1 Englishmetric conversions 2 pts each4 pts total

- Slides: 7

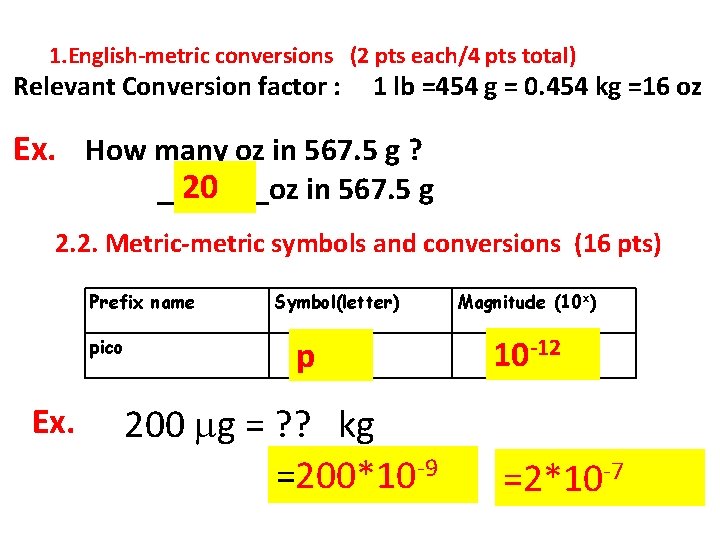
1. English-metric conversions (2 pts each/4 pts total) Relevant Conversion factor : 1 lb =454 g = 0. 454 kg =16 oz Ex. How many oz in 567. 5 g ? 20 _______oz in 567. 5 g 2. 2. Metric-metric symbols and conversions (16 pts) Prefix name pico Ex. Symbol(letter) p 200 g = ? ? kg =200*10 -9 Magnitude (10 x) 10 -12 =2*10 -7

2. 3. unknown metal density determination (4 pts) See density lab and review metal density determination 2. 4 egg arithmetic (2 pt each/8 pts total)) A dozen monstrously large eggs from Aldi’s Ex. weighs 2000 g. Assuming 1 dozen =12 count: If you have 50, 000 grams of eggs, how many eggs do you have ? _____ egg count 300

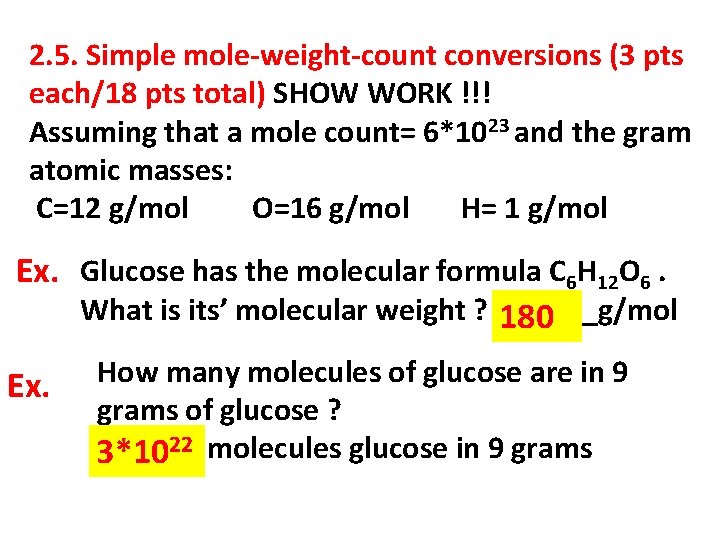
2. 5. Simple mole-weight-count conversions (3 pts each/18 pts total) SHOW WORK !!! Assuming that a mole count= 6*1023 and the gram atomic masses: C=12 g/mol O=16 g/mol H= 1 g/mol Ex. Glucose has the molecular formula C 6 H 12 O 6. What is its’ molecular weight ? 180 ______g/mol Ex. How many molecules of glucose are in 9 grams of glucose ? 22 molecules glucose in 9 grams ______ 3*10

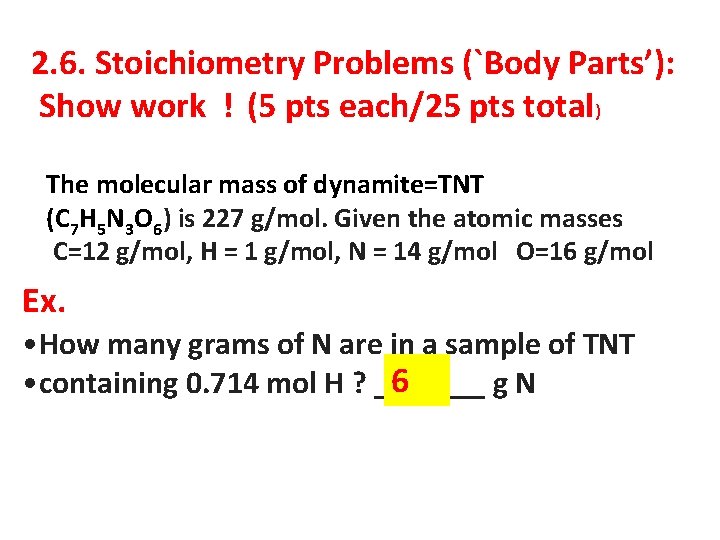
2. 6. Stoichiometry Problems (`Body Parts’): Show work ! (5 pts each/25 pts total) The molecular mass of dynamite=TNT (C 7 H 5 N 3 O 6) is 227 g/mol. Given the atomic masses C=12 g/mol, H = 1 g/mol, N = 14 g/mol O=16 g/mol Ex. • How many grams of N are in a sample of TNT 6 • containing 0. 714 mol H ? _______ g. N

