1 Chapter Thirteen Chemical Kinetics Rates and Mechanisms
![11 The Rate Law Rate = k[A]1 = k[A] Reaction is first order in 11 The Rate Law Rate = k[A]1 = k[A] Reaction is first order in](https://slidetodoc.com/presentation_image_h/2e24fd0ee7eb49f9f6f7b186271f3ef3/image-11.jpg)
![22 A Zero-Order Reaction rate = k[A]0 =k Rate is independent of initial concentration 22 A Zero-Order Reaction rate = k[A]0 =k Rate is independent of initial concentration](https://slidetodoc.com/presentation_image_h/2e24fd0ee7eb49f9f6f7b186271f3ef3/image-22.jpg)
![25 Example 13. 8 A Conceptual Example Shown here are graphs of [A] versus 25 Example 13. 8 A Conceptual Example Shown here are graphs of [A] versus](https://slidetodoc.com/presentation_image_h/2e24fd0ee7eb49f9f6f7b186271f3ef3/image-25.jpg)
- Slides: 50

1 Chapter Thirteen Chemical Kinetics: Rates and Mechanisms of Chemical Reactions Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

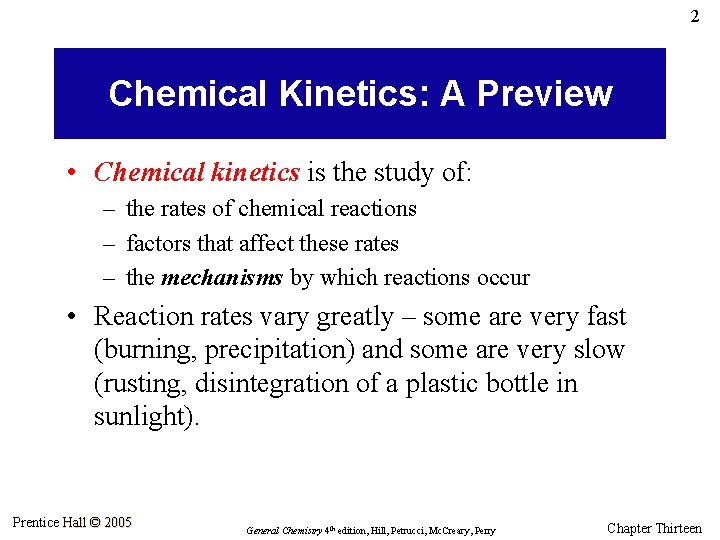
2 Chemical Kinetics: A Preview • Chemical kinetics is the study of: – the rates of chemical reactions – factors that affect these rates – the mechanisms by which reactions occur • Reaction rates vary greatly – some are very fast (burning, precipitation) and some are very slow (rusting, disintegration of a plastic bottle in sunlight). Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

3 Variables in Reaction Rates • Concentrations of reactants: Reaction rates generally increase as the concentrations of the reactants are increased. • Temperature: Reaction rates generally increase rapidly as the temperature is increased. • Surface area: For reactions that occur on a surface rather than in solution, the rate increases as the surface area is increased. • Catalysts: Catalysts speed up reactions and inhibitors slow them down. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

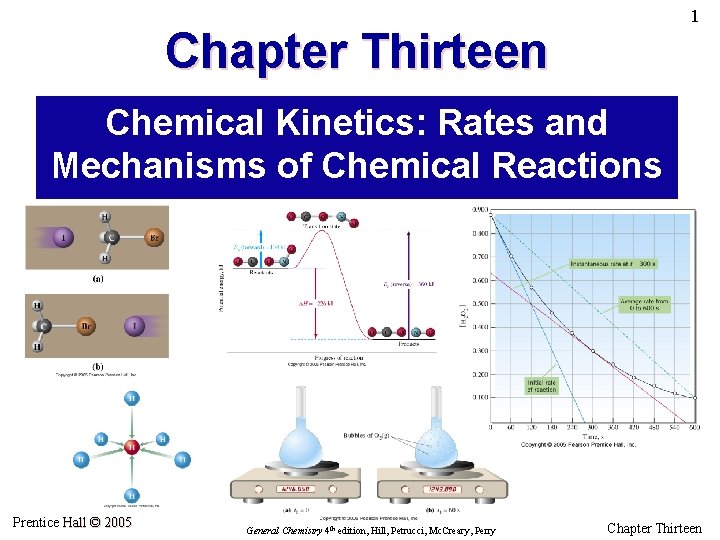
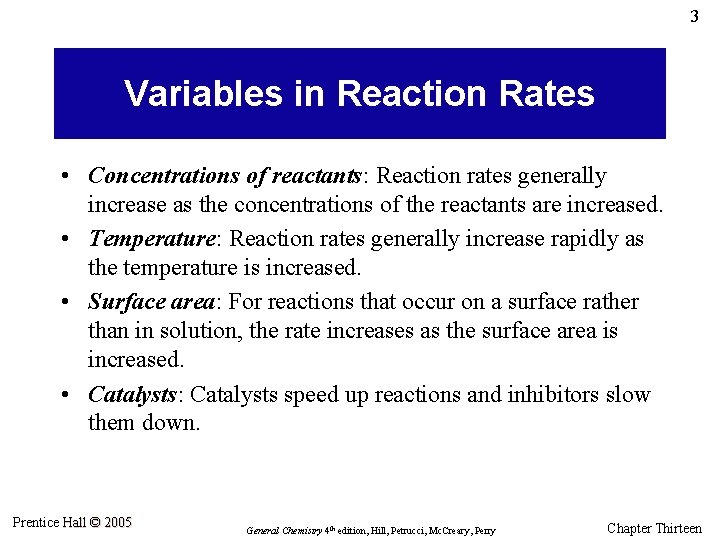
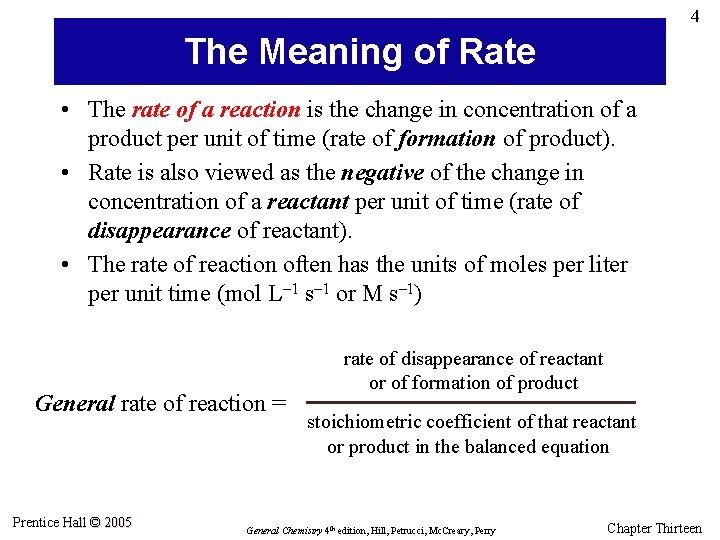
4 The Meaning of Rate • The rate of a reaction is the change in concentration of a product per unit of time (rate of formation of product). • Rate is also viewed as the negative of the change in concentration of a reactant per unit of time (rate of disappearance of reactant). • The rate of reaction often has the units of moles per liter per unit time (mol L– 1 s– 1 or M s– 1) General rate of reaction = Prentice Hall © 2005 rate of disappearance of reactant or of formation of product stoichiometric coefficient of that reactant or product in the balanced equation General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

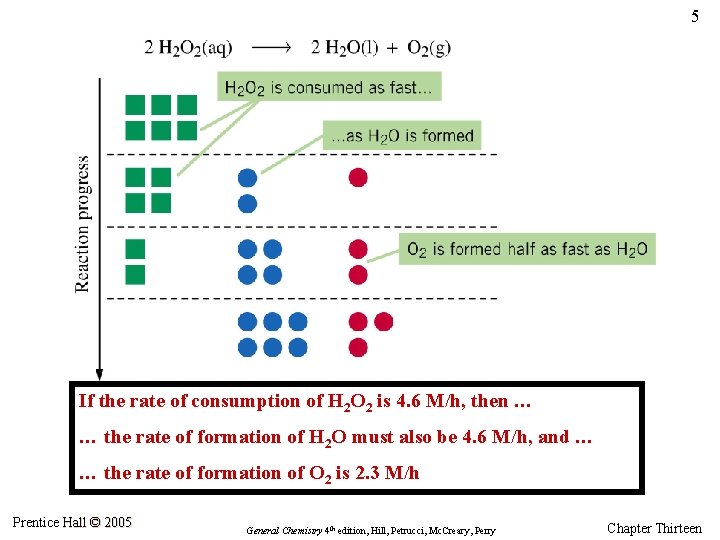
5 If the rate of consumption of H 2 O 2 is 4. 6 M/h, then … … the rate of formation of H 2 O must also be 4. 6 M/h, and … … the rate of formation of O 2 is 2. 3 M/h Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

