1 Electromagnetic Radiation Visible light is just one

1
Electromagnetic Radiation • Visible light is just one form of electromagnetic radiation. • Light travels in space as a wave. • In vacuum, speed of light is constant and given the symbol “c”, c = 3. 00 x 108 m/s. • Light waves have amplitude, frequency, and wavelength. • Wavelength ( ) : distance between consecutive crests. • Frequency ( ) : number of waves that pass a given point in one second (SI Unit is s-1 or Hz). c = and = c/ 2
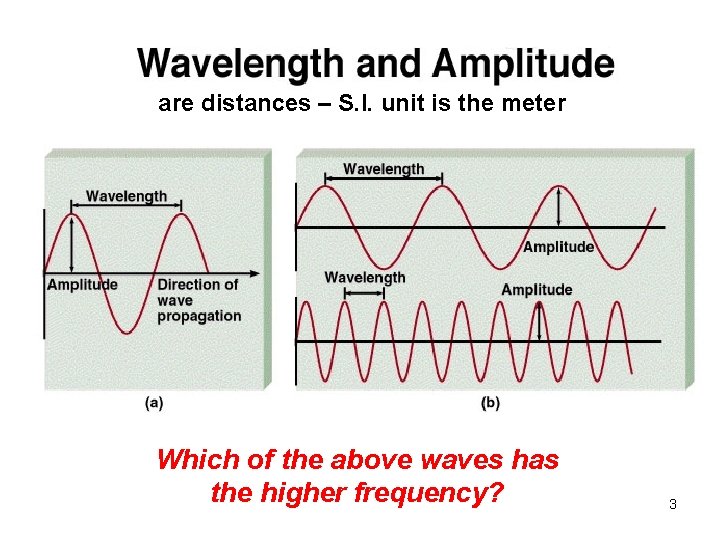
are distances – S. I. unit is the meter Which of the above waves has the higher frequency? 3
The Electromagnetic Spectrum The electromagnetic spectrum consists of all the different wavelengths of electromagnetic radiation, The only region in the entire electromagnetic spectrum that our eyes are sensitive to is the visible region. Gamma rays have the shortest wavelengths, < 0. 01 nanometers (about the size of an atomic nucleus). This is the highest frequency and most energetic region of the electromagnetic spectrum. Gamma rays can result from nuclear reactions. 4

The Electromagnetic Spectrum 5
The Electromagnetic Spectrum X-rays range in wavelength from 0. 01 – 10 nm (about the size of an atom). They are generated, when matter is irradiated by a beam of high-energy charged particles such as electrons. Ultraviolet radiation has wavelengths of 10 – 310 nm (about the size of a virus). Stars produce a lot of ultraviolet light. Visible light covers the range of wavelengths from 400 – 700 nm (from the size of a molecule to a protozoan). Our sun emits the most of its radiation in the visible range, which our eyes perceive as the colors of the rainbow. Our eyes are sensitive only to this small portion of the electromagnetic spectrum. 6

• Microwaves are a form of electromagnetic radiation with wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz (0. 3 GHz) and 300 GHz. • A microwave oven, often called microwave, is a kitchen appliance that heats food by bombarding it with electromagnetic radiation in the microwave spectrum causing polarized molecules in the food to rotate and build up thermal energy in a process known as dielectric heating. Microwave ovens heat foods quickly and efficiently because excitation is fairly uniform in the outer 25– 38 mm of a dense (high water content) food item; food is more evenly heated. 7
The Electromagnetic Spectrum Infrared wavelengths span from 710 nm – 1 millimeter (from the width of a pinpoint to the size of small plant seeds). At a temperature of 37 o. C, our bodies give off infrared wavelengths with a peak intensity near 900 nm. Radio waves are longer than 1 mm waves, have the lowest energy. Radio stations use radio wavelengths of electromagnetic radiation to send signals that our radios then translate into sound. Radio stations transmit electromagnetic radiation, not sound. Our radios receive the electromagnetic radiation, decode the pattern and translate the pattern into sound. 8

Which one of the following types of radiation has the shortest wavelength, the greatest energy, and the highest frequency? a) ultraviolet radiation b) infrared radiation c) visible red light d) visible blue light e) none because short wavelength is associated with low energy and low frequency, not high energy and high frequency 9
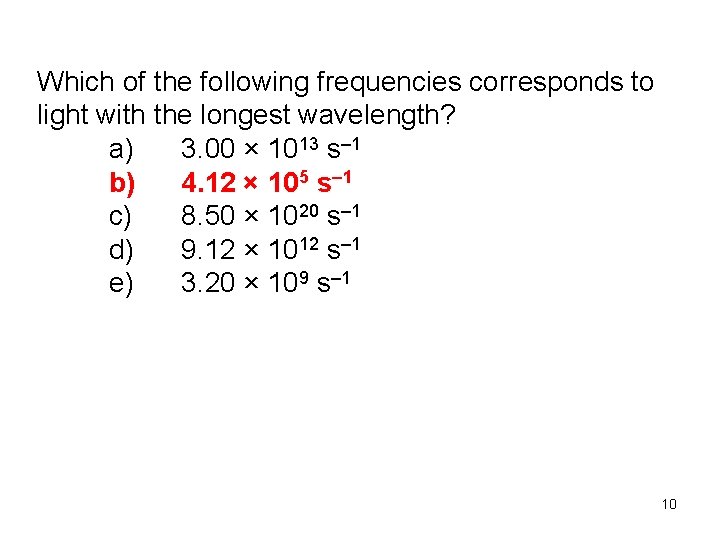
Which of the following frequencies corresponds to light with the longest wavelength? a) 3. 00 × 1013 s– 1 b) 4. 12 × 105 s– 1 c) 8. 50 × 1020 s– 1 d) 9. 12 × 1012 s– 1 e) 3. 20 × 109 s– 1 10
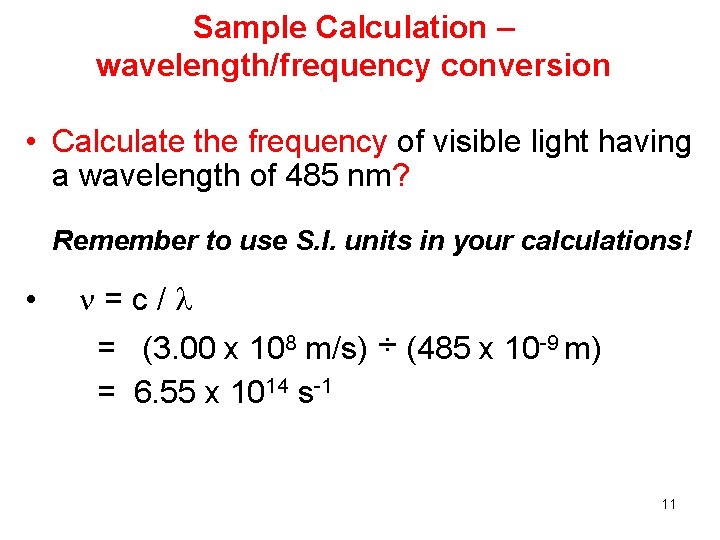
Sample Calculation – wavelength/frequency conversion • Calculate the frequency of visible light having a wavelength of 485 nm? Remember to use S. I. units in your calculations! • =c/ = (3. 00 x 108 m/s) ÷ (485 x 10 -9 m) = 6. 55 x 1014 s-1 11
The Nature of Light Particles or Waves? ? • Light must be made of particles because it… – travels in a vacuum – reflects off of objects – exerts force – Light must consist of waves because it… – reflects like waves – refracts and diffracts – exhibits interference By the end of the 19 th century, scientists had concluded that light is composed of WAVES! 12

History of Optics & Light Studies Ibn Alhazen is considered the “Father of Optics. ” He wrote the “Book of Optics”, which correctly explained and proved the modern theory of vision. His experiments included ones on lenses, mirrors, refraction, reflection, and the dispersion of light into its constituent colors. He studied the electromagnetic aspects of light, and argued that rays of light are streams of energy particles traveling in straight lines. Ibn Alhazen (965 – 1039) Arab Muslim Scientist “Father of Optics” 13

