1 Course code 2 Course title ERT 430




















- Slides: 33

(1) Course code: (2) Course title: ERT 430 Introduction ERT 430 Pharmaceutical Process Engineering (3) Number of unit: 3 (4) Course type: Elective (5) Prerequisite: Nil (6) Course synopsis: The course includes the principles of drug pharmacokinetics: absorption, distribution, metabolism and excretion of drugs. This course also covers the scientific and technological aspects of the designing and manufacturing of pharmaceutical products. (7) ) Learning Outcomes: At the end of the course, students are expected to be: 1. Able to explain the basic concept of drug absorption and disposition and analyze the related pharmacokinetics. 2. Able to evaluate the pharmaceutical engineering processes in pharmaceutical formulation and production. 3. Able to design pharmaceutical facilities. (8) List of possible experiments: Nil (9) Learning approach: Lecture : 100% (42 hours) (10) Evaluation contribution: (i) Examination: 70% Midterm Examinations = 20% Final Examination = 50% (ii) Continual Assessment: 30% Assignments = 20% Quizzes = 10%

ERT 430 Introduction (11) Lecturers i. Dr Khairul Farihan Kasim (C) ii. Mrs Anis Atikah binti Ahmad iii. Mrs Syazwani binti Mahmad Puzi (12) List of text books and references Textbook: i) Bennet, B. and Cole, G. Pharmaceutical Production: An Engineering Guide. Warwickshire: Institution of Chemical Engineers (IChem. E). , 2003. Reference Books: i) Aulton M. E. , Pharmaceutics. The science of dosage form design. 2 nd Edition. London: Churchill Livingstone. , 2002. ii) David J. am Ende, Chemical Engineering in the Pharmaceutical Industry: R&D to Manufacturing, New Jersey, USA: John Wiley & Sons, Inc. , 2011. iii) Blacker A. John, Williams Mike T. , Pharmaceutical Process Development - Current Chemical and Engineering Challenges, Royal Society of Chemistry, UK: Cambridge. , 2011. iv) Anthony J. Hickey, David Ganderton. Pharmaceutical Process Engineering: 2 nd Edition. New York: Informa Healthcare. , 2009 v) Sambamurthy K. , Pharmaceutical Engineering. New Delhi: New Age International Publishers. 2012

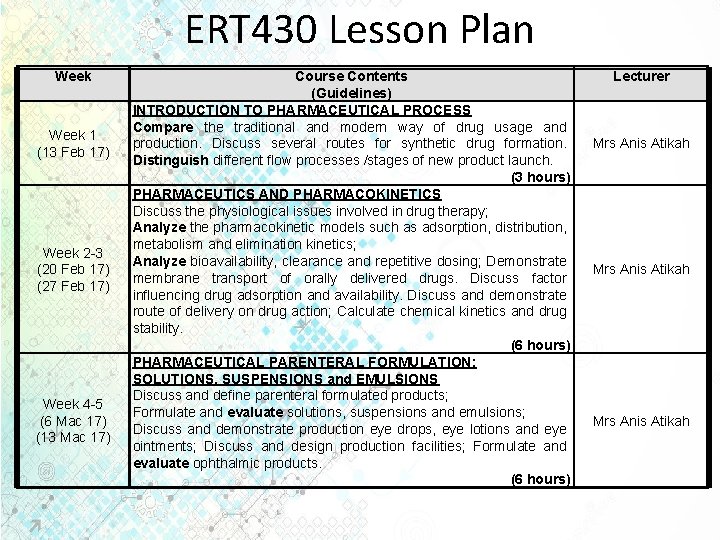
ERT 430 Lesson Plan Week 1 (13 Feb 17) Week 2 -3 (20 Feb 17) (27 Feb 17) Week 4 -5 (6 Mac 17) (13 Mac 17) Course Contents (Guidelines) INTRODUCTION TO PHARMACEUTICAL PROCESS Compare the traditional and modern way of drug usage and production. Discuss several routes for synthetic drug formation. Distinguish different flow processes /stages of new product launch. (3 hours) PHARMACEUTICS AND PHARMACOKINETICS Discuss the physiological issues involved in drug therapy; Analyze the pharmacokinetic models such as adsorption, distribution, metabolism and elimination kinetics; Analyze bioavailability, clearance and repetitive dosing; Demonstrate membrane transport of orally delivered drugs. Discuss factor influencing drug adsorption and availability. Discuss and demonstrate route of delivery on drug action; Calculate chemical kinetics and drug stability. (6 hours) PHARMACEUTICAL PARENTERAL FORMULATION: SOLUTIONS, SUSPENSIONS and EMULSIONS Discuss and define parenteral formulated products; Formulate and evaluate solutions, suspensions and emulsions; Discuss and demonstrate production eye drops, eye lotions and eye ointments; Discuss and design production facilities; Formulate and evaluate ophthalmic products. (6 hours) Lecturer Mrs Anis Atikah