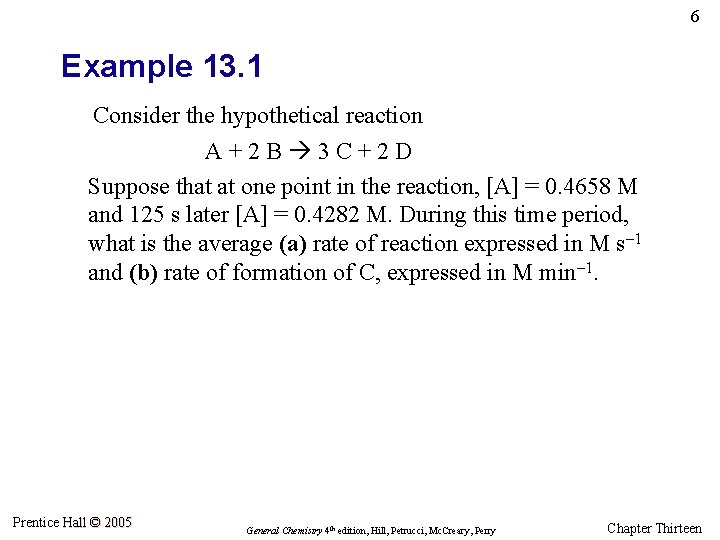
6 Example 13. 1 Consider the hypothetical reaction A+2 B 3 C+2 D Suppose that at one point in the reaction, [A] = 0. 4658 M and 125 s later [A] = 0. 4282 M. During this time period, what is the average (a) rate of reaction expressed in M s– 1 and (b) rate of formation of C, expressed in M min– 1. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

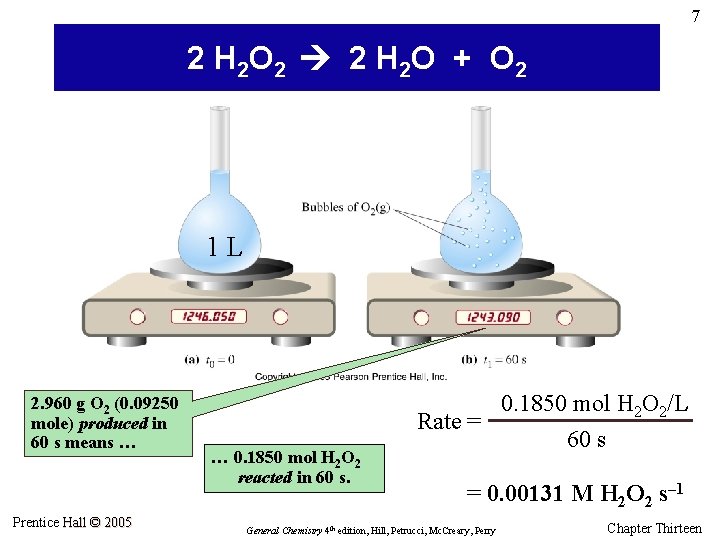
7 2 H 2 O 2 2 H 2 O + O 2 1 L 2. 960 g O 2 (0. 09250 mole) produced in 60 s means … Prentice Hall © 2005 … 0. 1850 mol H 2 O 2 reacted in 60 s. 0. 1850 mol H 2 O 2/L Rate = 60 s = 0. 00131 M H 2 O 2 s– 1 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

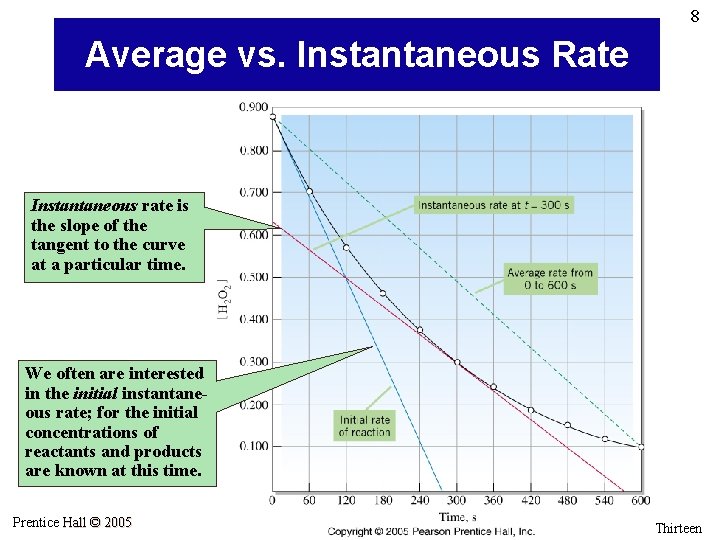
8 Average vs. Instantaneous Rate Instantaneous rate is the slope of the tangent to the curve at a particular time. We often are interested in the initial instantaneous rate; for the initial concentrations of reactants and products are known at this time. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

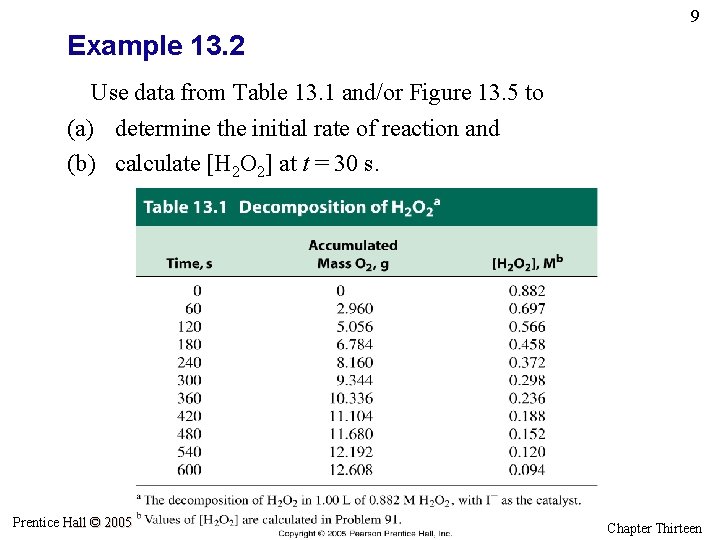
9 Example 13. 2 Use data from Table 13. 1 and/or Figure 13. 5 to (a) determine the initial rate of reaction and (b) calculate [H 2 O 2] at t = 30 s. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

10 The Rate Law of a Chemical Reaction • The rate law for a chemical reaction relates the rate of reaction to the concentrations of reactants. a. A + b. B + c. C … products rate = k[A]n[B]m[C]p … • The exponents (m, n, p…) are determined by experiment. • Exponents are not derived from the coefficients in the balanced chemical equation, though in some instances the exponents and the coefficients may be the same. • The value of an exponent in a rate law is the order of the reaction with respect to the reactant in question. • The proportionality constant, k, is the rate constant. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen
![11 The Rate Law Rate kA1 kA Reaction is first order in 11 The Rate Law Rate = k[A]1 = k[A] Reaction is first order in](https://slidetodoc.com/presentation_image_h/2e24fd0ee7eb49f9f6f7b186271f3ef3/image-11.jpg)
11 The Rate Law Rate = k[A]1 = k[A] Reaction is first order in A Rate = k[A]2 Reaction is second order in A Rate = k[A]3 Reaction is third order in A If we triple the concentration of A in a second-order reaction, the rate increases by a factor of ____. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

12 More About the Rate Constant k • The rate of a reaction is the change in concentration with time, whereas the rate constant is the proportionality constant relating reaction rate to the concentrations of reactants. • The rate constant remains constant throughout a reaction, regardless of the initial concentrations of the reactants. • The rate and the rate constant have the same numerical values and units only in zero-order reactions. • For reaction orders other than zero, the rate and rate constant are numerically equal only when the concentrations of all reactants are 1 M. Even then, their units are different. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

13 Method of Initial Rates • The method of initial rates is a method of establishing the rate law for a reaction—finding the values of the exponents in the rate law, and the value of k. • A series of experiments is performed in which the initial concentration of one reactant is varied. Concentrations of the other reactants are held constant. • When we double the concentration of a reactant A, if: – there is no effect on the rate, the reaction is zero-order in A. – the rate doubles, the reaction is first-order in A. – the rate quadruples, the reaction is second-order in A. – the rate increases eight times, the reaction is third-order in A. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

