1 Chromatography Affinity Chromatography This technique takes advantage









- Slides: 18


1. Chromatography의 종류 ◎ Affinity Chromatography – This technique takes advantage of the high affinity of many proteins for specific groups. Specific binding affinity ◎ Ion-Exchange Chromatography – Proteins can be separated on the basis of their net charge by ion-exchange chromatography. Charge ◎ Gel Filtration Chromatography – More discriminating separations on the basis of size can be achieved by the technique of gel filtration chromatography. Size

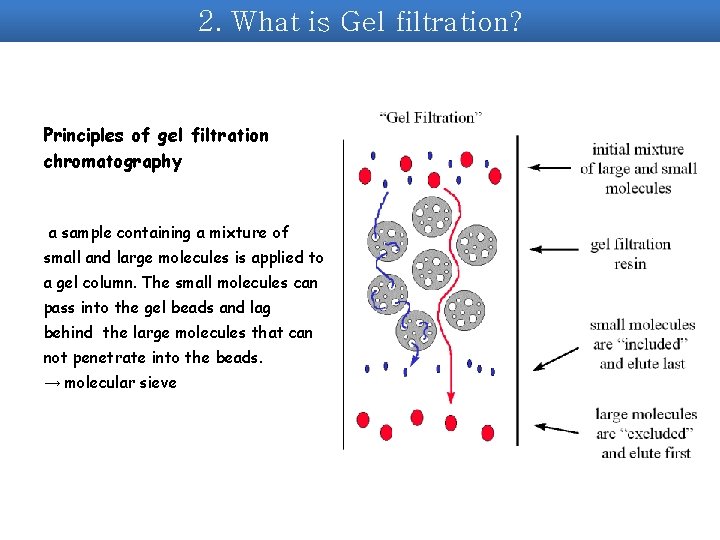
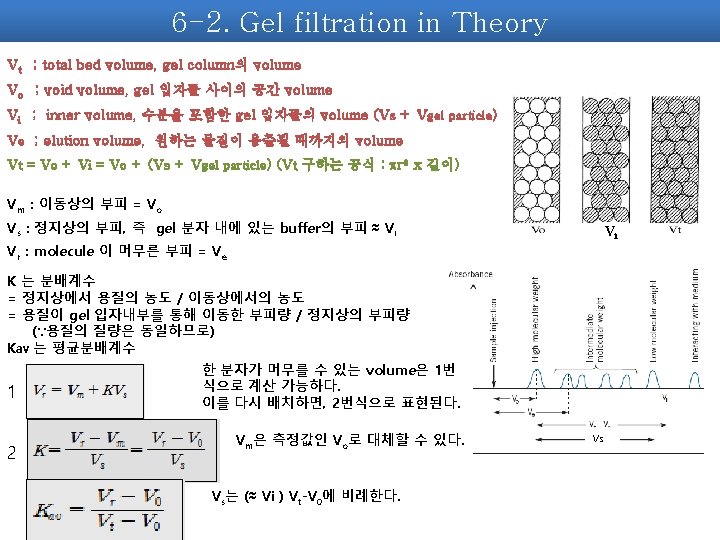
2. What is Gel filtration? Principles of gel filtration chromatography a sample containing a mixture of small and large molecules is applied to a gel column. The small molecules can pass into the gel beads and lag behind the large molecules that can not penetrate into the beads. → molecular sieve

3. What is resin? Gel is the stationary phase in the chromatographic bed. This has been cross-linked to form a three-dimensional network. Instead of dissolving in the liquid, they swell, taking up large amounts of liquid. 1. The requirements of gel filtration gel a. Chemical inertness and stability b. Low content of ionic groups c. Uniform pore and particle size d. High mechanical rigidity

3. What is resin? 2. 다양한 Gel 성분 a. Dextran gels b. Polyacrylamide gels c. Agar and agarose gels 3. Sephacryl gel This makes it possible to work small particles. Sephacryl is intended for use mainly with aqueous eluants. Gel filtration에 사용되는 gel의 종류

