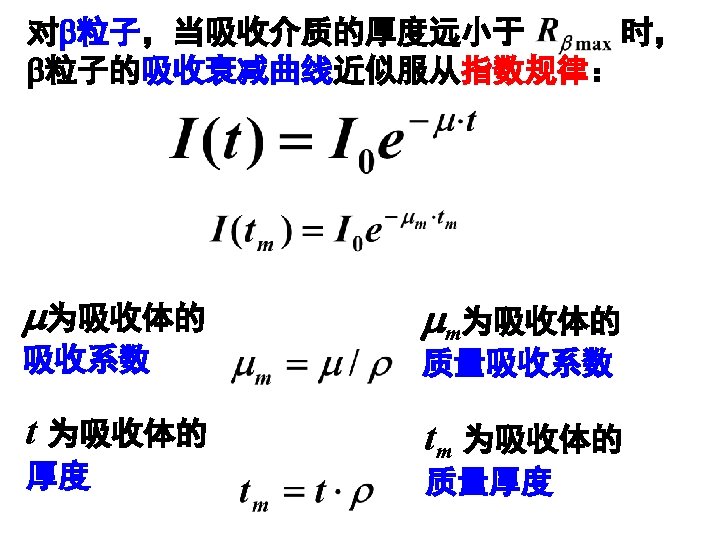
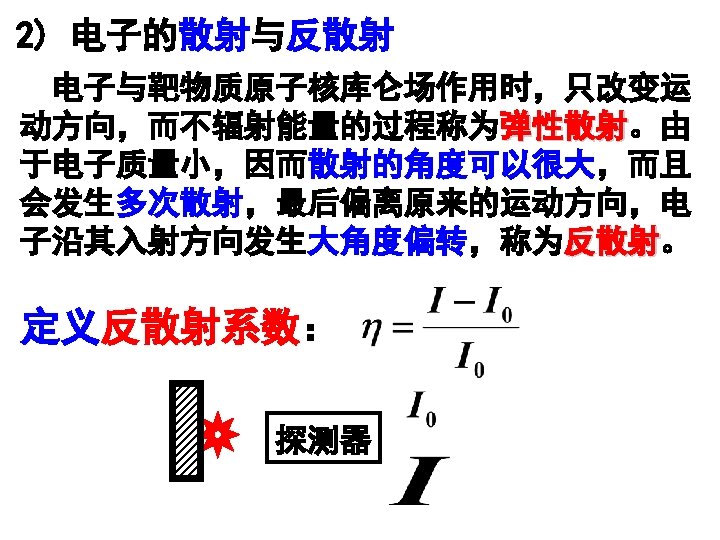
Fig. 2. 14 Range-energy plots for electrons in silicon and sodium iodide. (Calculated by Geant 4 in LIU Group)





因此,两个湮没光子的能量相同,各等 于0. 511 Me. V。 而两个湮没光子的发射方向相反,且发 射是各向同性的。 Pair Annihilation Positron 511 ke. V Electron Two photons travel in exactly opposite directions E = mc 2 511 ke. V


- Slides: 33