ERT 430 Lesson Plan – cont. Week 6 -7 (20 Mac 17) (27 Mac 17) Week 8 (3 April 17) Week 9 (10 April 17) Week 10 -11 (17 Apr 17) (24 Apr 17) PHARMACEUTICAL SOLID DOSE FORMULATION: TABLETS and CAPSULES Illustrate, formulate and evaluate of different types of solid dose formulation; Discuss, analyze and evaluate methods of preparation, processing problems, formulation and manufacturing techniques of different compressed tablets and capsules. (6 hours) Dr Khairul Farihan CUTI PERTENGAHAN SEMESTER/MID-TERM BREAK PHARMACEUTICAL SOLID DOSE FORMULATION: POWDERS and GRANULES Discuss, demonstrate and analyze methods of preparation, processing problems, formulation and manufacturing techniques of powders and granules. (3 hours) PHARMACEUTICAL PROCESSING Describe, discuss and illustrate general technique used for extraction and isolation of phytopharmaceuticals; illustrate principles and design pilot plant for Isolation of active pharmaceuticals. (6 hours) Dr Khairul Farihan

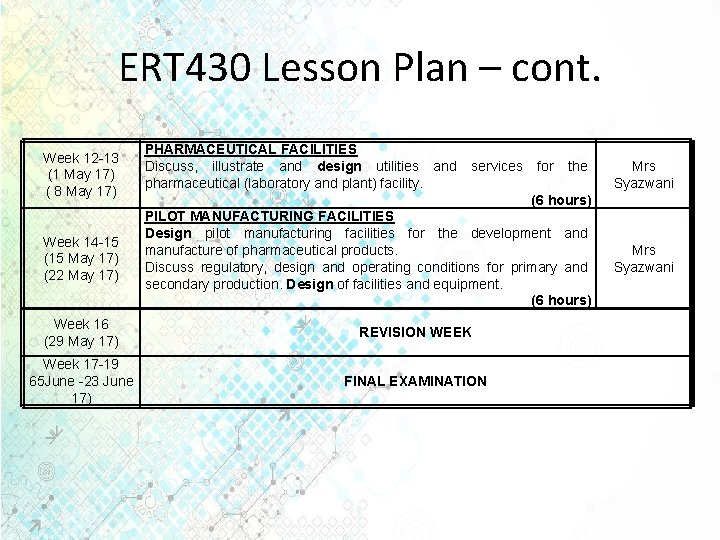
ERT 430 Lesson Plan – cont. Week 12 -13 (1 May 17) ( 8 May 17) Week 14 -15 (15 May 17) (22 May 17) PHARMACEUTICAL FACILITIES Discuss, illustrate and design utilities and services for the pharmaceutical (laboratory and plant) facility. (6 hours) PILOT MANUFACTURING FACILITIES Design pilot manufacturing facilities for the development and manufacture of pharmaceutical products. Discuss regulatory, design and operating conditions for primary and secondary production. Design of facilities and equipment. (6 hours) Week 16 (29 May 17) REVISION WEEK Week 17 -19 65 June -23 June 17) FINAL EXAMINATION Mrs Syazwani