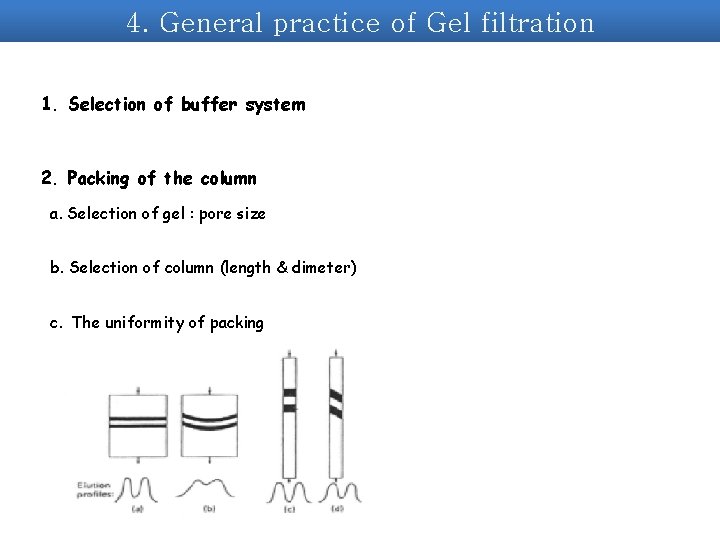
4. General practice of Gel filtration 1. Selection of buffer system 2. Packing of the column a. Selection of gel : pore size b. Selection of column (length & dimeter) c. The uniformity of packing

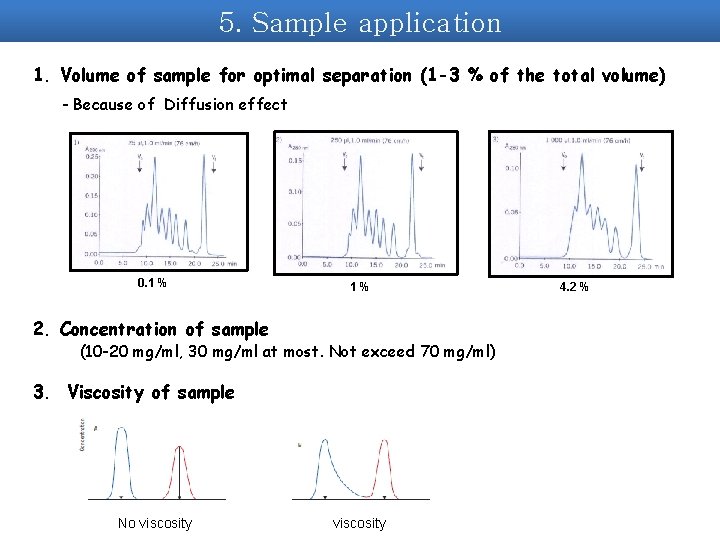
5. Sample application 1. Volume of sample for optimal separation (1 -3 % of the total volume) - Because of Diffusion effect 0. 1 % 1% 2. Concentration of sample (10 -20 mg/ml, 30 mg/ml at most. Not exceed 70 mg/ml) 3. Viscosity of sample No viscosity 4. 2 %



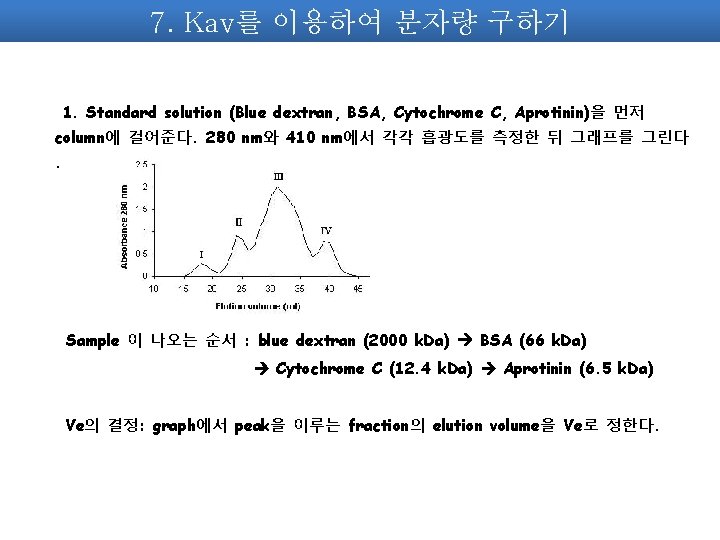
7. Kav를 이용하여 분자량 구하기 1. Standard solution (Blue dextran, BSA, Cytochrome C, Aprotinin)을 먼저 column에 걸어준다. 280 nm와 410 nm에서 각각 흡광도를 측정한 뒤 그래프를 그린다. Sample 이 나오는 순서 : blue dextran (2000 k. Da) BSA (66 k. Da) Cytochrome C (12. 4 k. Da) Aprotinin (6. 5 k. Da) Ve의 결정: graph에서 peak을 이루는 fraction의 elution volume을 Ve로 정한다.