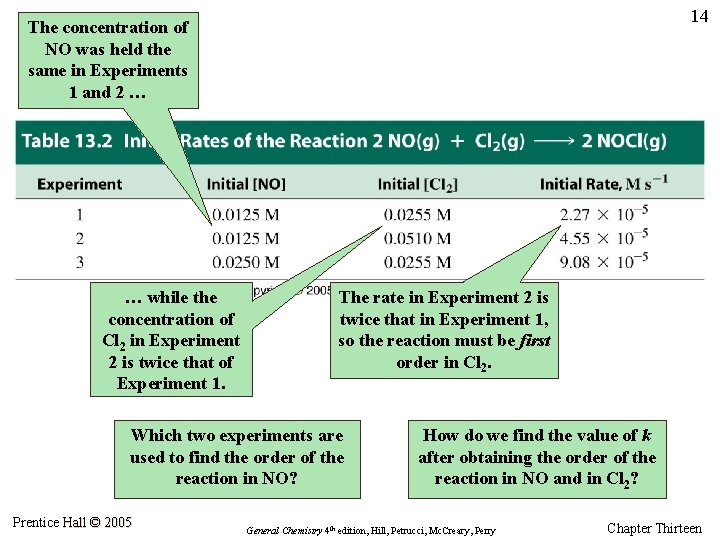
14 The concentration of NO was held the same in Experiments 1 and 2 … … while the concentration of Cl 2 in Experiment 2 is twice that of Experiment 1. The rate in Experiment 2 is twice that in Experiment 1, so the reaction must be first order in Cl 2. Which two experiments are used to find the order of the reaction in NO? Prentice Hall © 2005 How do we find the value of k after obtaining the order of the reaction in NO and in Cl 2? General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

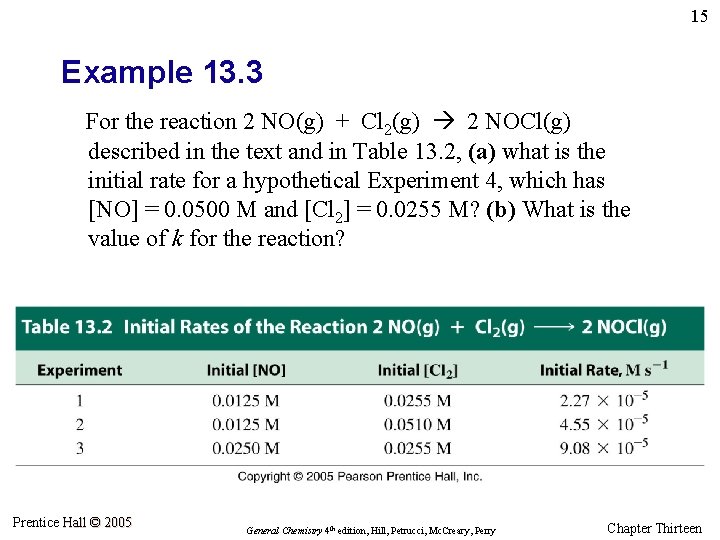
15 Example 13. 3 For the reaction 2 NO(g) + Cl 2(g) 2 NOCl(g) described in the text and in Table 13. 2, (a) what is the initial rate for a hypothetical Experiment 4, which has [NO] = 0. 0500 M and [Cl 2] = 0. 0255 M? (b) What is the value of k for the reaction? Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

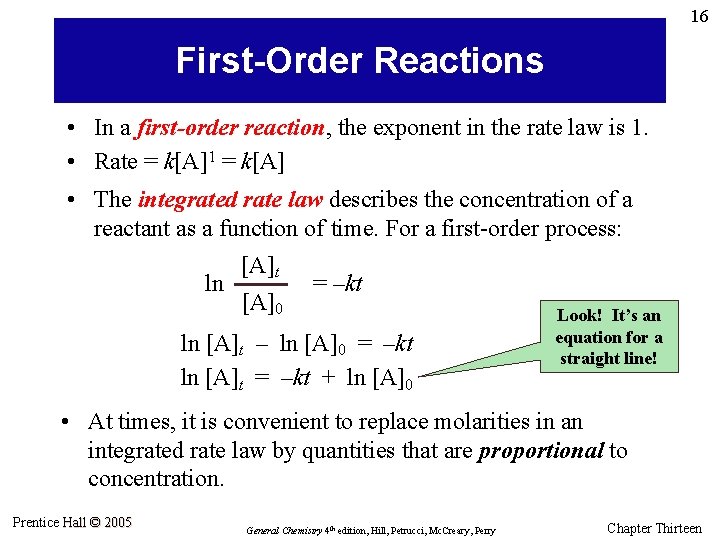
16 First-Order Reactions • In a first-order reaction, the exponent in the rate law is 1. • Rate = k[A]1 = k[A] • The integrated rate law describes the concentration of a reactant as a function of time. For a first-order process: ln [A]t [A]0 = –kt ln [A]t – ln [A]0 = –kt ln [A]t = –kt + ln [A]0 Look! It’s an equation for a straight line! • At times, it is convenient to replace molarities in an integrated rate law by quantities that are proportional to concentration. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

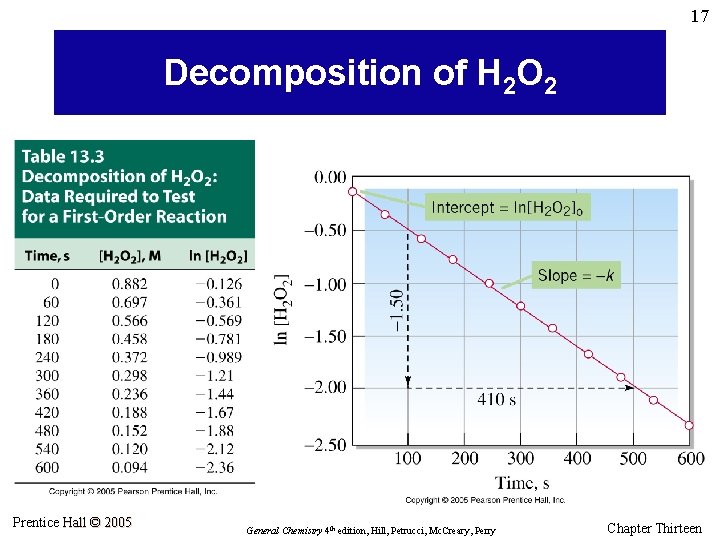
17 Decomposition of H 2 O 2 Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

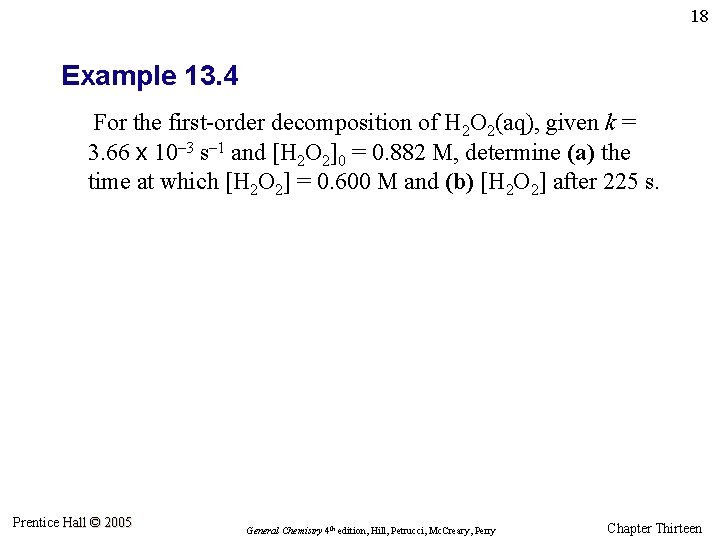
18 Example 13. 4 For the first-order decomposition of H 2 O 2(aq), given k = 3. 66 x 10– 3 s– 1 and [H 2 O 2]0 = 0. 882 M, determine (a) the time at which [H 2 O 2] = 0. 600 M and (b) [H 2 O 2] after 225 s. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