PHARMACEUTICAL PROCESS ENGINEERING CHAPTER 1 – INTRODUCTION MRS ANIS ATIKAH AHMAD anisatikah@unimap. edu. my

PHARMACEUTICAL ENGINEERING • Pharmaceutical process engineering: involves the manufacturing process of pharmaceuticals and related therapies. • Pharmaceutical engineers: are the professionals who help develop the manufacturing plants and design pharmaceutical products.

DEFINITION OF DRUG • Substances intended for use in the diagnosis, cure, mitigation or prevention of disease in man or animals; • substances (other than food) intended to affect the structure or any function of the body of man or animals; • substances intended for use as a component of any substances specified above but does not include devices or their components, parts or accessories

TRADITIONAL VS MODERN WAY OF DRUG USAGE & PRODUCTION 1. TRADITIONAL WAY: • Trial & error : by looking at the effect of consumption • Discovered that small quantities of drugs are useful and larger quantities intake are not necessarily better (usually harmful) • Direct consumption of medication: coca leaves (cocaine), poppy juice (morphine).

TRADITIONAL VS MODERN WAY OF DRUG USAGE & PRODUCTION MODERN WAY: • Needs to follow a path or process before can be sold • Once an active product has been discovered and proven to be medically effective, the manufacturer has to produce the active ingredient and process it into the most suitable dosage form • Very costly: can cost up to 300 million USD/drug (involves R&D, manufacturing, distribution, marketing & sales. )

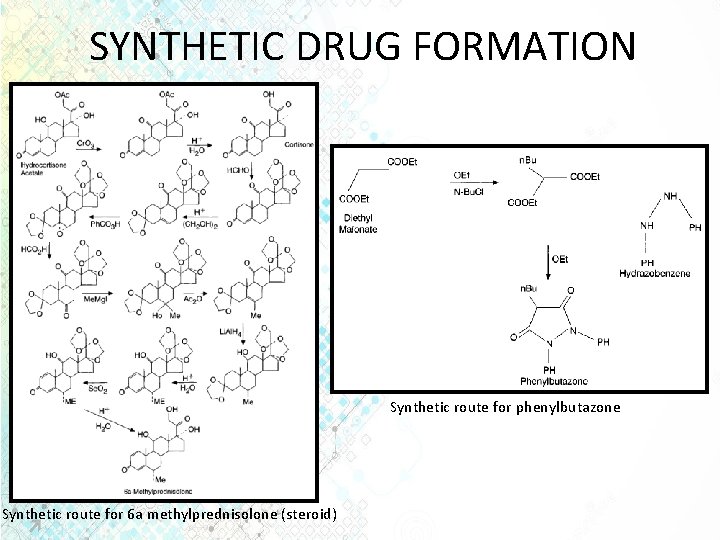
SYNTHETIC DRUG FORMATION Synthetic route for phenylbutazone Synthetic route for 6 a methylprednisolone (steroid)