A Quantitative Study of Light Sir Isaac Newton was one of the first man to study light scientifically. In 1672, Newton directed a beam of white light through a triangular bar of glass, called a “prism”. He discovered that the light coming out of the prism was separated into bands of colors. The arrangement of colors produced by a prism is called a “spectrum”. Sir Isaac Newton 1643 - 1727 Prior to this it was believed that “white light” was equal to purity. 14
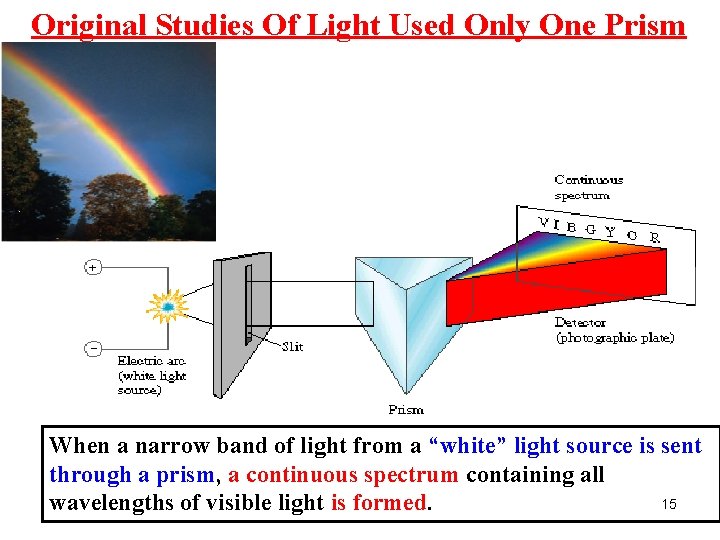
Original Studies Of Light Used Only One Prism . When a narrow band of light from a “white” light source is sent through a prism, a continuous spectrum containing all 15 wavelengths of visible light is formed.
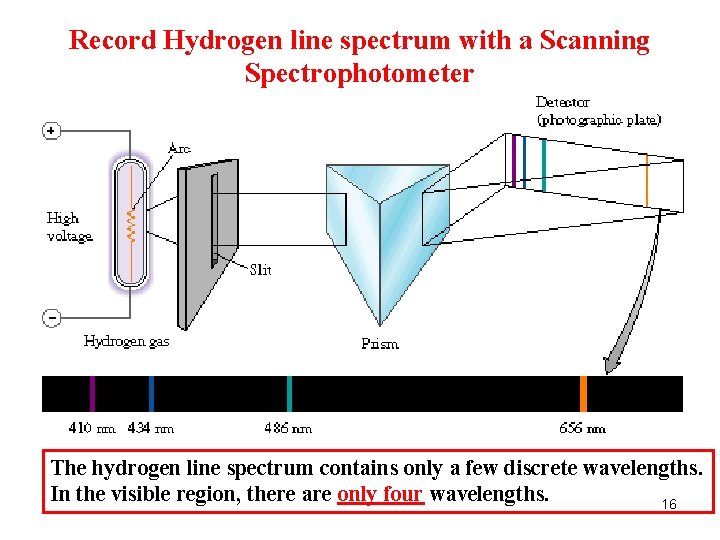
Record Hydrogen line spectrum with a Scanning Spectrophotometer The hydrogen line spectrum contains only a few discrete wavelengths. In the visible region, there are only four wavelengths. 16
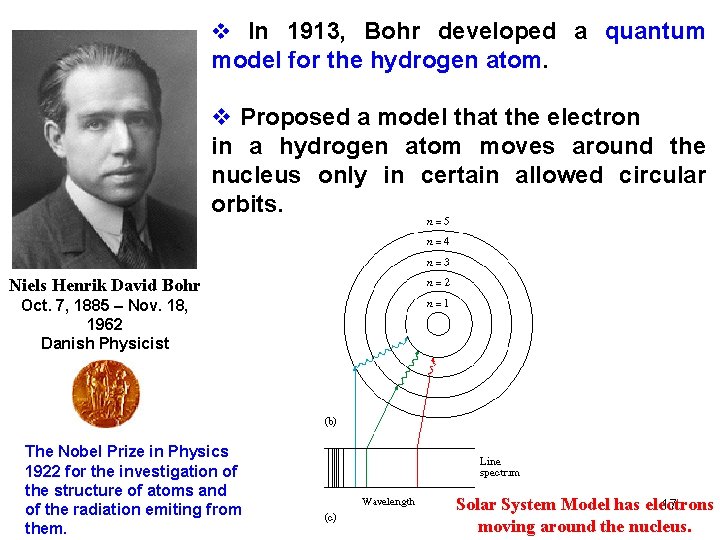
v In 1913, Bohr developed a quantum model for the hydrogen atom. v Proposed a model that the electron in a hydrogen atom moves around the nucleus only in certain allowed circular orbits. Niels Henrik David Bohr Oct. 7, 1885 – Nov. 18, 1962 Danish Physicist The Nobel Prize in Physics 1922 for the investigation of the structure of atoms and of the radiation emiting from them. 17 Solar System Model has electrons moving around the nucleus.