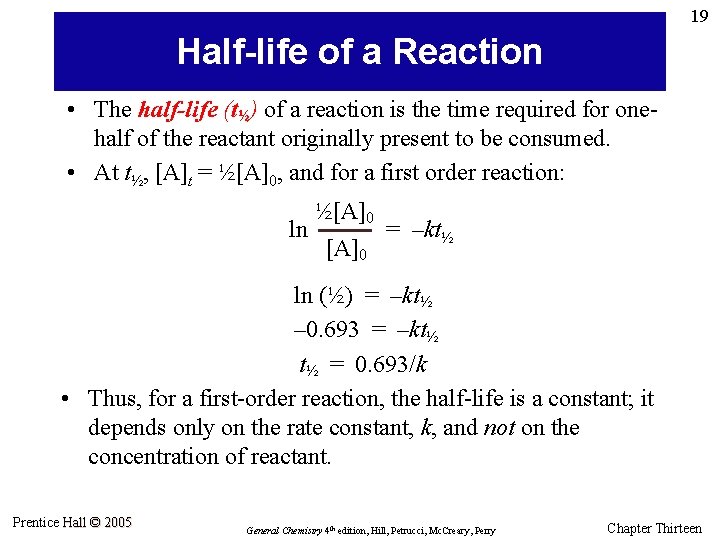
19 Half-life of a Reaction • The half-life (t½) of a reaction is the time required for onehalf of the reactant originally present to be consumed. • At t½, [A]t = ½[A]0, and for a first order reaction: ln ½[A]0 = –kt½ ln (½) = –kt½ – 0. 693 = –kt½ t½ = 0. 693/k • Thus, for a first-order reaction, the half-life is a constant; it depends only on the rate constant, k, and not on the concentration of reactant. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

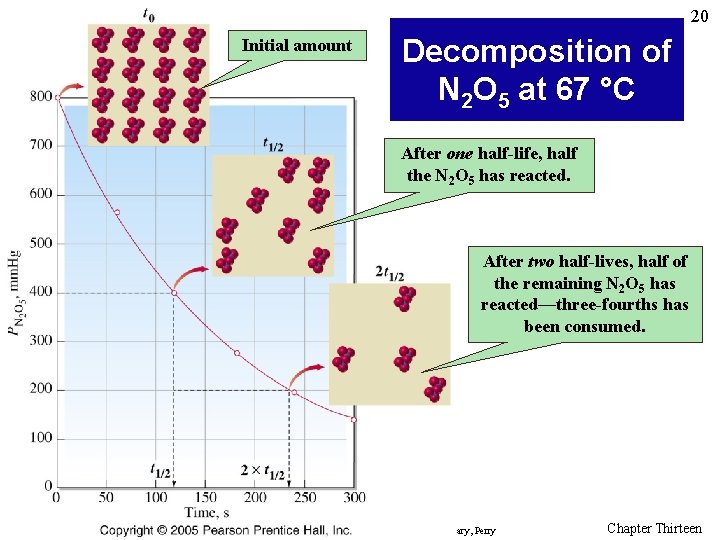
20 Initial amount Decomposition of N 2 O 5 at 67 °C After one half-life, half the N 2 O 5 has reacted. After two half-lives, half of the remaining N 2 O 5 has reacted—three-fourths has been consumed. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

21 Example 13. 5 A Conceptual Example Use data from Figure 13. 7 to evaluate the (a) half-life and (b) rate constant for the first-order decomposition of N 2 O 5 at 67 °C. Example 13. 6 An Estimation Example Which seems like a probable approximate time for 90% of a sample of N 2 O 5 to undergo decomposition at 67 °C: (a) 200 s, (b) 300 s, (c) 400 s, or (d) 500 s? Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen
![22 A ZeroOrder Reaction rate kA0 k Rate is independent of initial concentration 22 A Zero-Order Reaction rate = k[A]0 =k Rate is independent of initial concentration](https://slidetodoc.com/presentation_image_h/2e24fd0ee7eb49f9f6f7b186271f3ef3/image-22.jpg)
22 A Zero-Order Reaction rate = k[A]0 =k Rate is independent of initial concentration Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

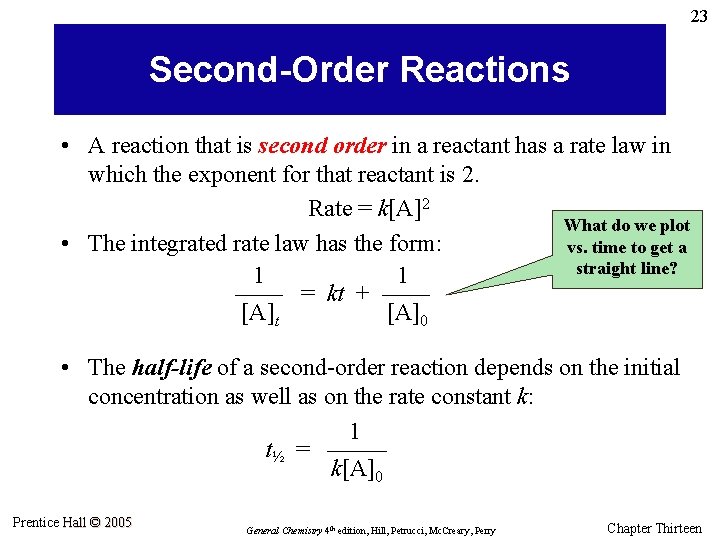
23 Second-Order Reactions • A reaction that is second order in a reactant has a rate law in which the exponent for that reactant is 2. Rate = k[A]2 What do we plot • The integrated rate law has the form: vs. time to get a straight line? 1 1 –––– = kt + –––– [A]t [A]0 • The half-life of a second-order reaction depends on the initial concentration as well as on the rate constant k: 1 t½ = ––––– k[A]0 Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

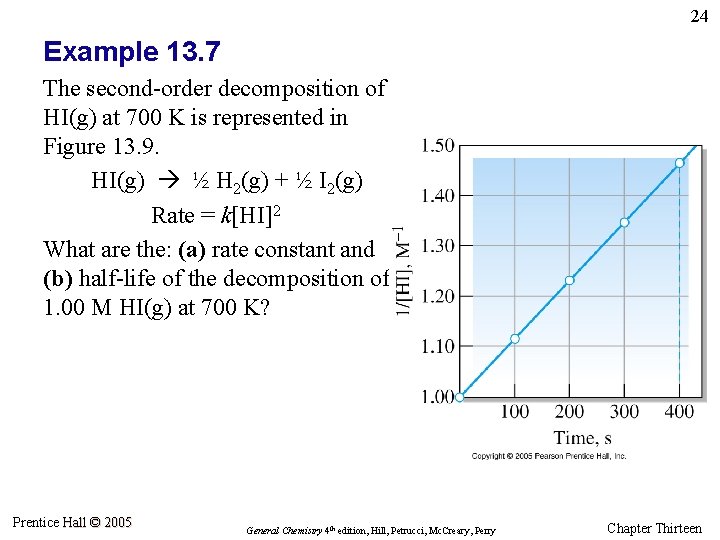
24 Example 13. 7 The second-order decomposition of HI(g) at 700 K is represented in Figure 13. 9. HI(g) ½ H 2(g) + ½ I 2(g) Rate = k[HI]2 What are the: (a) rate constant and (b) half-life of the decomposition of 1. 00 M HI(g) at 700 K? Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen
![25 Example 13 8 A Conceptual Example Shown here are graphs of A versus 25 Example 13. 8 A Conceptual Example Shown here are graphs of [A] versus](https://slidetodoc.com/presentation_image_h/2e24fd0ee7eb49f9f6f7b186271f3ef3/image-25.jpg)
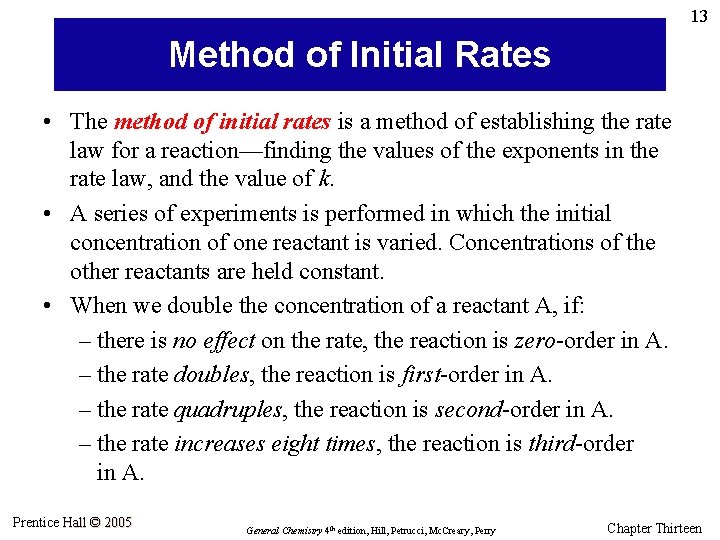
25 Example 13. 8 A Conceptual Example Shown here are graphs of [A] versus time for two different experiments dealing with the reaction A products. What is the order of this reaction? Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