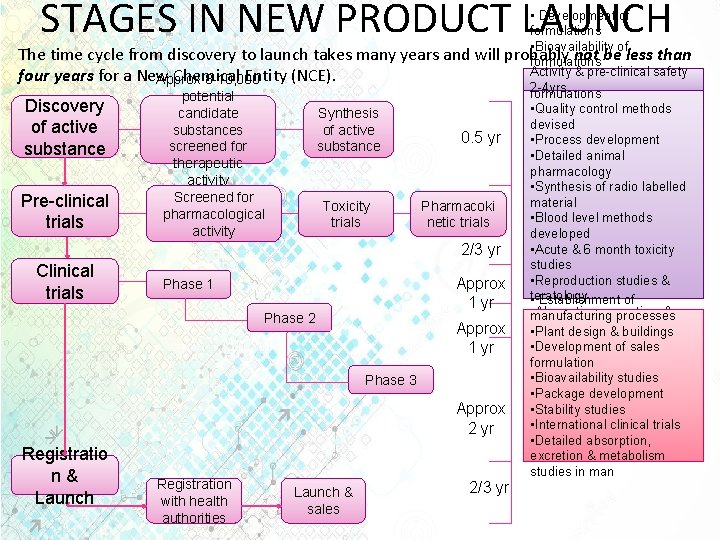
STAGES IN NEW PRODUCT LAUNCH • Development of formulations • Bioavailability of The time cycle from discovery to launch takes many years and will probably not be less than formulations Activity & pre-clinical safety four years for a New Chemical Entity (NCE). • Stability tests on drugs & Approx 8 -10, 000 2 -4 yrs formulations potential Discovery • Quality control methods Synthesis candidate devised of active substances 0. 5 yr • Process development substance screened for substance • Detailed animal therapeutic pharmacology activity • Synthesis of radio labelled Screened for material Pre-clinical Toxicity Pharmacoki pharmacological • Blood level methods trials netic trials activity developed • Acute & 6 month toxicity 2/3 yr studies Clinical Approx • Reproduction studies & Phase 1 trials teratology • Establishment of 1 yr • manufacturing processes Absorption, excretion, & Phase 2 Approx metabolism on animal • Plant design & buildings species • Development of sales 1 yr • formulation Outline clinical trial programmes • Bioavailability studies Phase 3 • Package development Approx • Stability studies • International clinical trials 2 yr • Detailed absorption, Registratio excretion & metabolism studies in man n & Registration 2/3 yr Launch & Launch with health sales authorities

Pre-Clinical Testing (on animal) • it is used as an aid to assessing whether initial human studies will be acceptably safe, and • to predict therapeutic activity of the drug • If the drug looks promising, human clinical studies are proposed.

Clinical Testing • PHASE 1: includes the initial introduction of an investigational drug into humans and consists of short-term studies in a small number of healthy subjects, or patients with the target disease, to determine the metabolism and basic pharmacological and toxicological properties of the drug, and if possible, to obtain preliminary evidence of effectiveness. • PHASE 2: consists of larger, more detailed studies; usually including the first controlled clinical studies intended to assess the effectiveness of the drug and to determine the common short-term side effects and risks of the drug. • PHASE 3 studies are expanded controlled and uncontrolled trials. They are performed after preliminary evidence of effectiveness has been established and are designed to gather the additional information necessary to evaluate the overall benefit-risk relationship of the drug and to provide an adequate basis for professional labelling.

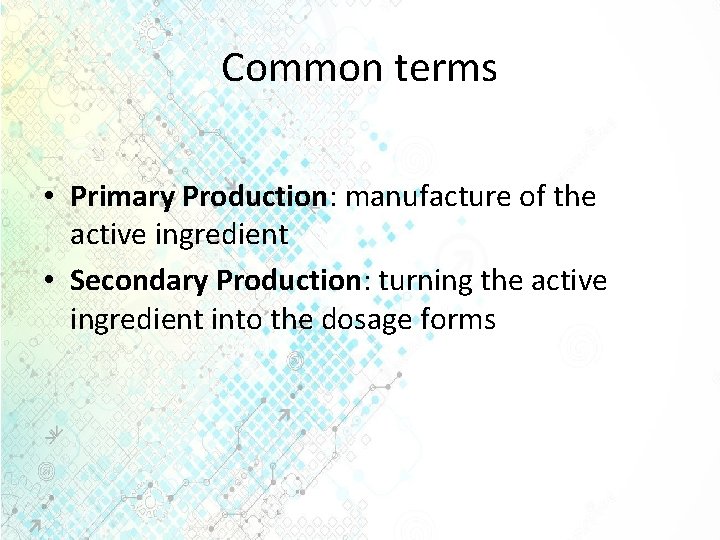
Common terms • Primary Production: manufacture of the active ingredient • Secondary Production: turning the active ingredient into the dosage forms

