1 CDK 4 6 INHIBITORS IN BREAST CANCER
1 CDK 4 -6 INHIBITORS IN BREAST CANCER: OPTIMAL TREATMENT SEQUENCING Mansoor A Khan, BS, MS, BCOP Oncology/Hematology/BMT Clinical Pharmacist King Abdulaziz Medical City, Jeddah Ministry of National Guard Health Affairs Satellite Symposium: 2 nd Saudi Oncology Pharmacy Association (SOPA 2019) September 14, 2019, Riyadh, Saudi Arabia
2 Objectives • Review role of cyclin dependent kinase 4 -6 (CDK 4 -6) in mediating endocrine resistance in breast cancer and mechanism of action of CDK 4 -6 inhibitors • Describe clinical pharmacology of CDK 4 -6 inhibitors • Review the monitoring of side effects of CDK 4 -6 inhibitors and toxicity dose adjustment • Review the landmark trials of CDK 4 -6 inhibitors and clinical efficacy of CDK 4 -6 inhibitors as well as place of therapy in NCCN guidelines • Selection criteria of choosing a certain CDK 4 -6 inhibitors for an individual patient Satellite Symposium: 2 nd Saudi Oncology Pharmacy Association (SOPA 2019) September 14, 2019, Riyadh, Saudi Arabia
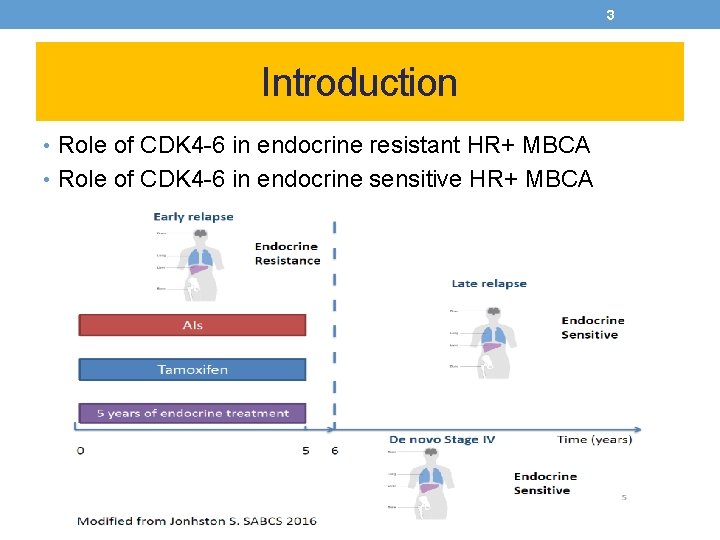
3 Introduction • Role of CDK 4 -6 in endocrine resistant HR+ MBCA • Role of CDK 4 -6 in endocrine sensitive HR+ MBCA
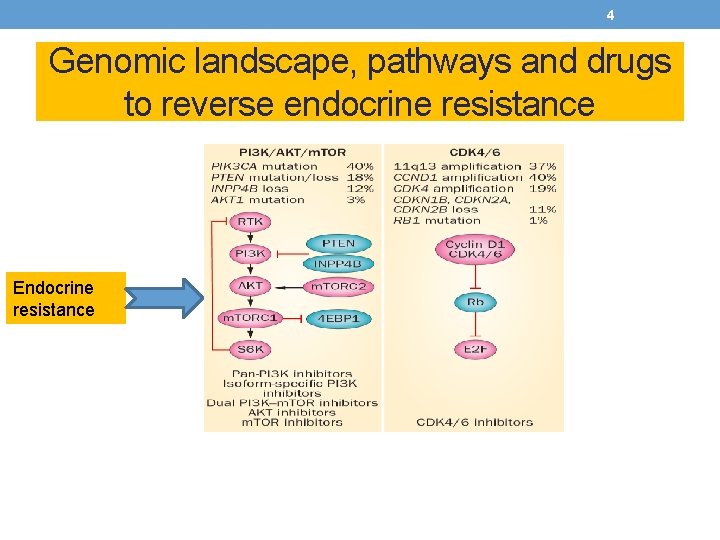
4 Genomic landscape, pathways and drugs to reverse endocrine resistance Endocrine resistance
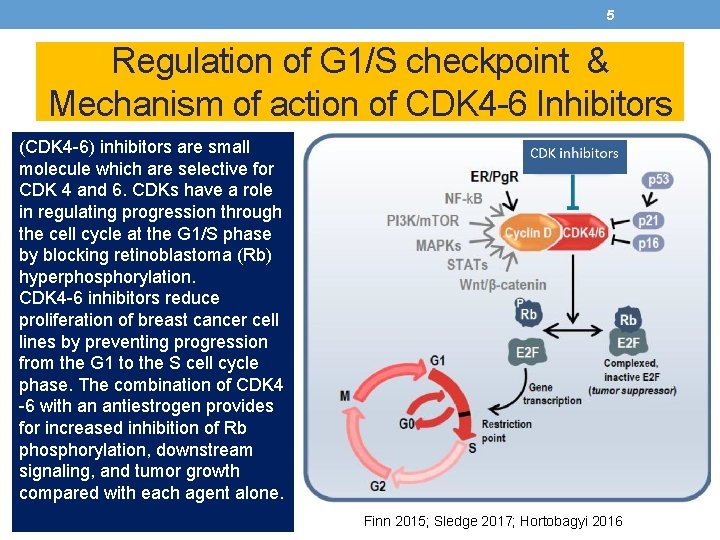
5 Regulation of G 1/S checkpoint & Mechanism of action of CDK 4 -6 Inhibitors (CDK 4 -6) inhibitors are small molecule which are selective for CDK 4 and 6. CDKs have a role in regulating progression through the cell cycle at the G 1/S phase by blocking retinoblastoma (Rb) hyperphosphorylation. CDK 