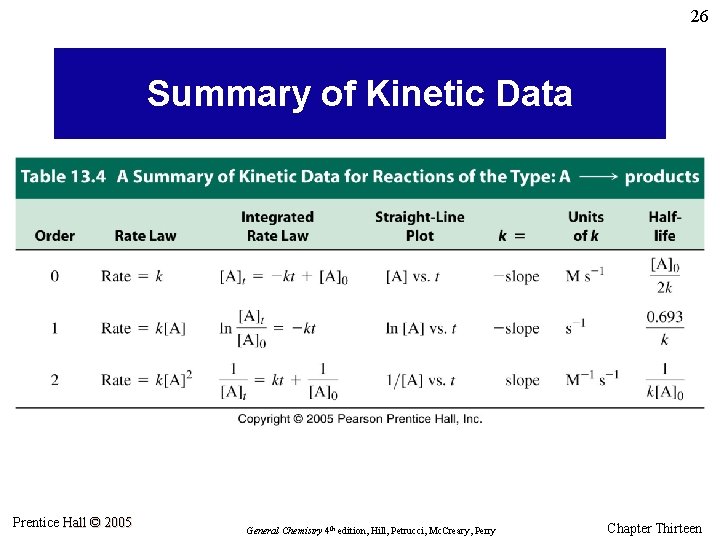
26 Summary of Kinetic Data Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

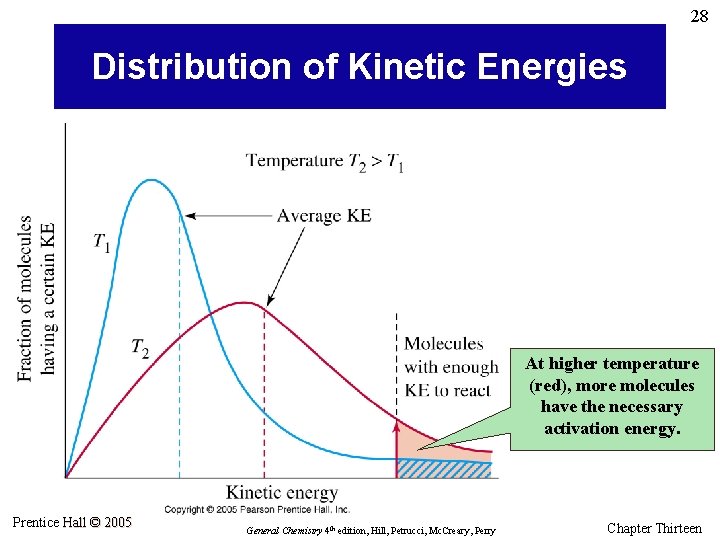
27 Theories of Chemical Kinetics: Collision Theory • Before atoms, molecules, or ions can react, they must first collide. • An effective collision between two molecules puts enough energy into key bonds to break them. • The activation energy (Ea) is the minimum energy that must be supplied by collisions for a reaction to occur. • A certain fraction of all molecules in a sample will have the necessary activation energy to react; that fraction increases with increasing temperature. • The spatial orientations of the colliding species may also determine whether a collision is effective. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

28 Distribution of Kinetic Energies At higher temperature (red), more molecules have the necessary activation energy. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

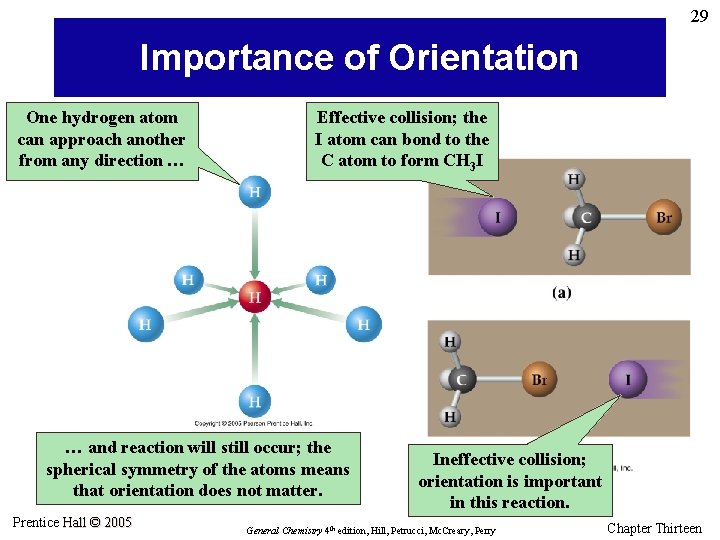
29 Importance of Orientation One hydrogen atom can approach another from any direction … Effective collision; the I atom can bond to the C atom to form CH 3 I … and reaction will still occur; the spherical symmetry of the atoms means that orientation does not matter. Prentice Hall © 2005 Ineffective collision; orientation is important in this reaction. General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

30 Transition State Theory • The configuration of the atoms of the colliding species at the time of the collision is called the transition state. • The transitory species having this configuration is called the activated complex. • A reaction profile shows potential energy plotted as a function of a parameter called the progress of the reaction. • Reactant molecules must have enough energy to surmount the energy “hill” separating products from reactants. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

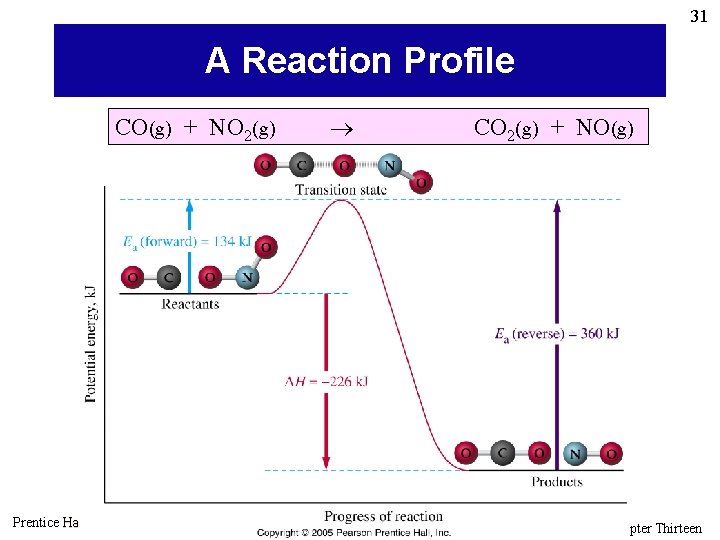
31 A Reaction Profile CO(g) + NO 2(g) Prentice Hall © 2005 CO 2(g) + NO(g) General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

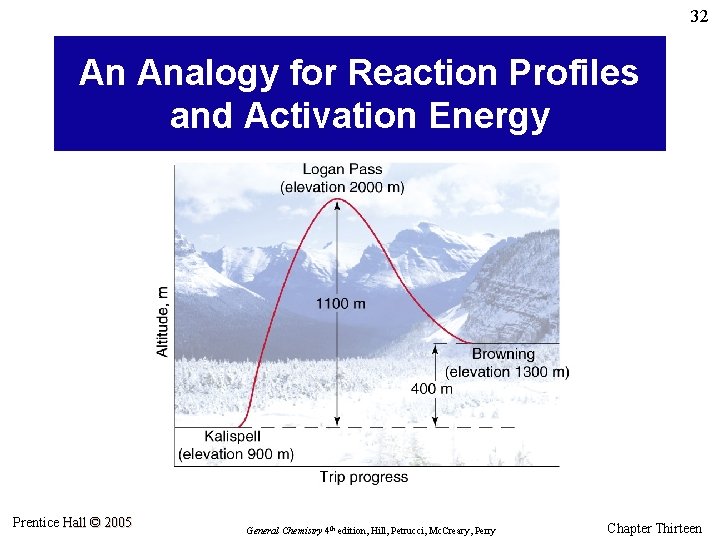
32 An Analogy for Reaction Profiles and Activation Energy Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

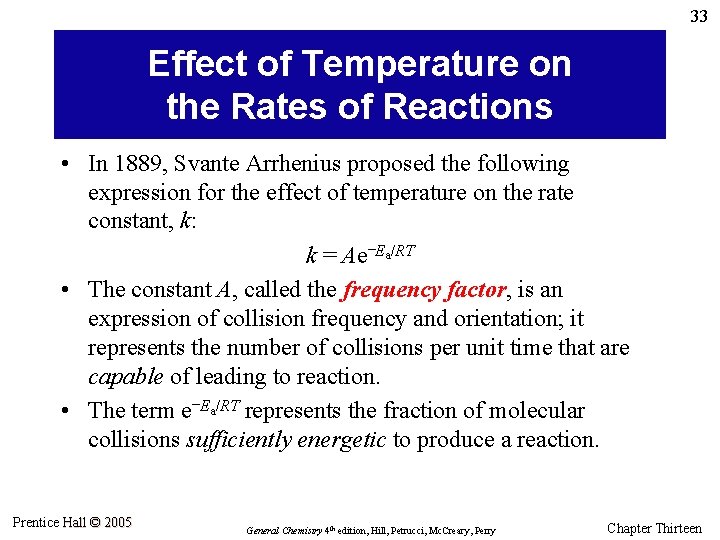
33 Effect of Temperature on the Rates of Reactions • In 1889, Svante Arrhenius proposed the following expression for the effect of temperature on the rate constant, k: k = Ae–Ea/RT • The constant A, called the frequency factor, is an expression of collision frequency and orientation; it represents the number of collisions per unit time that are capable of leading to reaction. • The term e–Ea/RT represents the fraction of molecular collisions sufficiently energetic to produce a reaction. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