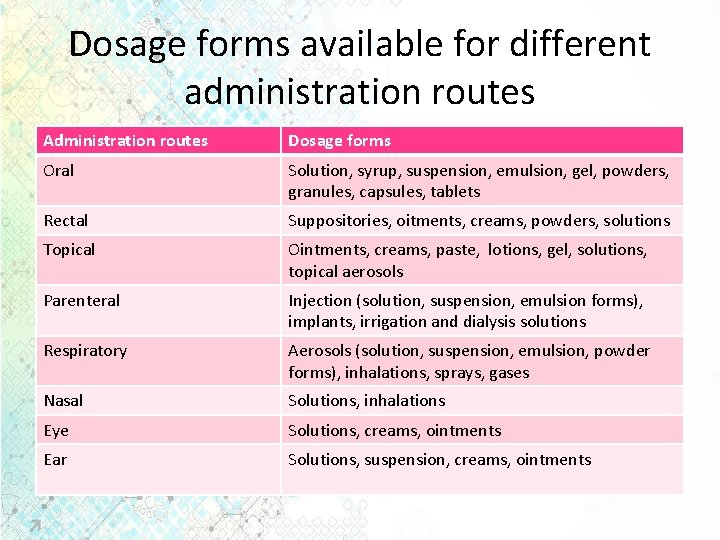
Dosage forms available for different administration routes Administration routes Dosage forms Oral Solution, syrup, suspension, emulsion, gel, powders, granules, capsules, tablets Rectal Suppositories, oitments, creams, powders, solutions Topical Ointments, creams, paste, lotions, gel, solutions, topical aerosols Parenteral Injection (solution, suspension, emulsion forms), implants, irrigation and dialysis solutions Respiratory Aerosols (solution, suspension, emulsion, powder forms), inhalations, sprays, gases Nasal Solutions, inhalations Eye Solutions, creams, ointments Ear Solutions, suspension, creams, ointments

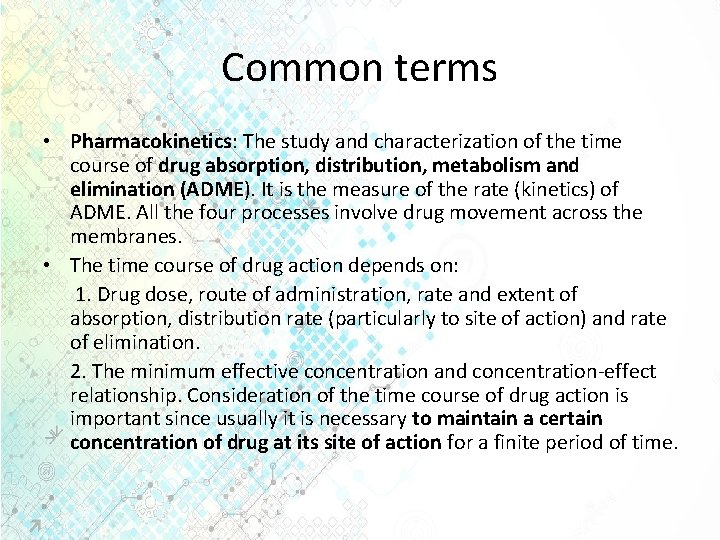
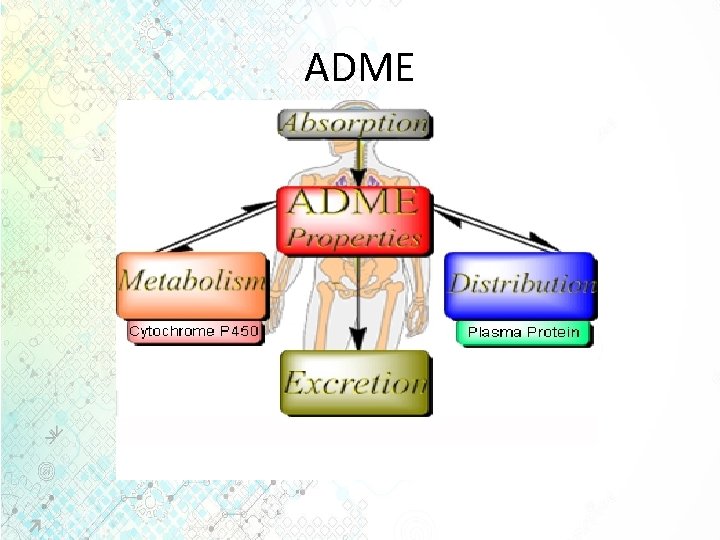
Common terms • Pharmacokinetics: The study and characterization of the time course of drug absorption, distribution, metabolism and elimination (ADME). It is the measure of the rate (kinetics) of ADME. All the four processes involve drug movement across the membranes. • The time course of drug action depends on: 1. Drug dose, route of administration, rate and extent of absorption, distribution rate (particularly to site of action) and rate of elimination. 2. The minimum effective concentration and concentration-effect relationship. Consideration of the time course of drug action is important since usually it is necessary to maintain a certain concentration of drug at its site of action for a finite period of time.