How are the electrons arranged in a Bohr Model of an atom? • Electrons orbit the nucleus. • Electrons are arranged in specific pathways called energy levels. • There’s a fixed number of energy levels. • Each energy level is capable of holding certain number of electrons. • An electron cannot be found between energy levels. 18
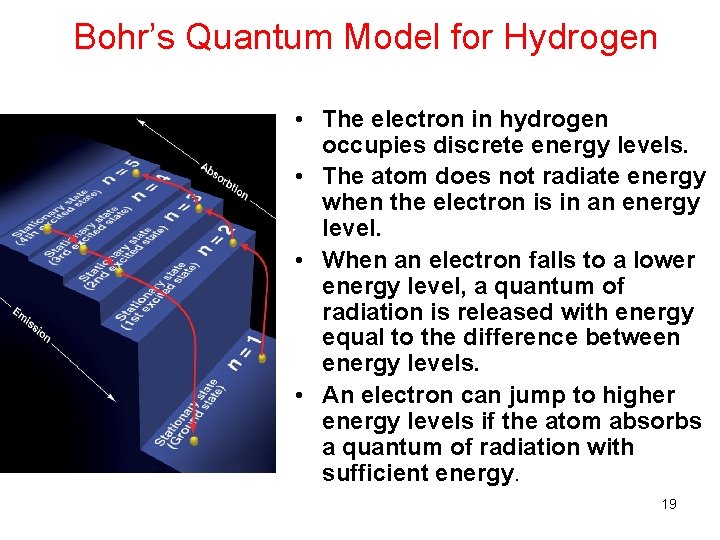
Bohr’s Quantum Model for Hydrogen • The electron in hydrogen occupies discrete energy levels. • The atom does not radiate energy when the electron is in an energy level. • When an electron falls to a lower energy level, a quantum of radiation is released with energy equal to the difference between energy levels. • An electron can jump to higher energy levels if the atom absorbs a quantum of radiation with sufficient energy. 19
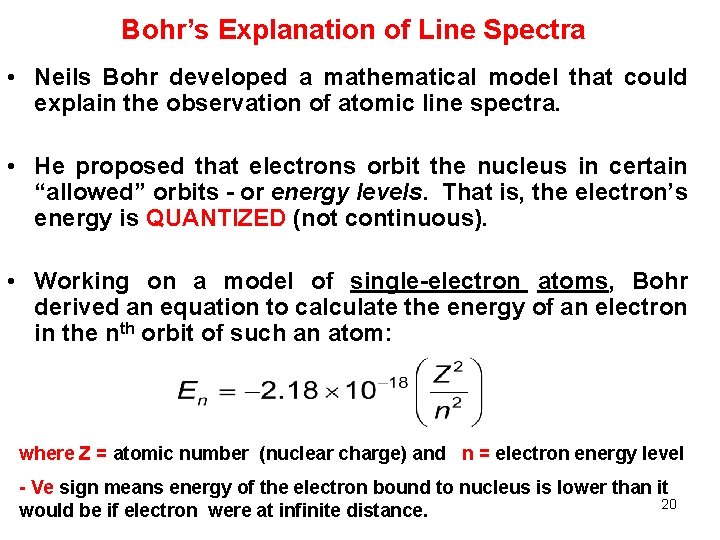
Bohr’s Explanation of Line Spectra • Neils Bohr developed a mathematical model that could explain the observation of atomic line spectra. • He proposed that electrons orbit the nucleus in certain “allowed” orbits - or energy levels. That is, the electron’s energy is QUANTIZED (not continuous). • Working on a model of single-electron atoms, Bohr derived an equation to calculate the energy of an electron in the nth orbit of such an atom: where Z = atomic number (nuclear charge) and n = electron energy level - Ve sign means energy of the electron bound to nucleus is lower than it 20 would be if electron were at infinite distance.
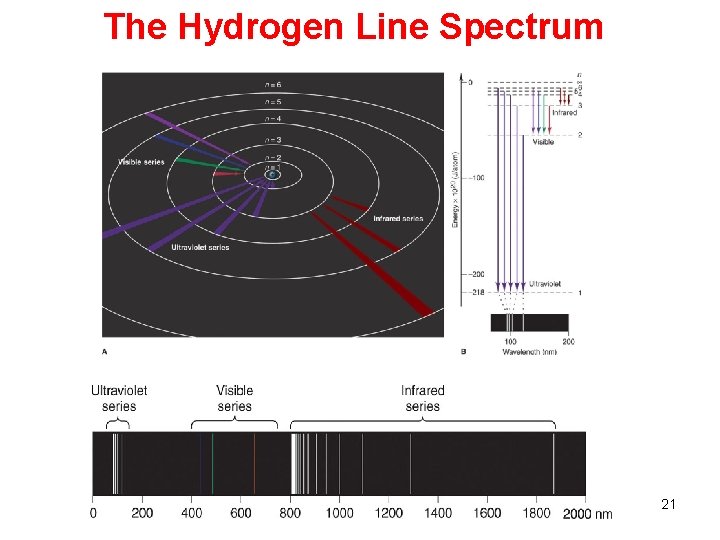
The Hydrogen Line Spectrum 21
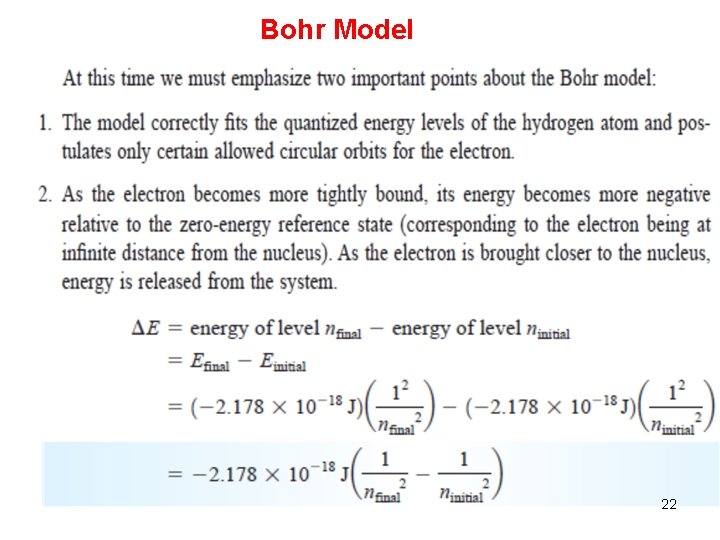
Bohr Model 22
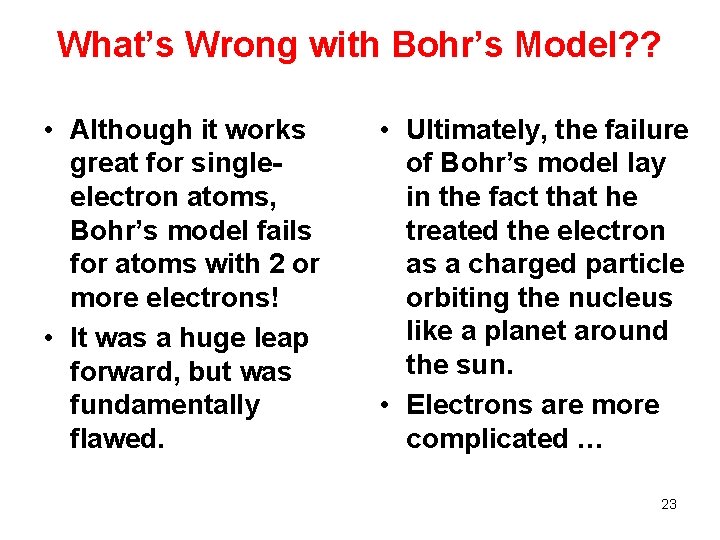
What’s Wrong with Bohr’s Model? ? • Although it works great for singleelectron atoms, Bohr’s model fails for atoms with 2 or more electrons! • It was a huge leap forward, but was fundamentally flawed. • Ultimately, the failure of Bohr’s model lay in the fact that he treated the electron as a charged particle orbiting the nucleus like a planet around the sun. • Electrons are more complicated … 23

Which of the following statements is (are) true? I. An excited atom can return to its ground state by absorbing electromagnetic radiation. II. The energy of an atom is increased when electromagnetic radiation is emitted from it. III. The energy of electromagnetic radiation increases as its frequency increases. IV. An electron in the n = 4 state in the hydrogen atom can go to the n = 2 state by emitting electromagnetic radiation at the appropriate frequency. V. The frequency and wavelength of electromagnetic radiation are inversely proportional to each other. a) II, IV b) III, V c) I, III d) III, IV, V e) I, IV 24

In Bohr’s atomic theory, when an electron moves from one energy level to another energy level more distant from the nucleus a) energy is emitted. b) energy is absorbed. c) no change in energy occurs. d) light is emitted. e) none of these 25
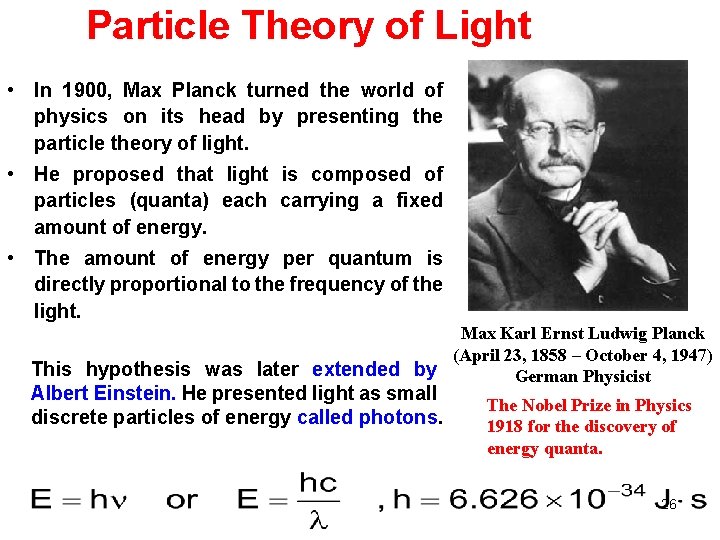
Particle Theory of Light • In 1900, Max Planck turned the world of physics on its head by presenting the particle theory of light. • He proposed that light is composed of particles (quanta) each carrying a fixed amount of energy. • The amount of energy per quantum is directly proportional to the frequency of the light. Max Karl Ernst Ludwig Planck (April 23, 1858 – October 4, 1947) This hypothesis was later extended by German Physicist Albert Einstein. He presented light as small discrete particles of energy called photons. The Nobel Prize in Physics 1918 for the discovery of energy quanta. 26
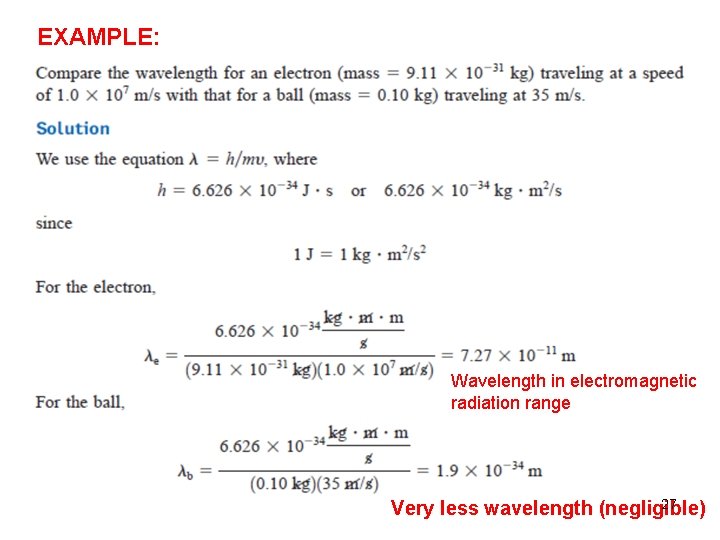
EXAMPLE: Wavelength in electromagnetic radiation range 27 Very less wavelength (negligible)