4 -6 inhibitors reduce proliferation of breast cancer cell lines by preventing progression from the G 1 to the S cell cycle phase. The combination of CDK 4 -6 with an antiestrogen provides for increased inhibition of Rb phosphorylation, downstream signaling, and tumor growth compared with each agent alone. Finn 2015; Sledge 2017; Hortobagyi 2016
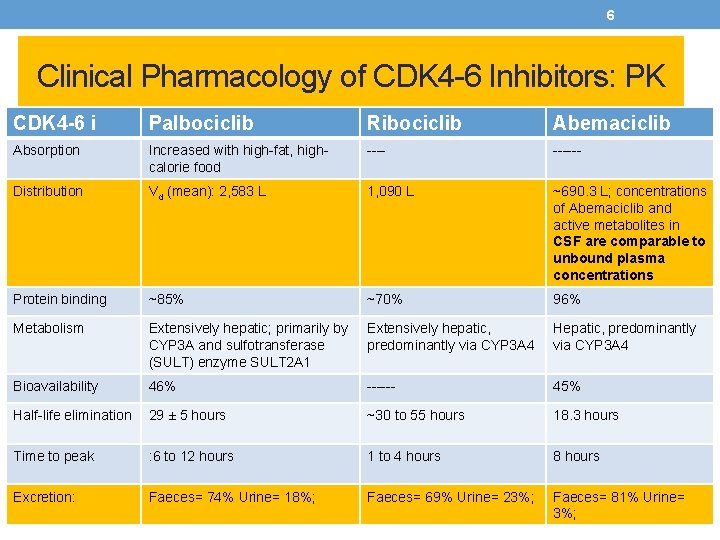
6 Clinical Pharmacology of CDK 4 -6 Inhibitors: PK CDK 4 -6 i Palbociclib Ribociclib Abemaciclib Absorption Increased with high-fat, highcalorie food ------ Distribution Vd (mean): 2, 583 L 1, 090 L ~690. 3 L; concentrations of Abemaciclib and active metabolites in CSF are comparable to unbound plasma concentrations Protein binding ~85% ~70% 96% Metabolism Extensively hepatic; primarily by CYP 3 A and sulfotransferase (SULT) enzyme SULT 2 A 1 Extensively hepatic, predominantly via CYP 3 A 4 Hepatic, predominantly via CYP 3 A 4 Bioavailability 46% ------ 45% Half-life elimination 29 ± 5 hours ~30 to 55 hours 18. 3 hours Time to peak : 6 to 12 hours 1 to 4 hours 8 hours Excretion: Faeces= 74% Urine= 18%; Faeces= 69% Urine= 23%; Faeces= 81% Urine= 3%;
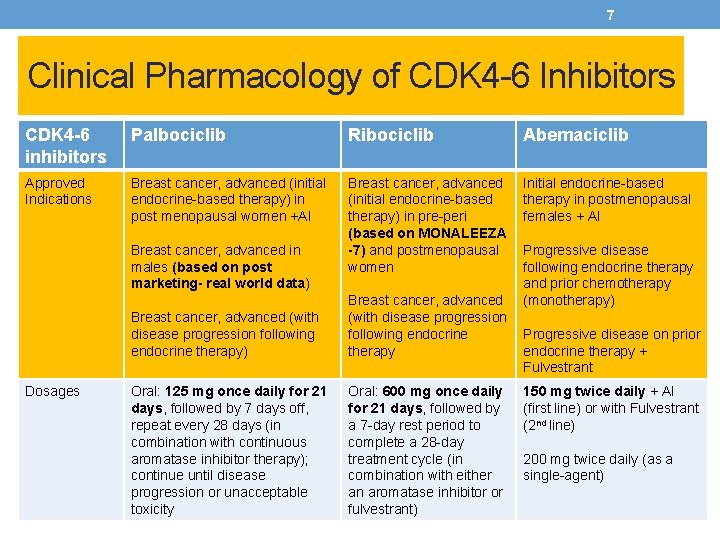
7 Clinical Pharmacology of CDK 4 -6 Inhibitors CDK 4 -6 inhibitors Palbociclib Ribociclib Abemaciclib Approved Indications Breast cancer, advanced (initial endocrine-based therapy) in post menopausal women +AI Breast cancer, advanced (initial endocrine-based therapy) in pre-peri (based on MONALEEZA -7) and postmenopausal women Initial endocrine-based therapy in postmenopausal females + AI Breast cancer, advanced in males (based on post marketing- real world