ADME

ADME • Absorption: is the process by which a drug enters the bloodstream without being chemically altered. • Absorption is the movement of a drug from its site of administration into the blood. Most drugs are absorbed by passive absorption but some drugs need carrier mediated transport. Small molecules diffuse more rapidly than large molecules. • Most drugs are absorbed in small intestine.

ADME • Distribution is the movement of drugs throughout the body. Determined by the blood flow to the tissues, it is ability of the drug to enter the vasculature system and the ability of the drug to enter the cell if required.

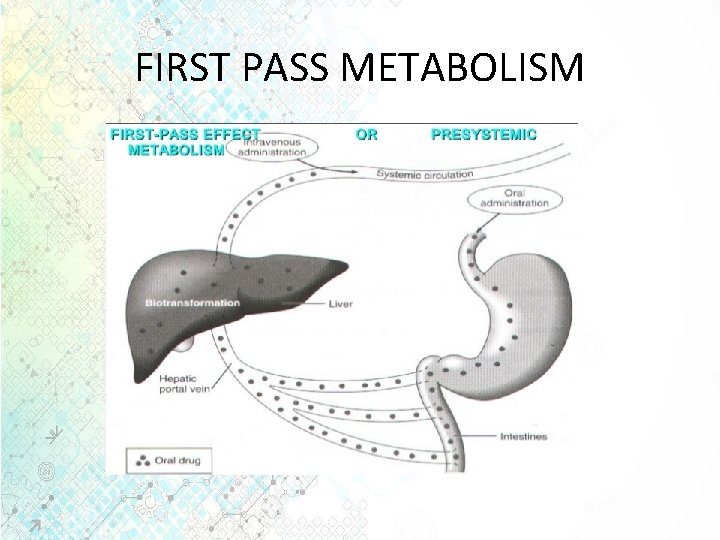
ADME • Metabolism or Biotransformation • It is the process of transformation of a drug within the body to make it more hydrophilic so that it can be excreted out from the body by the kidneys. This needs to be done since drugs and chemicals are foreign substances in our body. • Metabolism is one of the most important mechanisms that the body has for detoxifying and eliminating drugs and other foreign substances. • Drugs delivered by the oral route must pass through the liver before reaching the general circulation. • Metabolism at this point is called “first-pass metabolism, ” which can limit systemic exposure for drugs despite good absorption. • The greater the first pass effect, the lesser the drug that will reach the systemic circulation when the drug is administered orally.

FIRST PASS METABOLISM

FIRST PASS METABOLISM

ADME • Elimination/Excretion • Excretion is the removal of the substance from the body. Some drugs are either excreted out unchanged or some are excreted out as metabolites in urine or bile. • Drugs may also leave the body by natural routes such as tears, sweat, breath and saliva. Patients with kidney or liver problem can have elevated levels of drug in the system and it may be necessary to monitor the dose of the drug appropriately since a high dose in the blood can lead to drug toxicity.