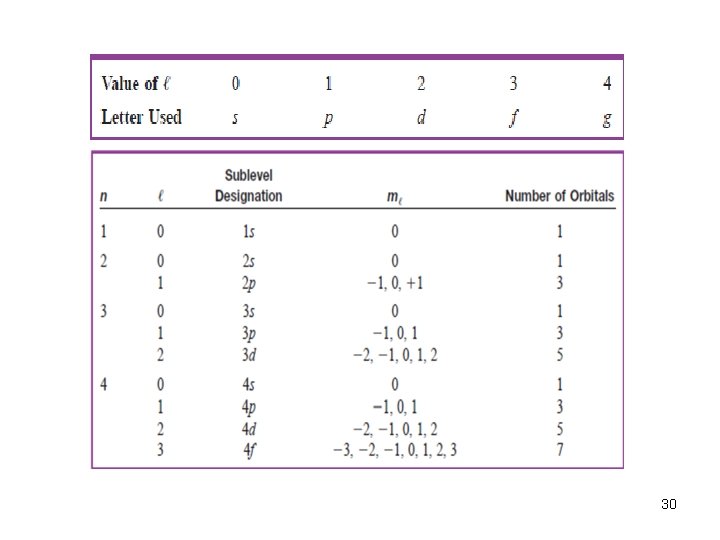
QUANTUM NUMBERS Total four quantum numbers were developed to better understand the movement and pathways of electrons in its orbital within an atom. Each quantum numbers indicates the trait of electron in atom. 1 - The principal quantum number (n): It has integral values: 1, 2, 3…. The principal quantum number is related to the size and energy of the orbital. An increase in n means higher energy, because the electron is less tightly bound to the nucleus, and the energy is less negative. Electrons with same value of n are said to be in the same “electron shell” 28
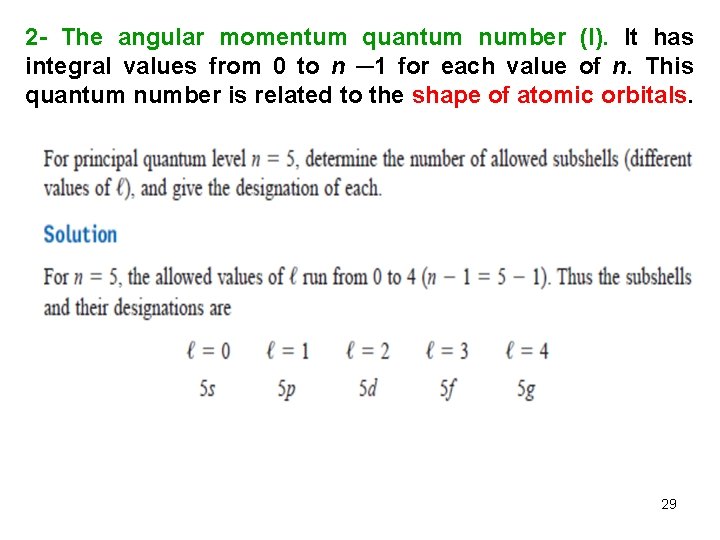
2 - The angular momentum quantum number (l). It has integral values from 0 to n ─1 for each value of n. This quantum number is related to the shape of atomic orbitals. 29
30
3 - The magnetic quantum number (m): It has integral values between + and─, including zero. The value of m is related to the orientation of the orbital in space relative to the other orbitals in the atom. 31
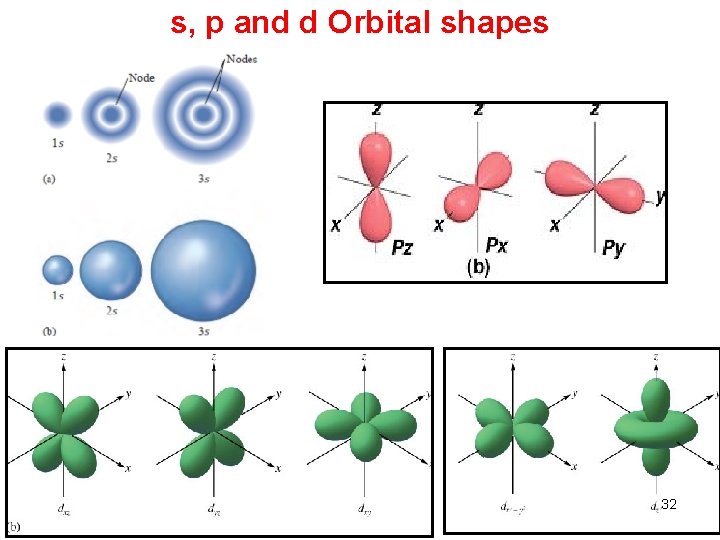
s, p and d Orbital shapes 32
4 - Electron Spin Quantum Number (ms) • A fourth quantum number, ms describes electron spin (either clockwise +½ or anticlockwise – ½) • Each electron in atom has a unique set of these four quantum A spinning negative numbers. charge creates a magnetic field. The • Electrons in orbitals with same n and l values are said to be in the direction of spin determines the direction of the field. same subs hell. • Electrons with all three numbers the same, n, l , and ml , are in the same orbital. 33

Pauli Exclusion Principle • Electrons have negative charge and repel each other. How are the electrons in an atom distributed? • Wolfgang Pauli proposed that no two electrons in a given atom can be described by the same four quantum numbers! • The first three quantum numbers determine an orbital – therefore spins must be opposite! • Practical result is that each orbital can hold a maximum of two electrons, with opposite spins. 34
Electron Configurations • Electrons orbitals are defined by their quantum numbers, n, l and m. • Each electron in an atom has a unique set of 4 quantum numbers. • No two electrons can have the same “address”, i. e. , the same 4 quantum #’s • Rules define how multiple electrons will be distributed among the possible energy levels 35
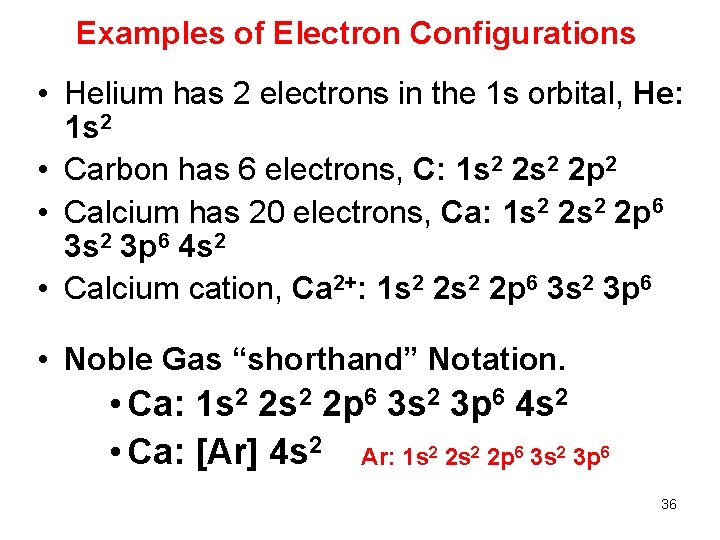
Examples of Electron Configurations • Helium has 2 electrons in the 1 s orbital, He: 1 s 2 • Carbon has 6 electrons, C: 1 s 2 2 p 2 • Calcium has 20 electrons, Ca: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 • Calcium cation, Ca 2+: 1 s 2 2 p 6 3 s 2 3 p 6 • Noble Gas “shorthand” Notation. • Ca: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 • Ca: [Ar] 4 s 2 Ar: 1 s 2 2 p 6 3 s 2 3 p 6 36