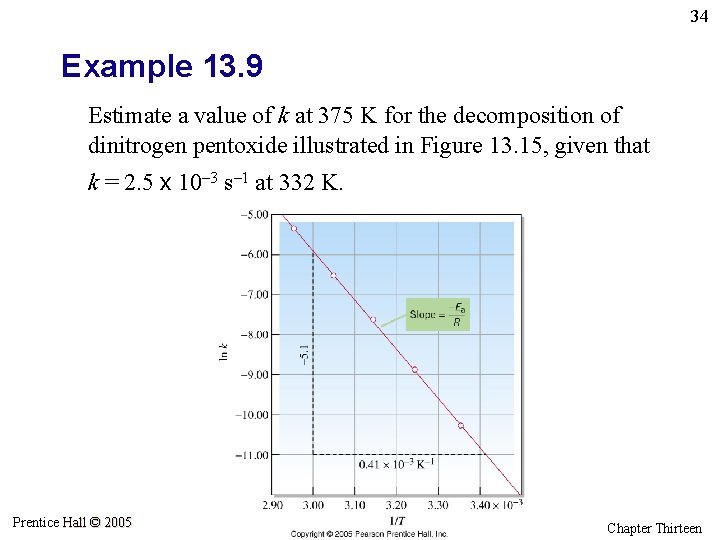
34 Example 13. 9 Estimate a value of k at 375 K for the decomposition of dinitrogen pentoxide illustrated in Figure 13. 15, given that k = 2. 5 x 10– 3 s– 1 at 332 K. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

35 Reaction Mechanisms • Analogy: a banana split is made by steps in sequence: slice banana; three scoops ice cream; chocolate sauce; strawberries; pineapple; whipped cream; end with cherry. • A chemical reaction occurs according to a reaction mechanism—a series of collisions or dissociations—that lead from initial reactants to the final products. • An elementary reaction represents, at the molecular level, a single step in the progress of the overall reaction. • A proposed mechanism must: – account for the experimentally determined rate law. – be consistent with the stoichiometry of the overall or net reaction. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

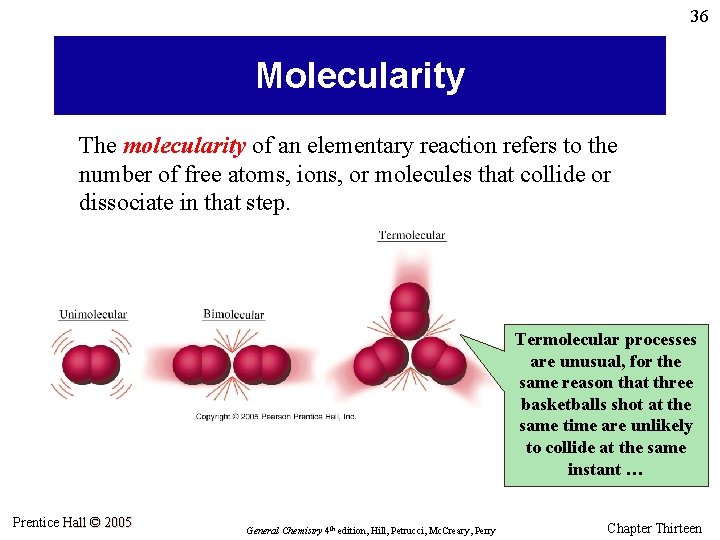
36 Molecularity The molecularity of an elementary reaction refers to the number of free atoms, ions, or molecules that collide or dissociate in that step. Termolecular processes are unusual, for the same reason that three basketballs shot at the same time are unlikely to collide at the same instant … Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

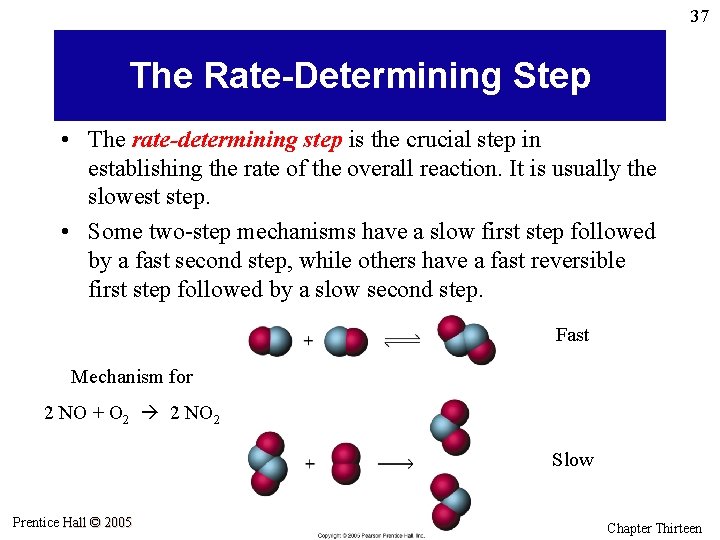
37 The Rate-Determining Step • The rate-determining step is the crucial step in establishing the rate of the overall reaction. It is usually the slowest step. • Some two-step mechanisms have a slow first step followed by a fast second step, while others have a fast reversible first step followed by a slow second step. Fast Mechanism for 2 NO + O 2 2 NO 2 Slow Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

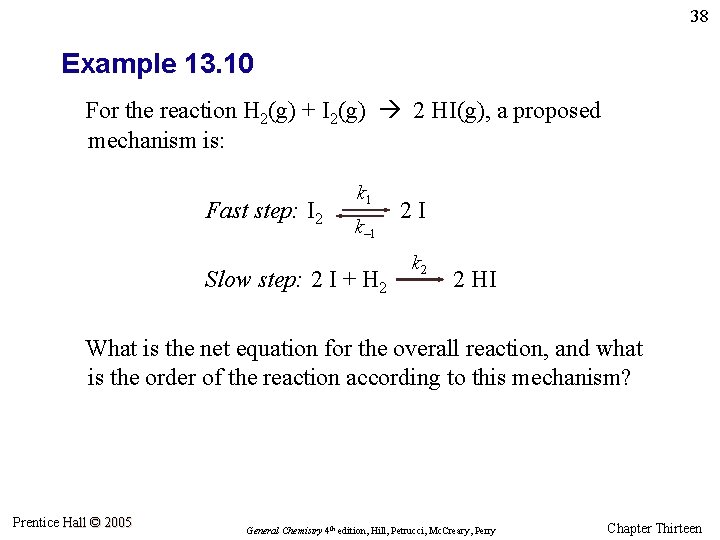
38 Example 13. 10 For the reaction H 2(g) + I 2(g) 2 HI(g), a proposed mechanism is: Fast step: I 2 k 1 k– 1 Slow step: 2 I + H 2 2 I k 2 2 HI What is the net equation for the overall reaction, and what is the order of the reaction according to this mechanism? Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