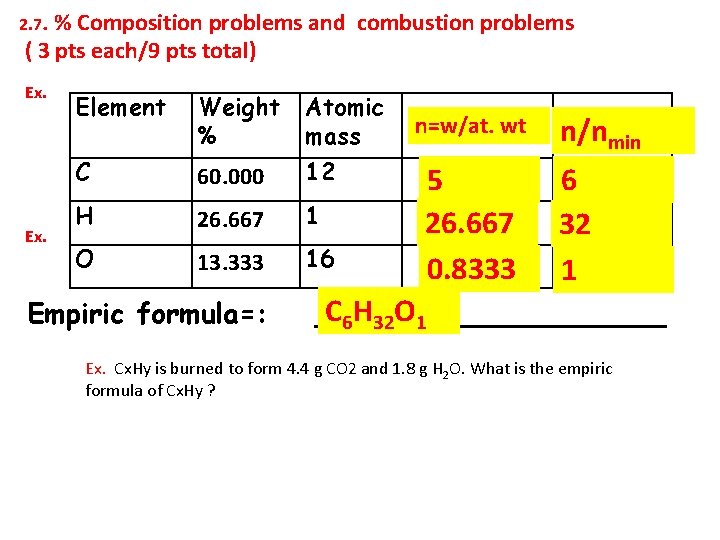
2. 7. % Composition problems and combustion problems ( 3 pts each/9 pts total) Ex. Element Weight % Atomic mass C 60. 000 12 H 26. 667 1 O 13. 333 16 Empiric formula=: n=w/at. wt 5 26. 667 0. 8333 n/nmin 6 32 1 C 6 H 32 O 1 __________ Ex. Cx. Hy is burned to form 4. 4 g CO 2 and 1. 8 g H 2 O. What is the empiric formula of Cx. Hy ?

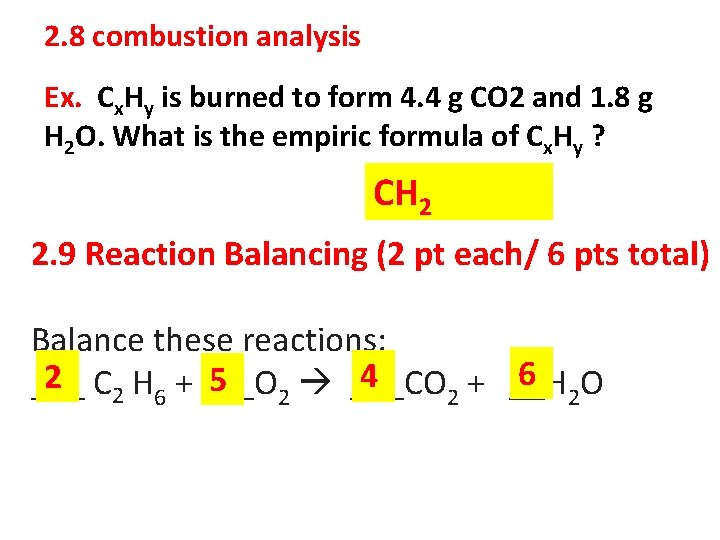
2. 8 combustion analysis Ex. Cx. Hy is burned to form 4. 4 g CO 2 and 1. 8 g H 2 O. What is the empiric formula of Cx. Hy ? CH 2 2. 9 Reaction Balancing (2 pt each/ 6 pts total) Balance these reactions: 6 O 4 2 C 2 H 6 + ___O 5 2 ___CO ___ + __H 2 2

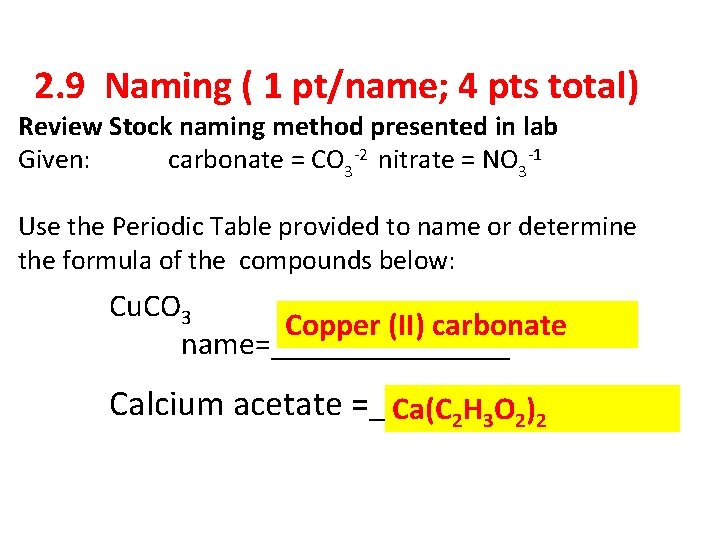
2. 9 Naming ( 1 pt/name; 4 pts total) Review Stock naming method presented in lab Given: carbonate = CO 3 -2 nitrate = NO 3 -1 Use the Periodic Table provided to name or determine the formula of the compounds below: Cu. CO 3 Copper (II) carbonate name=________ Calcium acetate =_______ Ca(C 2 H 3 O 2)2