Aufbau Principle • In the ground state, the electrons occupy the lowest available energy levels. An atom is in an excited state if one or more electrons are in higher energy orbitals. • In atoms with more than one electron the lower energy orbitals get filled by electrons first! • This is the Aufbau principle, which is named after the German word which means “to build up”. • When one describes the locations of the electrons in an atom, start with the lowest energy electron and work up to the highest energy electron. 37
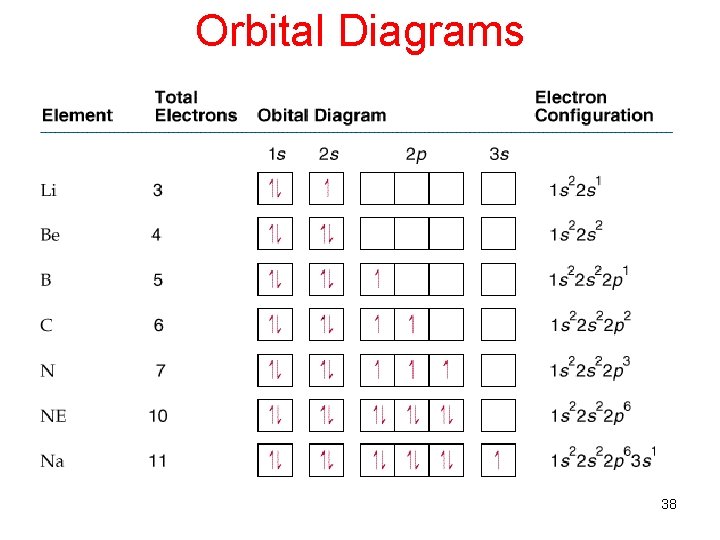
Orbital Diagrams 38
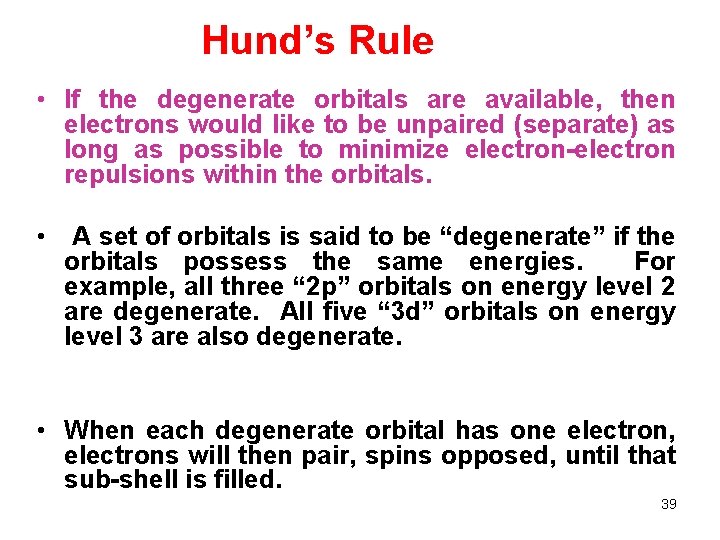
Hund’s Rule • If the degenerate orbitals are available, then electrons would like to be unpaired (separate) as long as possible to minimize electron-electron repulsions within the orbitals. • A set of orbitals is said to be “degenerate” if the orbitals possess the same energies. For example, all three “ 2 p” orbitals on energy level 2 are degenerate. All five “ 3 d” orbitals on energy level 3 are also degenerate. • When each degenerate orbital has one electron, electrons will then pair, spins opposed, until that sub-shell is filled. 39
Summary of Distribution Rules • Electrons distribute to lower energy levels until they are filled, before occupying higher levels. (Aufbau Principle) • Electrons will spread out as much as possible within a sub-shell. (Hund’s Rule) • Electrons will pair up, two to an orbital, spins opposed, until that sub-shell is filled. (Pauli Exclusion) • Every electron will have unique set of 4 quantum numbers. 40

How many f orbitals have the value n = 3? a) 0 b) 3 c) 5 d) 7 e) 1 41
How many electrons in an atom can have the quantum numbers n = 3, l = 2? a) 2 b) 5 c) 10 d) 18 e) 6 42
A given set of p orbitals consists of ______ orbitals. a) 1 b) 2 c) 3 d) 4 e) 5 43
Periodicity in Periodic Table • “Periodicity” refers to similarities in behavior and reactivity due to similar outer shell electron configurations. • All the Alkali Metals have one unpaired valence electron; all the Noble Gases have completely filled sub-shells. • We will examine periodic trends in atomic radius, ionization energy, electronegativity, and electron affinity. 44

Atomic Size Atomic radii increase within a group (column) as the principal quantum number of Outermost shell increases. Atomic size decreases across a row (period) from Left to Right, because the effective nuclear charge increases. 45
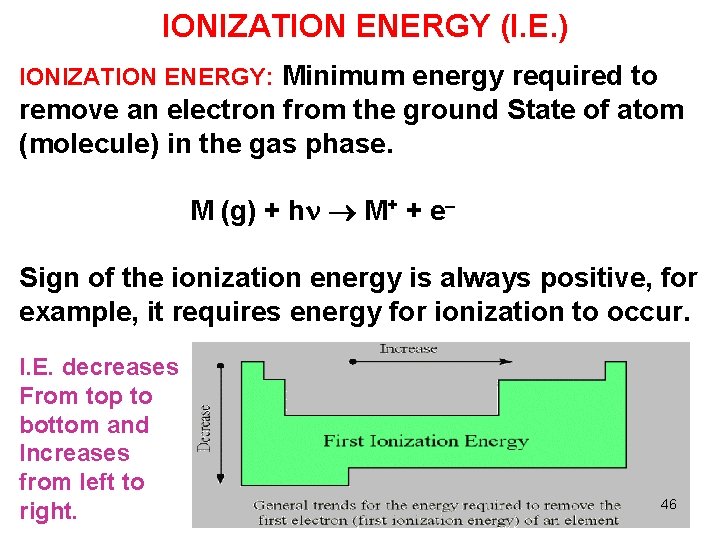
IONIZATION ENERGY (I. E. ) IONIZATION ENERGY: Minimum energy required to remove an electron from the ground State of atom (molecule) in the gas phase. M (g) + h M+ + e Sign of the ionization energy is always positive, for example, it requires energy for ionization to occur. I. E. decreases From top to bottom and Increases from left to right. 46
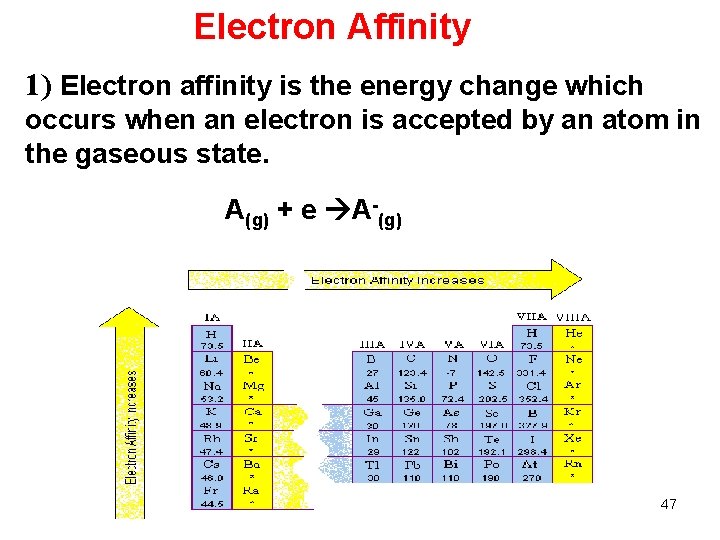
Electron Affinity 1) Electron affinity is the energy change which occurs when an electron is accepted by an atom in the gaseous state. A(g) + e A-(g) 47