data) Dosages Breast cancer, advanced (with disease progression following endocrine therapy) Breast cancer, advanced (with disease progression following endocrine therapy Oral: 125 mg once daily for 21 days, followed by 7 days off, repeat every 28 days (in combination with continuous aromatase inhibitor therapy); continue until disease progression or unacceptable toxicity Oral: 600 mg once daily for 21 days, followed by a 7 -day rest period to complete a 28 -day treatment cycle (in combination with either an aromatase inhibitor or fulvestrant) Progressive disease following endocrine therapy and prior chemotherapy (monotherapy) Progressive disease on prior endocrine therapy + Fulvestrant 150 mg twice daily + AI (first line) or with Fulvestrant (2 nd line) 200 mg twice daily (as a single-agent)
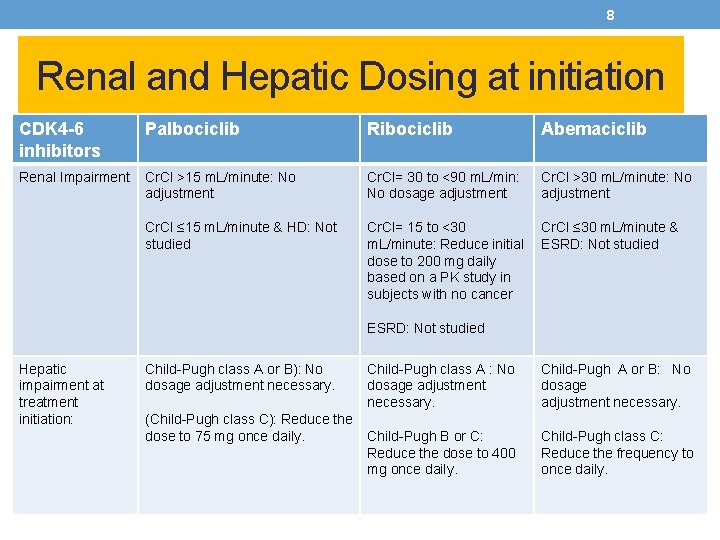
8 Renal and Hepatic Dosing at initiation CDK 4 -6 inhibitors Palbociclib Ribociclib Abemaciclib Renal Impairment Cr. Cl >15 m. L/minute: No adjustment Cr. Cl= 30 to <90 m. L/min: No dosage adjustment Cr. Cl >30 m. L/minute: No adjustment Cr. Cl ≤ 15 m. L/minute & HD: Not studied Cr. Cl= 15 to <30 m. L/minute: Reduce initial dose to 200 mg daily based on a PK study in subjects with no cancer Cr. Cl ≤ 30 m. L/minute & ESRD: Not studied Hepatic impairment at treatment initiation: Child-Pugh class A or B): No dosage adjustment necessary. (Child-Pugh class C): Reduce the dose to 75 mg once daily. Child-Pugh class A : No dosage adjustment necessary. Child-Pugh A or B: No dosage adjustment necessary. Child-Pugh B or C: Reduce the dose to 400 mg once daily. Child-Pugh class C: Reduce the frequency to once daily.