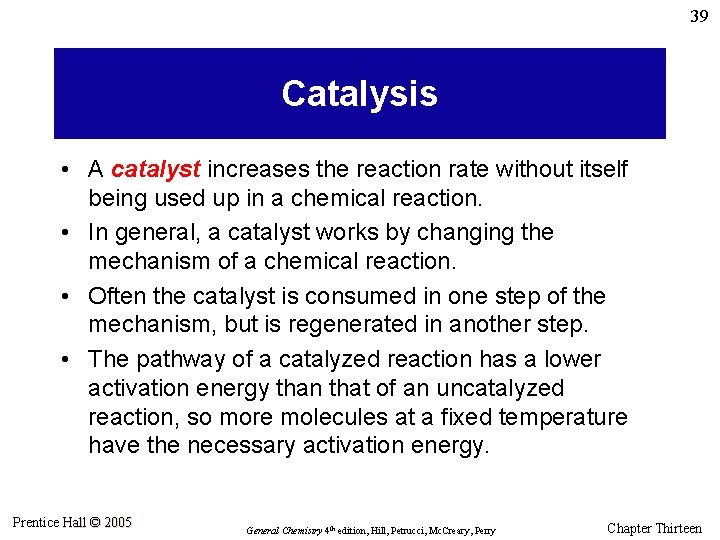
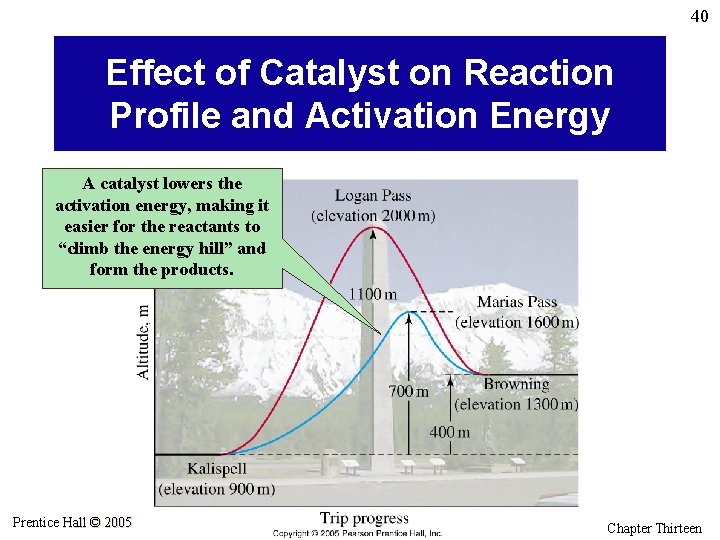
39 Catalysis • A catalyst increases the reaction rate without itself being used up in a chemical reaction. • In general, a catalyst works by changing the mechanism of a chemical reaction. • Often the catalyst is consumed in one step of the mechanism, but is regenerated in another step. • The pathway of a catalyzed reaction has a lower activation energy than that of an uncatalyzed reaction, so more molecules at a fixed temperature have the necessary activation energy. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

40 Effect of Catalyst on Reaction Profile and Activation Energy A catalyst lowers the activation energy, making it easier for the reactants to “climb the energy hill” and form the products. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

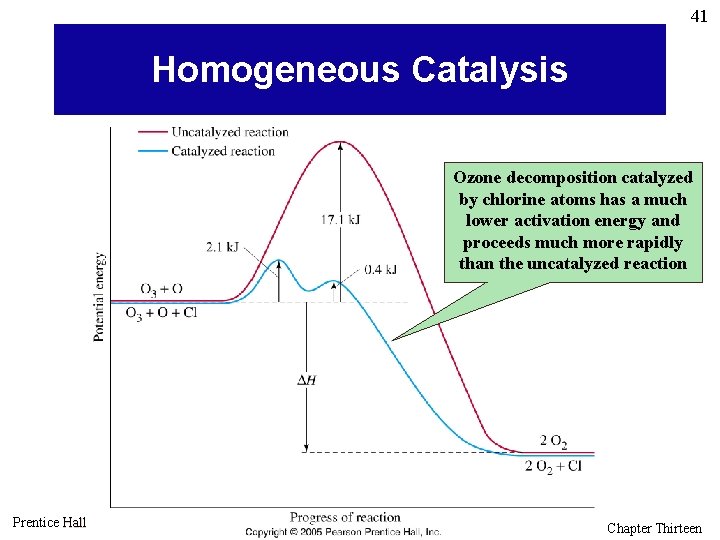
41 Homogeneous Catalysis Ozone decomposition catalyzed by chlorine atoms has a much lower activation energy and proceeds much more rapidly than the uncatalyzed reaction Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

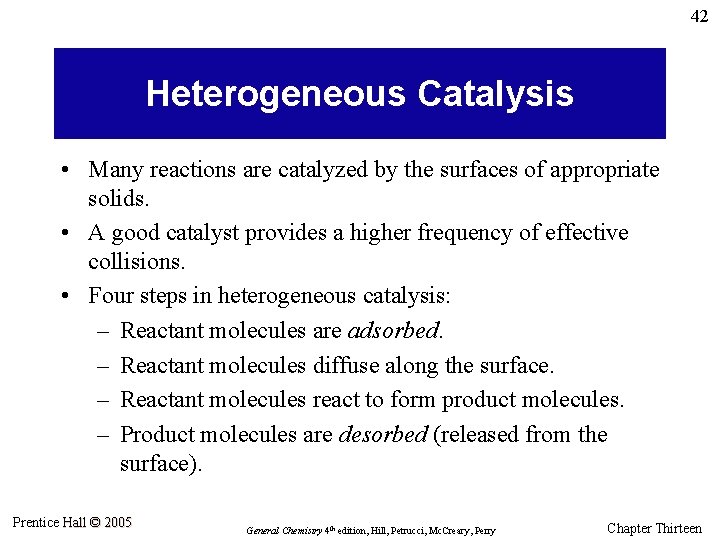
42 Heterogeneous Catalysis • Many reactions are catalyzed by the surfaces of appropriate solids. • A good catalyst provides a higher frequency of effective collisions. • Four steps in heterogeneous catalysis: – Reactant molecules are adsorbed. – Reactant molecules diffuse along the surface. – Reactant molecules react to form product molecules. – Product molecules are desorbed (released from the surface). Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

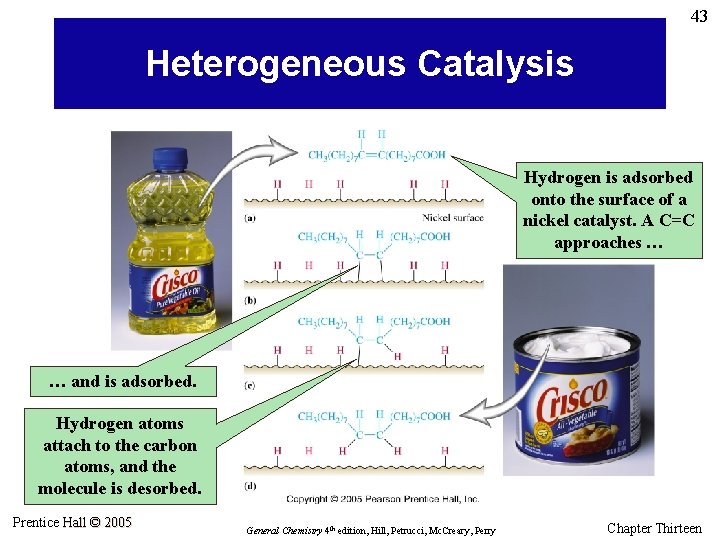
43 Heterogeneous Catalysis Hydrogen is adsorbed onto the surface of a nickel catalyst. A C=C approaches … … and is adsorbed. Hydrogen atoms attach to the carbon atoms, and the molecule is desorbed. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

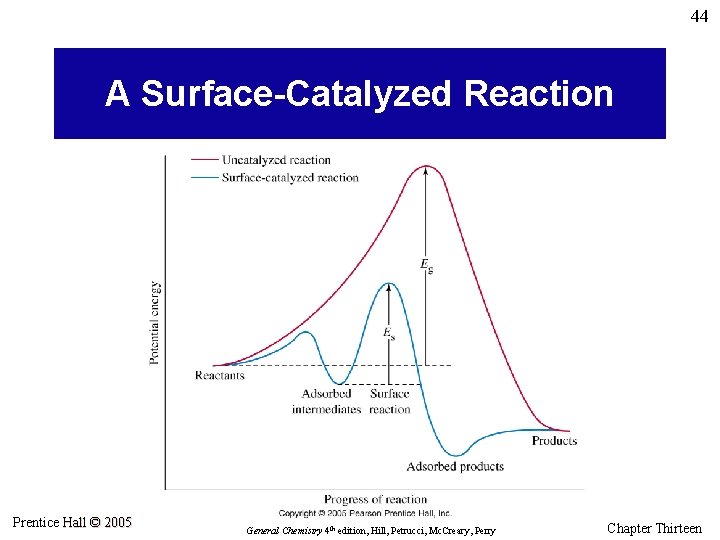
44 A Surface-Catalyzed Reaction Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

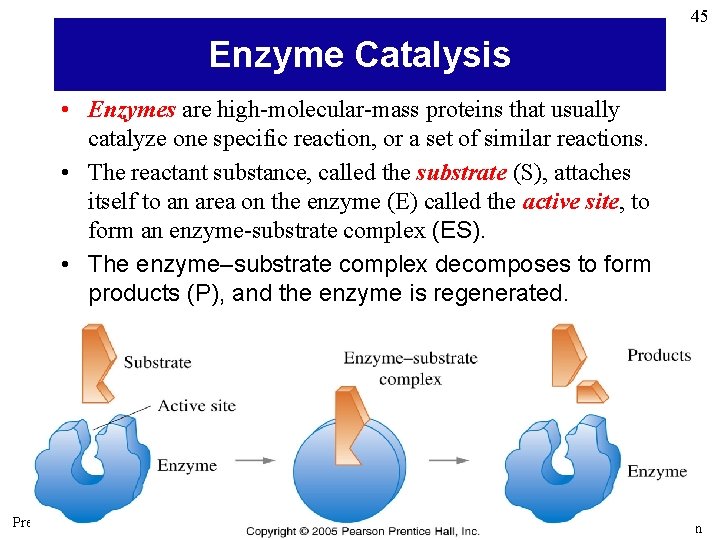
45 Enzyme Catalysis • Enzymes are high-molecular-mass proteins that usually catalyze one specific reaction, or a set of similar reactions. • The reactant substance, called the substrate (S), attaches itself to an area on the enzyme (E) called the active site, to form an enzyme-substrate complex (ES). • The enzyme–substrate complex decomposes to form products (P), and the enzyme is regenerated. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