Electronegativity: The ability of an atom in a bond to pull on the electron. (Linus Pauling) When the atoms are the same they pull on the electrons equally. Example, H-H. When the atoms are different, the atoms pull on the electrons unevenly. Example, HCl. 48
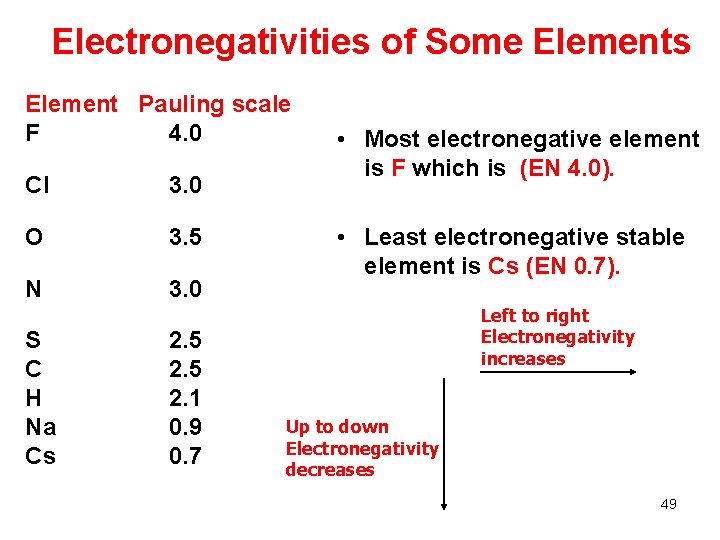
Electronegativities of Some Elements Element Pauling scale F 4. 0 Cl 3. 0 O 3. 5 N 3. 0 S C H Na Cs 2. 5 2. 1 0. 9 0. 7 • Most electronegative element is F which is (EN 4. 0). • Least electronegative stable element is Cs (EN 0. 7). Left to right Electronegativity increases Up to down Electronegativity decreases 49
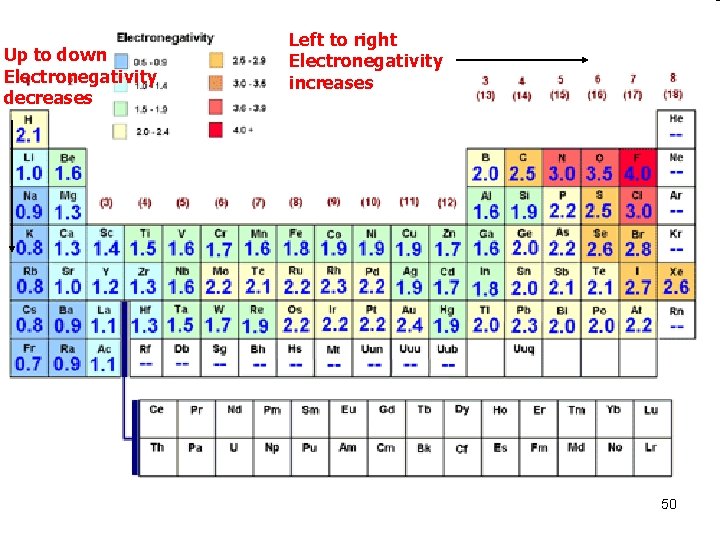
Up to down Electronegativity decreases Left to right Electronegativity increases 50
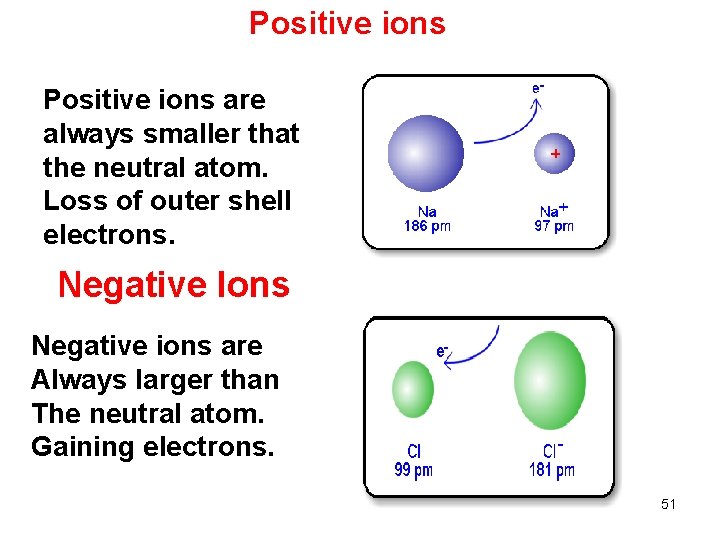
Positive ions are always smaller that the neutral atom. Loss of outer shell electrons. Negative Ions Negative ions are Always larger than The neutral atom. Gaining electrons. 51
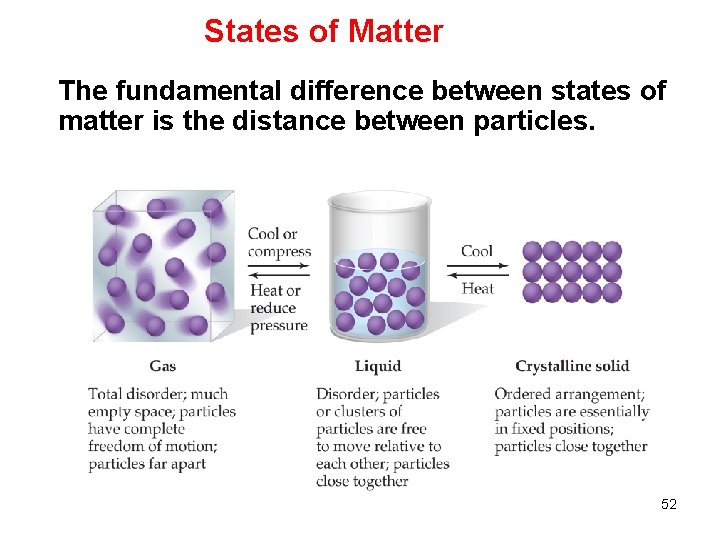
States of Matter The fundamental difference between states of matter is the distance between particles. 52
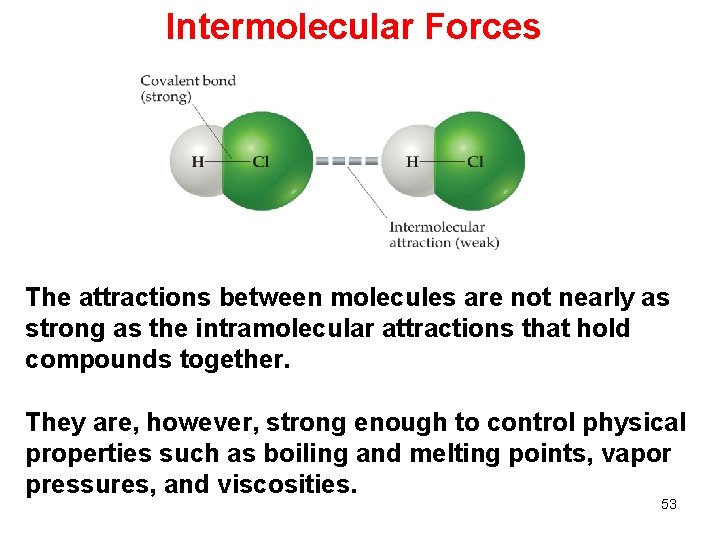
Intermolecular Forces The attractions between molecules are not nearly as strong as the intramolecular attractions that hold compounds together. They are, however, strong enough to control physical properties such as boiling and melting points, vapor pressures, and viscosities. 53
Intermolecular Forces These intermolecular forces as a group are referred to as van der Waals forces. 1 - Dipole-dipole interactions 2 - London dispersion forces 3 - Hydrogen bonding 54
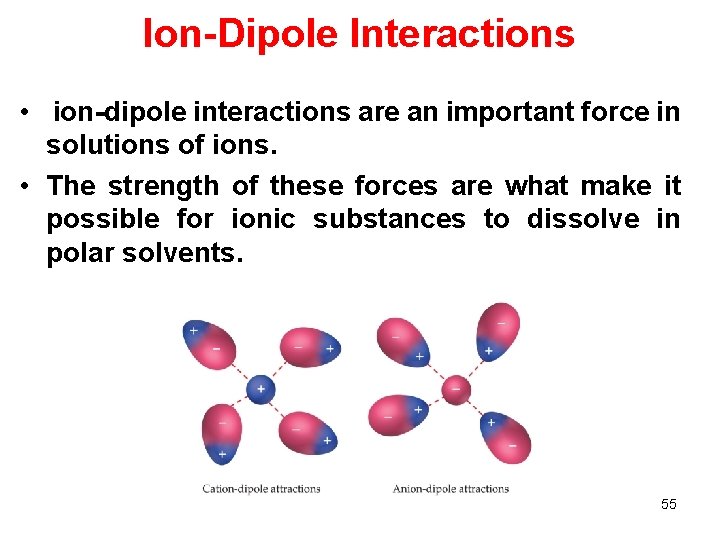
Ion-Dipole Interactions • ion-dipole interactions are an important force in solutions of ions. • The strength of these forces are what make it possible for ionic substances to dissolve in polar solvents. 55
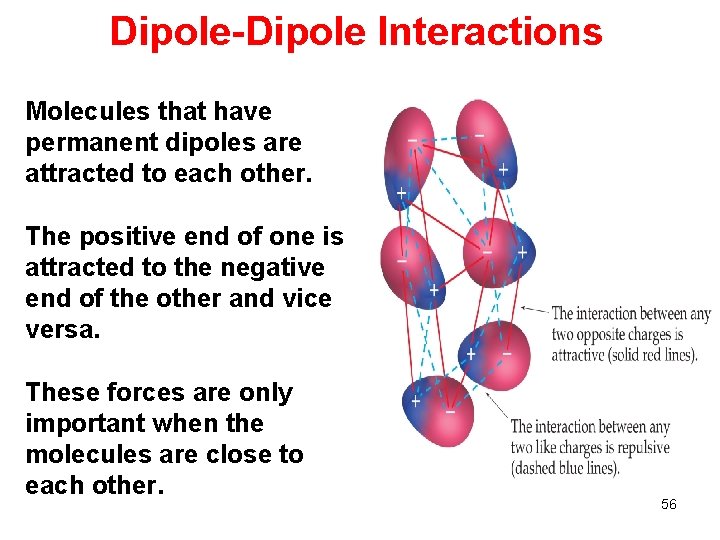
Dipole-Dipole Interactions Molecules that have permanent dipoles are attracted to each other. The positive end of one is attracted to the negative end of the other and vice versa. These forces are only important when the molecules are close to each other. 56
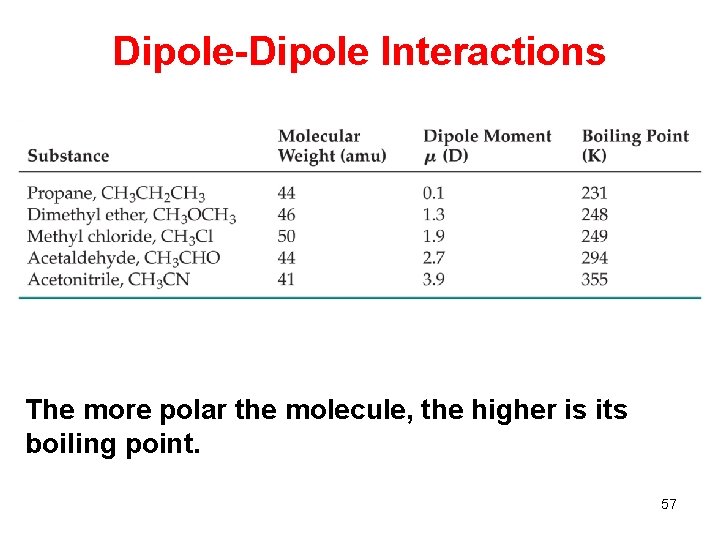
Dipole-Dipole Interactions The more polar the molecule, the higher is its boiling point. 57

