1 Engineering Measurements of Particles Volumetric measure Units



- Slides: 11

1 Engineering Measurements of Particles Volumetric measure – Units: m. L/L • Settleable solids – 30 minutes settling in Imhoff cone – Large particles

2 Gravimetric measures – mass concentration = mass per volume of suspension mg/L = ppm by mass (approx) ü Total solids – Mass of everything except the water – Evaporate the water off; weigh the residual

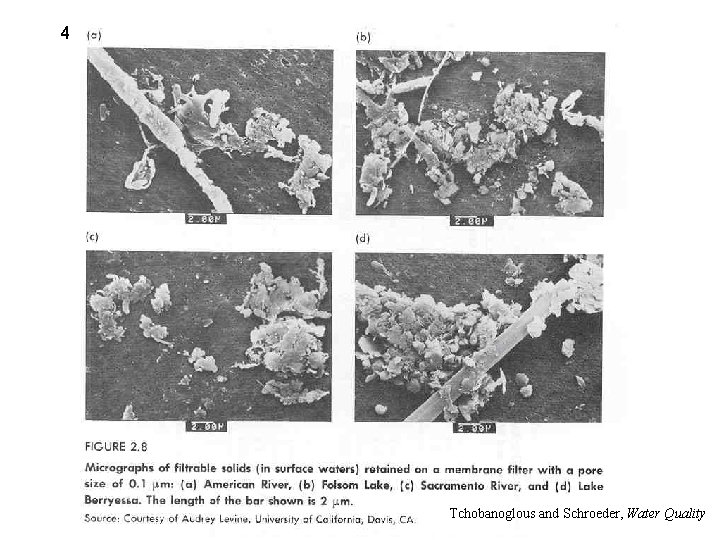
3 ü Total suspended solids (TSS) • Filter sample – 0. 45 to 1 m glass fiber filter – Dry at 105 C Tchobanoglous and Schroeder, Water Quality

4 Tchobanoglous and Schroeder, Water Quality

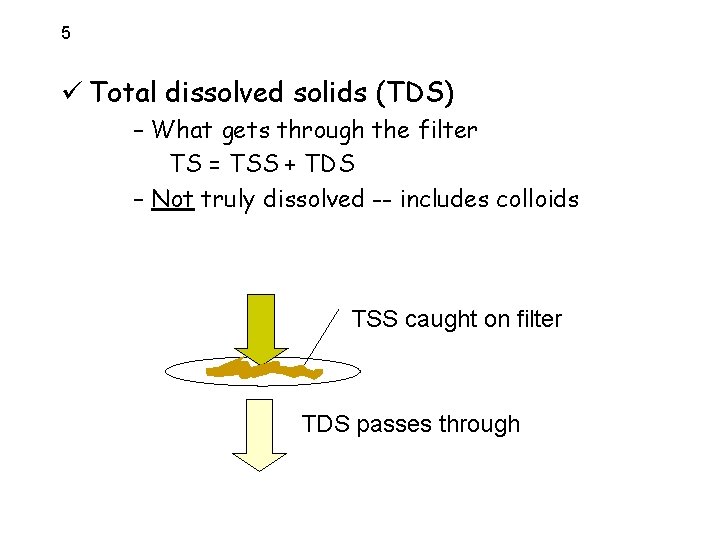
5 ü Total dissolved solids (TDS) – What gets through the filter TS = TSS + TDS – Not truly dissolved -- includes colloids TSS caught on filter TDS passes through

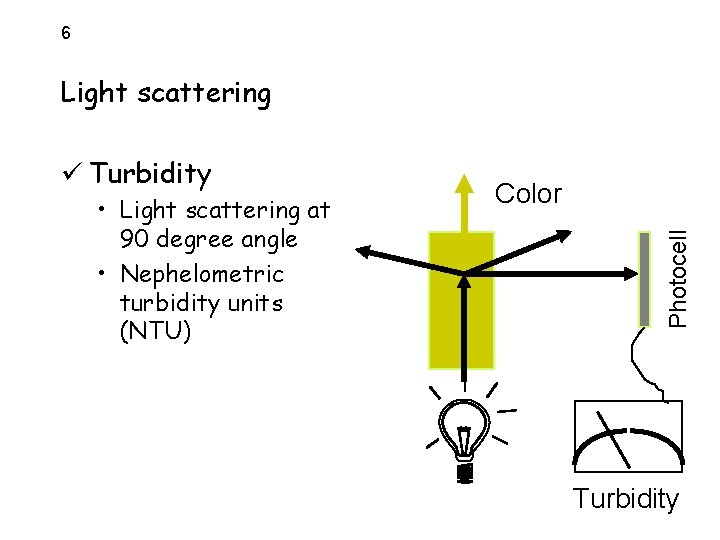
6 Light scattering • Light scattering at 90 degree angle • Nephelometric turbidity units (NTU) Color Photocell ü Turbidity