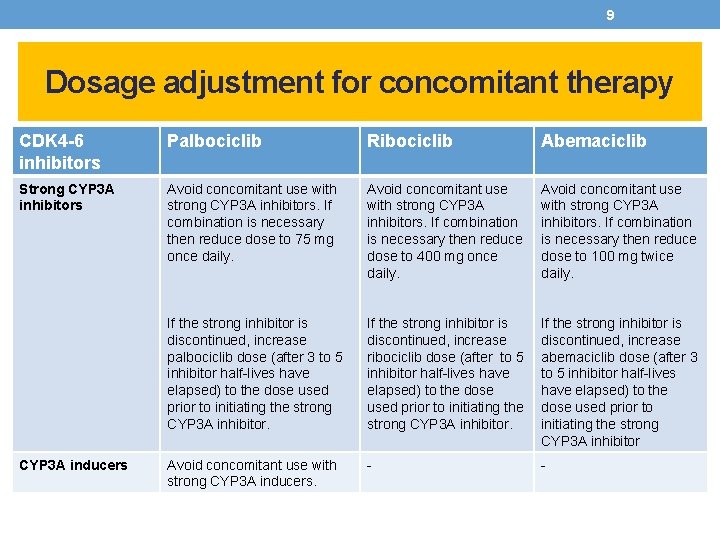
9 Dosage adjustment for concomitant therapy CDK 4 -6 inhibitors Palbociclib Ribociclib Abemaciclib Strong CYP 3 A inhibitors Avoid concomitant use with strong CYP 3 A inhibitors. If combination is necessary then reduce dose to 75 mg once daily. Avoid concomitant use with strong CYP 3 A inhibitors. If combination is necessary then reduce dose to 400 mg once daily. Avoid concomitant use with strong CYP 3 A inhibitors. If combination is necessary then reduce dose to 100 mg twice daily. If the strong inhibitor is discontinued, increase palbociclib dose (after 3 to 5 inhibitor half-lives have elapsed) to the dose used prior to initiating the strong CYP 3 A inhibitor. If the strong inhibitor is discontinued, increase ribociclib dose (after to 5 inhibitor half-lives have elapsed) to the dose used prior to initiating the strong CYP 3 A inhibitor. If the strong inhibitor is discontinued, increase abemaciclib dose (after 3 to 5 inhibitor half-lives have elapsed) to the dose used prior to initiating the strong CYP 3 A inhibitor Avoid concomitant use with strong CYP 3 A inducers. - - CYP 3 A inducers
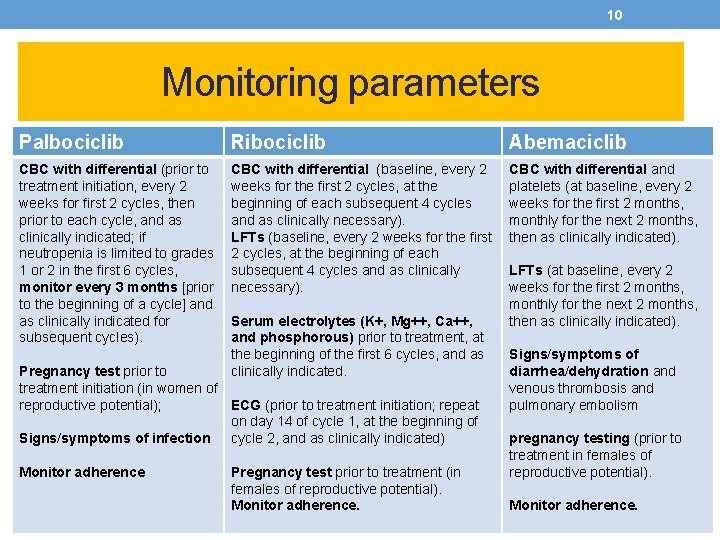
10 Monitoring parameters Palbociclib Ribociclib Abemaciclib CBC with differential (prior to treatment initiation, every 2 weeks for first 2 cycles, then prior to each cycle, and as clinically indicated; if neutropenia is limited to grades 1 or 2 in the first 6 cycles, monitor every 3 months [prior to the beginning of a cycle] and as clinically indicated for subsequent cycles). CBC with differential (baseline, every 2 weeks for the first 2 cycles, at the beginning of each subsequent 4 cycles and as clinically necessary). LFTs (baseline, every 2 weeks for the first 2 cycles, at the beginning of each subsequent 4 cycles and as clinically necessary). CBC with differential and platelets (at baseline, every 2 weeks for the first 2 months, monthly for the next 2 months, then as clinically indicated). Serum electrolytes (K+, Mg++, Ca++, and