London Dispersion Forces While the electrons in the 1 s orbital of helium would repel each other (and, therefore, tend to stay far away from each other), it does happen that they occasionally wind up on the same side of the atom. 58
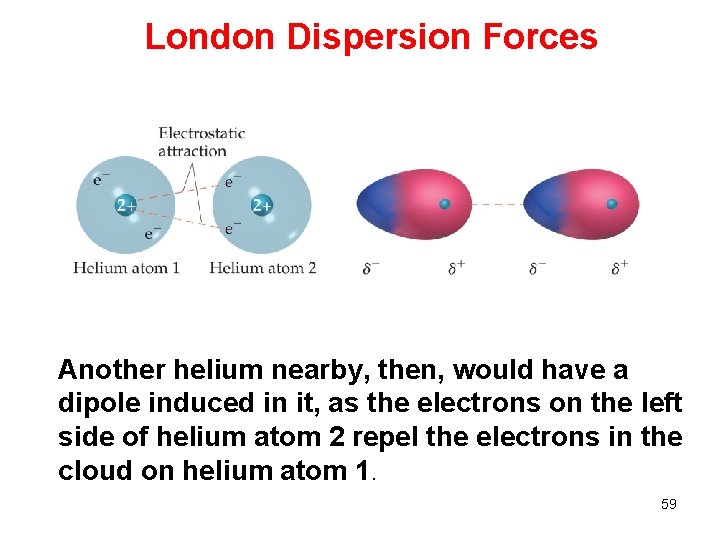
London Dispersion Forces Another helium nearby, then, would have a dipole induced in it, as the electrons on the left side of helium atom 2 repel the electrons in the cloud on helium atom 1. 59