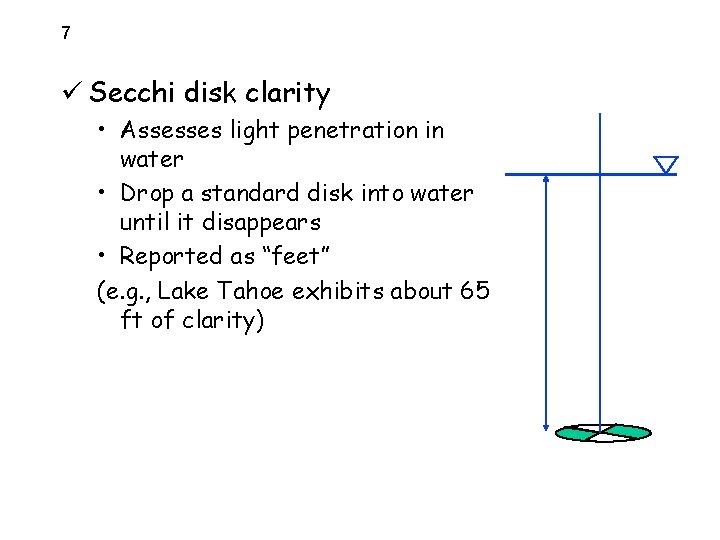
7 ü Secchi disk clarity • Assesses light penetration in water • Drop a standard disk into water until it disappears • Reported as “feet” (e. g. , Lake Tahoe exhibits about 65 ft of clarity)

8 Chemical Measures • Units for dissolved constituents – Molar concentration = moles per unit volume – Mass concentrations = mg/L or g/L • Units for particles – Mass concentrations = mg/L ü Many constituent-specific methods • Standard Methods for the Examination of Water and Wastewater • EPA Methods

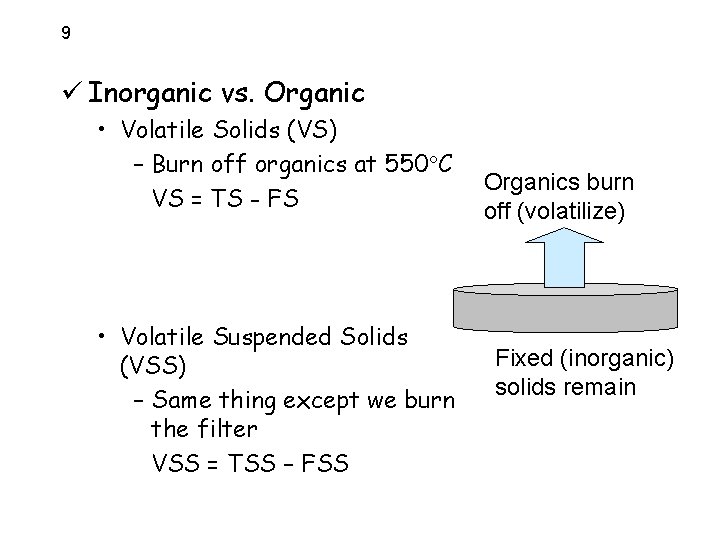
9 ü Inorganic vs. Organic • Volatile Solids (VS) – Burn off organics at 550 C VS = TS - FS • Volatile Suspended Solids (VSS) – Same thing except we burn the filter VSS = TSS – FSS Organics burn off (volatilize) Fixed (inorganic) solids remain

10 ü Total Organic Carbon (TOC) • Chemically oxidize organics to CO 2 • Infrared detector reads the CO 2 • Works for: – Dissolved – Small particles CO 2 detector Sample Oxidizers (ozone, UV light, hydrogen peroxide, etc.

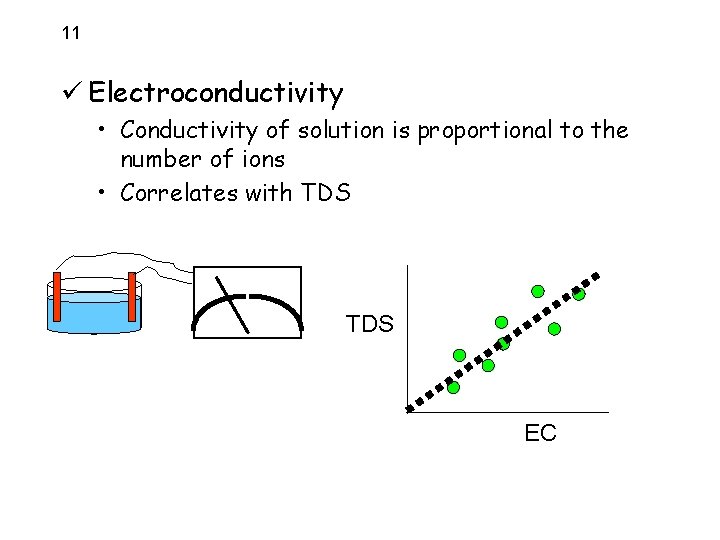
11 ü Electroconductivity • Conductivity of solution is proportional to the number of ions • Correlates with TDS EC