phosphorous) prior to treatment, at the beginning of the first 6 cycles, and as clinically indicated. Pregnancy test prior to treatment initiation (in women of reproductive potential); ECG (prior to treatment initiation; repeat on day 14 of cycle 1, at the beginning of Signs/symptoms of infection cycle 2, and as clinically indicated) Monitor adherence Pregnancy test prior to treatment (in females of reproductive potential). Monitor adherence. LFTs (at baseline, every 2 weeks for the first 2 months, monthly for the next 2 months, then as clinically indicated). Signs/symptoms of diarrhea/dehydration and venous thrombosis and pulmonary embolism pregnancy testing (prior to treatment in females of reproductive potential). Monitor adherence.
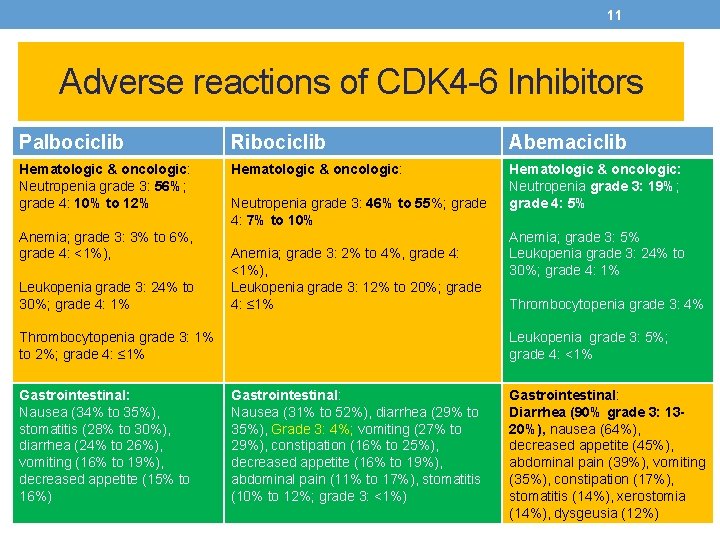
11 Adverse reactions of CDK 4 -6 Inhibitors Palbociclib Ribociclib Abemaciclib Hematologic & oncologic: Neutropenia grade 3: 56%; grade 4: 10% to 12% Hematologic & oncologic: Neutropenia grade 3: 19%; grade 4: 5% Anemia; grade 3: 3% to 6%, grade 4: <1%), Leukopenia grade 3: 24% to 30%; grade 4: 1% Neutropenia grade 3: 46% to 55%; grade 4: 7% to 10% Anemia; grade 3: 2% to 4%, grade 4: <1%), Leukopenia grade 3: 12% to 20%; grade 4: ≤ 1% Thrombocytopenia grade 3: 1% to 2%; grade 4: ≤ 1% Gastrointestinal: Nausea (34% to 35%), stomatitis (28% to 30%), diarrhea (24% to 26%), vomiting (16% to 19%), decreased appetite (15% to 16%) Anemia; grade 3: 5% Leukopenia grade 3: 24% to 30%; grade 4: 1% Thrombocytopenia grade 3: 4% Leukopenia grade 3: 5%; grade 4: <1% Gastrointestinal: Nausea (31% to 52%), diarrhea (29% to 35%), Grade 3: 4%; vomiting (27% to 29%), constipation (16% to 25%), decreased appetite (16% to 19%), abdominal pain (11% to 17%), stomatitis (10% to 12%; grade 3: <1%) Gastrointestinal: Diarrhea (90% grade 3: 1320%), nausea (64%), decreased appetite (45%), abdominal pain (39%), vomiting (35%), constipation (17%), stomatitis (14%), xerostomia (14%), dysgeusia (12%)
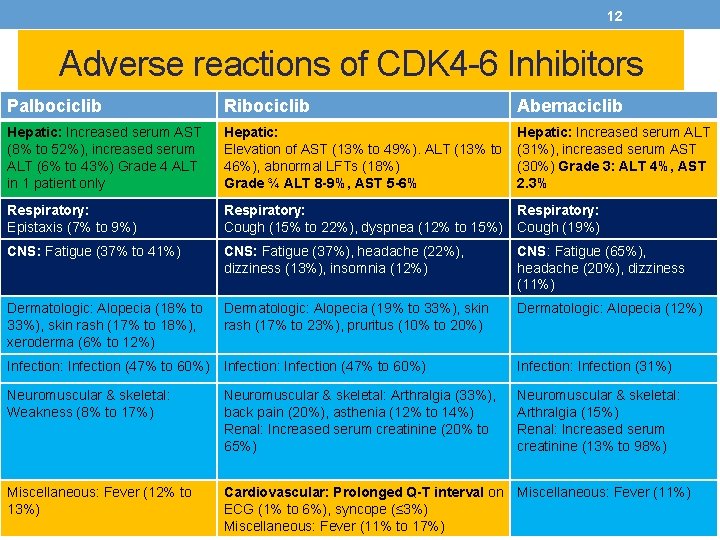