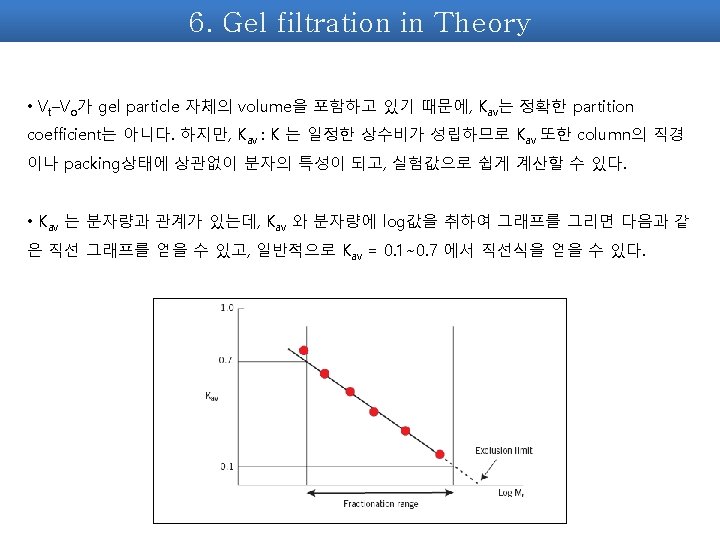
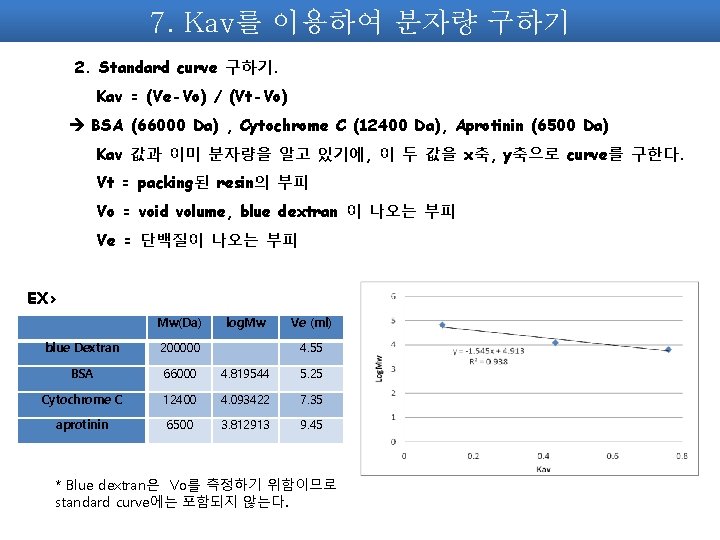
7. Kav를 이용하여 분자량 구하기 2. Standard curve 구하기. Kav = (Ve-Vo) / (Vt-Vo) BSA (66000 Da) , Cytochrome C (12400 Da), Aprotinin (6500 Da) Kav 값과 이미 분자량을 알고 있기에, 이 두 값을 x축, y축으로 curve를 구한다. Vt = packing된 resin의 부피 Vo = void volume, blue dextran 이 나오는 부피 Ve = 단백질이 나오는 부피 EX> Mw(Da) log. Mw Ve (ml) blue Dextran 200000 4. 55 BSA 66000 4. 819544 5. 25 Cytochrome C 12400 4. 093422 7. 35 aprotinin 6500 3. 812913 9. 45 * Blue dextran은 Vo를 측정하기 위함이므로 standard curve에는 포함되지 않는다.