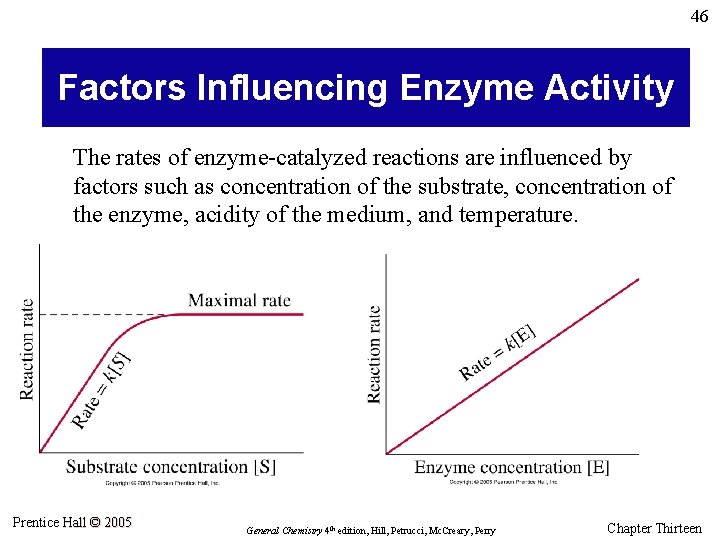
46 Factors Influencing Enzyme Activity The rates of enzyme-catalyzed reactions are influenced by factors such as concentration of the substrate, concentration of the enzyme, acidity of the medium, and temperature. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

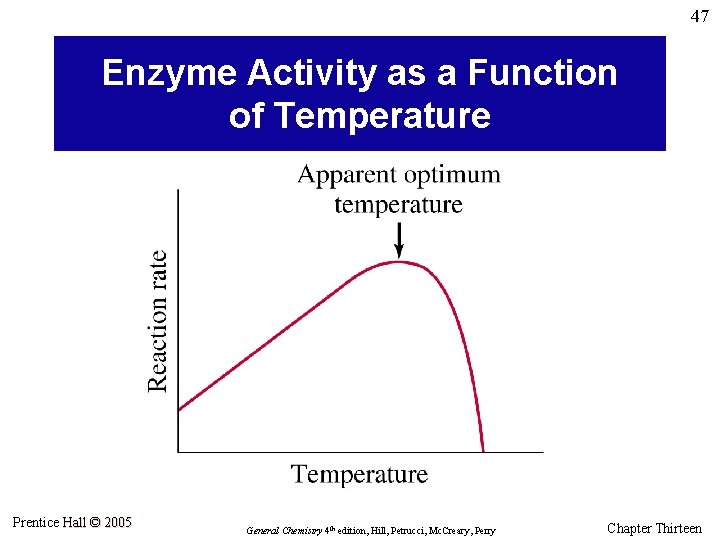
47 Enzyme Activity as a Function of Temperature Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

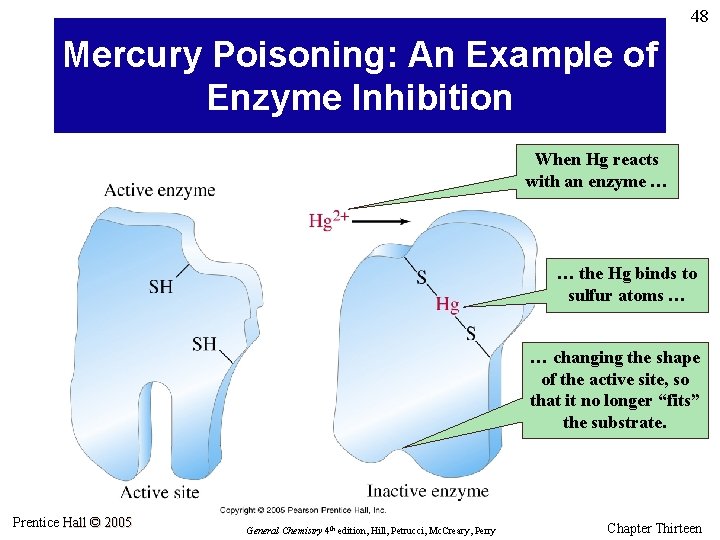
48 Mercury Poisoning: An Example of Enzyme Inhibition When Hg reacts with an enzyme … … the Hg binds to sulfur atoms … … changing the shape of the active site, so that it no longer “fits” the substrate. Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

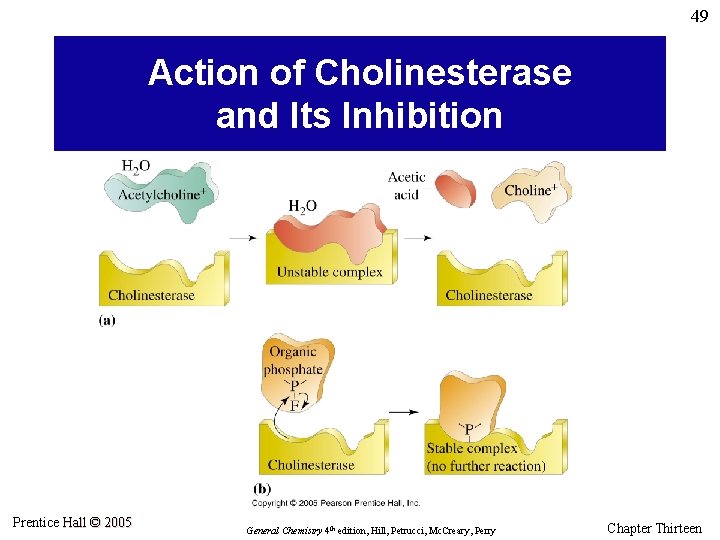
49 Action of Cholinesterase and Its Inhibition Prentice Hall © 2005 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen

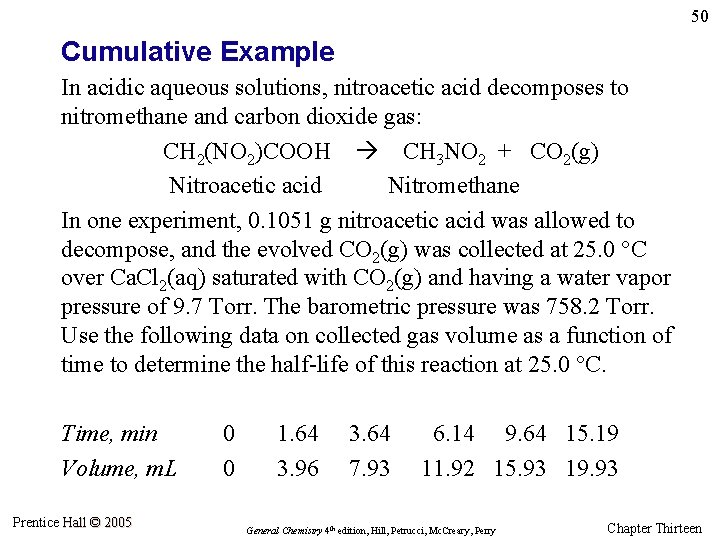
50 Cumulative Example In acidic aqueous solutions, nitroacetic acid decomposes to nitromethane and carbon dioxide gas: CH 2(NO 2)COOH CH 3 NO 2 + CO 2(g) Nitroacetic acid Nitromethane In one experiment, 0. 1051 g nitroacetic acid was allowed to decompose, and the evolved CO 2(g) was collected at 25. 0 °C over Ca. Cl 2(aq) saturated with CO 2(g) and having a water vapor pressure of 9. 7 Torr. The barometric pressure was 758. 2 Torr. Use the following data on collected gas volume as a function of time to determine the half-life of this reaction at 25. 0 °C. Time, min Volume, m. L Prentice Hall © 2005 0 0 1. 64 3. 96 3. 64 7. 93 6. 14 9. 64 15. 19 11. 92 15. 93 19. 93 General Chemistry 4 th edition, Hill, Petrucci, Mc. Creary, Perry Chapter Thirteen