12 Adverse reactions of CDK 4 -6 Inhibitors Palbociclib Ribociclib Abemaciclib Hepatic: Increased serum AST (8% to 52%), increased serum ALT (6% to 43%) Grade 4 ALT in 1 patient only Hepatic: Elevation of AST (13% to 49%). ALT (13% to 46%), abnormal LFTs (18%) Grade ¾ ALT 8 -9%, AST 5 -6% Hepatic: Increased serum ALT (31%), increased serum AST (30%) Grade 3: ALT 4%, AST 2. 3% Respiratory: Epistaxis (7% to 9%) Respiratory: Cough (15% to 22%), dyspnea (12% to 15%) Respiratory: Cough (19%) CNS: Fatigue (37% to 41%) CNS: Fatigue (37%), headache (22%), dizziness (13%), insomnia (12%) CNS: Fatigue (65%), headache (20%), dizziness (11%) Dermatologic: Alopecia (18% to 33%), skin rash (17% to 18%), xeroderma (6% to 12%) Dermatologic: Alopecia (19% to 33%), skin rash (17% to 23%), pruritus (10% to 20%) Dermatologic: Alopecia (12%) Infection: Infection (47% to 60%) Infection: Infection (31%) Neuromuscular & skeletal: Weakness (8% to 17%) Neuromuscular & skeletal: Arthralgia (33%), back pain (20%), asthenia (12% to 14%) Renal: Increased serum creatinine (20% to 65%) Neuromuscular & skeletal: Arthralgia (15%) Renal: Increased serum creatinine (13% to 98%) Miscellaneous: Fever (12% to 13%) Cardiovascular: Prolonged Q-T interval on Miscellaneous: Fever (11%) ECG (1% to 6%), syncope (≤ 3%) Miscellaneous: Fever (11% to 17%)
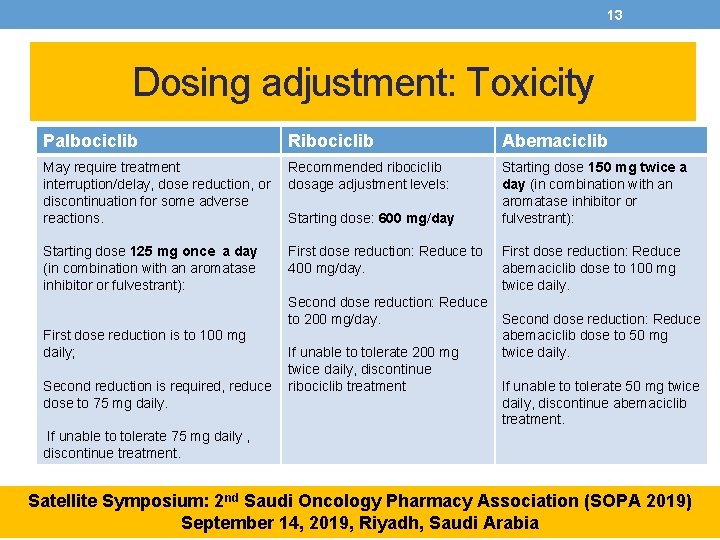
13 Dosing adjustment: Toxicity Palbociclib Ribociclib Abemaciclib May require treatment interruption/delay, dose reduction, or discontinuation for some adverse reactions. Recommended ribociclib dosage adjustment levels: Starting dose 150 mg twice a day (in combination with an aromatase inhibitor or fulvestrant): Starting dose 125 mg once a day (in combination with an aromatase inhibitor or fulvestrant): First dose reduction: Reduce to 400 mg/day. Starting dose: 600 mg/day Second dose reduction: Reduce to 200 mg/day. First dose reduction is to 100 mg daily; Second reduction is required, reduce dose to 75 mg daily. If unable to tolerate 200 mg twice