London Dispersion Forces At that instant, then, the helium atom is polar, with an excess of electrons on the left side and a shortage on the right side. 60
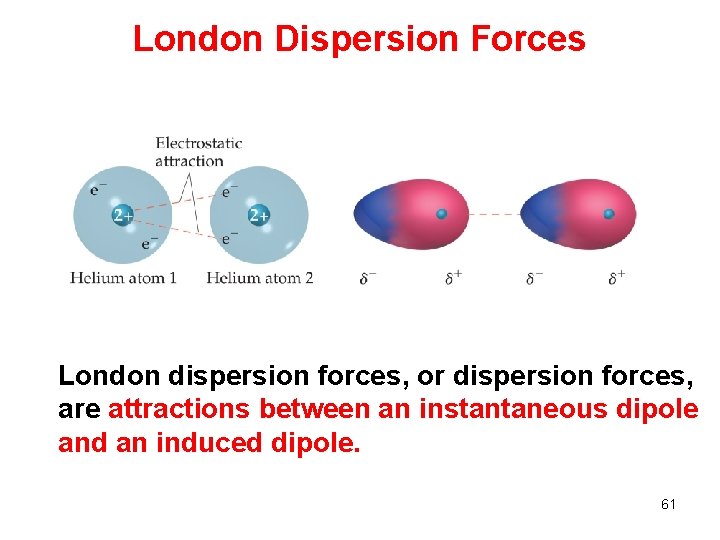
London Dispersion Forces London dispersion forces, or dispersion forces, are attractions between an instantaneous dipole and an induced dipole. 61
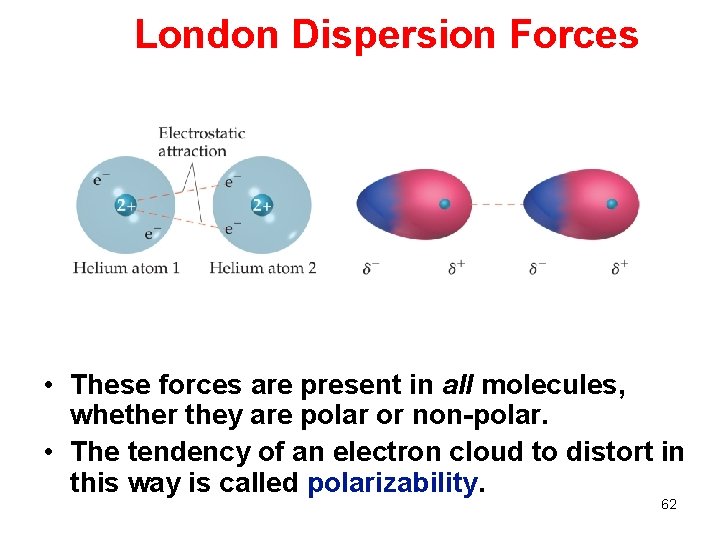
London Dispersion Forces • These forces are present in all molecules, whether they are polar or non-polar. • The tendency of an electron cloud to distort in this way is called polarizability. 62
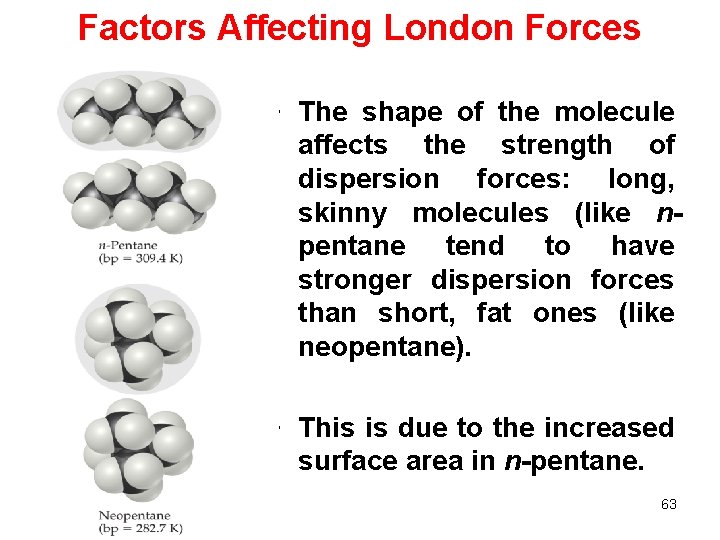
Factors Affecting London Forces • The shape of the molecule affects the strength of dispersion forces: long, skinny molecules (like npentane tend to have stronger dispersion forces than short, fat ones (like neopentane). • This is due to the increased surface area in n-pentane. 63
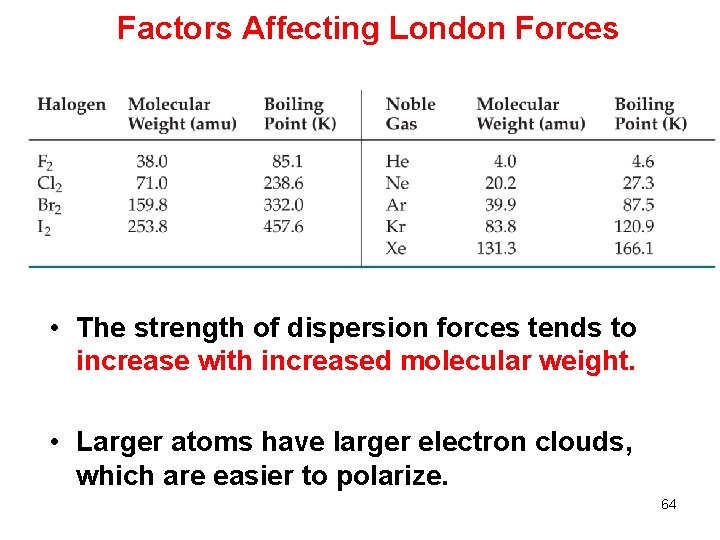
Factors Affecting London Forces • The strength of dispersion forces tends to increase with increased molecular weight. • Larger atoms have larger electron clouds, which are easier to polarize. 64

Which Have a Greater Effect Dipole-Dipole Interactions or Dispersion Forces? • If two molecules are of comparable size and shape, dipole-dipole interactions will likely be the dominating force. • If one molecule is much larger than another, dispersion forces will likely determine its physical properties. 65
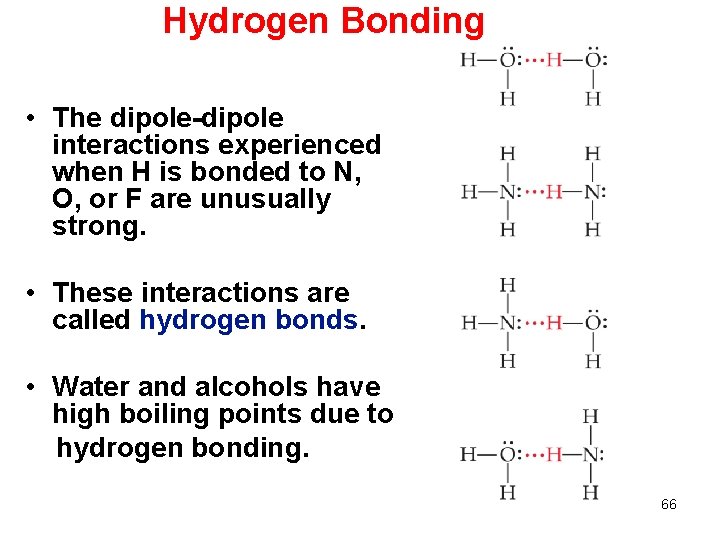
Hydrogen Bonding • The dipole-dipole interactions experienced when H is bonded to N, O, or F are unusually strong. • These interactions are called hydrogen bonds. • Water and alcohols have high boiling points due to hydrogen bonding. 66
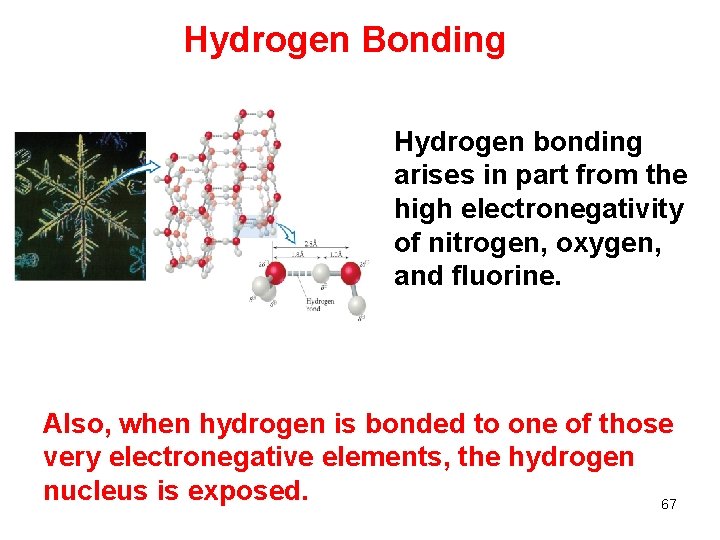
Hydrogen Bonding Hydrogen bonding arises in part from the high electronegativity of nitrogen, oxygen, and fluorine. Also, when hydrogen is bonded to one of those very electronegative elements, the hydrogen nucleus is exposed. 67
- Slides: 67