Common Terms (Cont. ) • Bioavailability: The relative amount/percentage/fraction of an administered dose of a particular drug that reaches the systemic circulation in unchanged form and the rate at which this occurs (the rate and extent of drug absorption). (the amount of drug that is available to the body to produce a therapeutic effect. ) • Half life of a drug : is the time for the drug to decrease to half of its concentration. • Minimum effective concentration: below which there will be no therapeutic effect. • Maximum safe concentration: above which there will be a toxic effect. The larger therapeutic index the more safer the drug. • Onset of action : it is the time taken for the drug to reach the minimum effective concentration after a drug has been administered. • Duration of action: is the length of time the drug has a pharmacological action.

Tablet / Pill

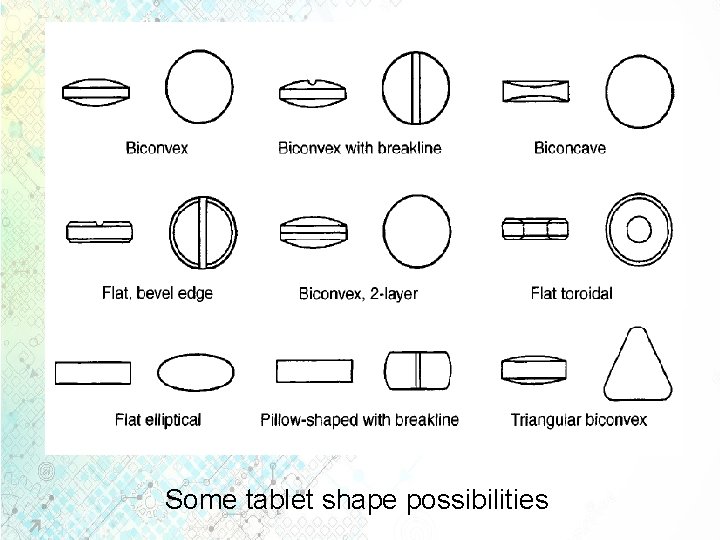
Some tablet shape possibilities

Capsules

Pellets and other extrudates

THANK YOU

QUIZ 1 1. After administering a drug, the time when the body first starts to respond to the medication is called: A. Plateau B. Peak plasma level C. Onset of action D. Drug half-life 2. Two most important sites for drug elimination: A) pulmonary and liver B) liver and gastrointestinal tract C) kidney and liver D) skin and liver E) pulmonary and kidney 3. Parenteral administration: A) Cannot be used with unconsciousness patients B) Generally results in a less accurate dosage than oral administration C) Usually produces a more rapid response than oral administration D) Is too slow for emergency use

QUIZ 1 4. Which route of drug administration is most likely to lead to the first-pass effect? A) Sublingual B) Oral C) Intravenous D) Intramuscular 5. Pharmacokinetics is: A) The study of biological and therapeutic effects of drugs B) The study of absorption, distribution, metabolism and excretion of drugs C) The study of mechanisms of drug action D) The study of methods of new drug development 6. What does the term “bioavailability” mean? A) Plasma protein binding degree of substance B) Permeability through the brain-blood barrier C) Fraction of an uncharged drug reaching the systemic circulation following any route administration D) Amount of a substance in urine relative to the initial doze

REFERENCES • Goodman & Gilman’s The Pharmacological Basis of Therapeutics by. Joel Griffith Hardman, Lee E. Limbird, Alfred G. Gilman. 10 th Ed. • Rang & Dale’s Pharmacology by. Humphrey Rang, Maureen Dale, James Ritter, Rod Flower. 6 th Ed. • PK/DB – Database for Pharmacokinetic Properties – IFSC/USP (URL= http: //miro. ifsc. usp. br/pkdb/) accessed