daily, discontinue ribociclib treatment First dose reduction: Reduce abemaciclib dose to 100 mg twice daily. Second dose reduction: Reduce abemaciclib dose to 50 mg twice daily. If unable to tolerate 50 mg twice daily, discontinue abemaciclib treatment. If unable to tolerate 75 mg daily , discontinue treatment. Satellite Symposium: 2 nd Saudi Oncology Pharmacy Association (SOPA 2019) September 14, 2019, Riyadh, Saudi Arabia
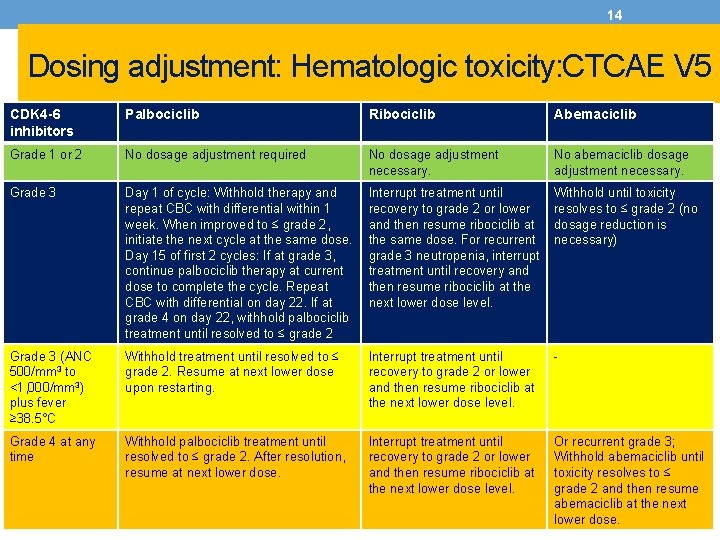
14 Dosing adjustment: Hematologic toxicity: CTCAE V 5 CDK 4 -6 inhibitors Palbociclib Ribociclib Abemaciclib Grade 1 or 2 No dosage adjustment required No dosage adjustment necessary. No abemaciclib dosage adjustment necessary. Grade 3 Day 1 of cycle: Withhold therapy and repeat CBC with differential within 1 week. When improved to ≤ grade 2, initiate the next cycle at the same dose. Day 15 of first 2 cycles: If at grade 3, continue palbociclib therapy at current dose to complete the cycle. Repeat CBC with differential on day 22. If at grade 4 on day 22, withhold palbociclib treatment until resolved to ≤ grade 2 Interrupt treatment until recovery to grade 2 or lower and then resume ribociclib at the same dose. For recurrent grade 3 neutropenia, interrupt treatment until recovery and then resume ribociclib at the next lower dose level. Withhold until toxicity resolves to ≤ grade 2 (no dosage reduction is necessary) Grade 3 (ANC 500/mm 3 to <1, 000/mm 3) plus fever ≥ 38. 5°C Withhold treatment until resolved to ≤ grade 2. Resume at next lower dose upon restarting. Interrupt treatment until recovery to grade 2 or lower and then resume ribociclib at the next lower dose level. - Grade 4 at any time Withhold palbociclib treatment until resolved to ≤ grade 2. After resolution, resume at next lower dose. Interrupt treatment until recovery to grade 2 or lower and then resume ribociclib at the next lower dose level. Or recurrent grade 3; Withhold abemaciclib until toxicity resolves to ≤ grade 2 and then resume abemaciclib at the next lower dose.
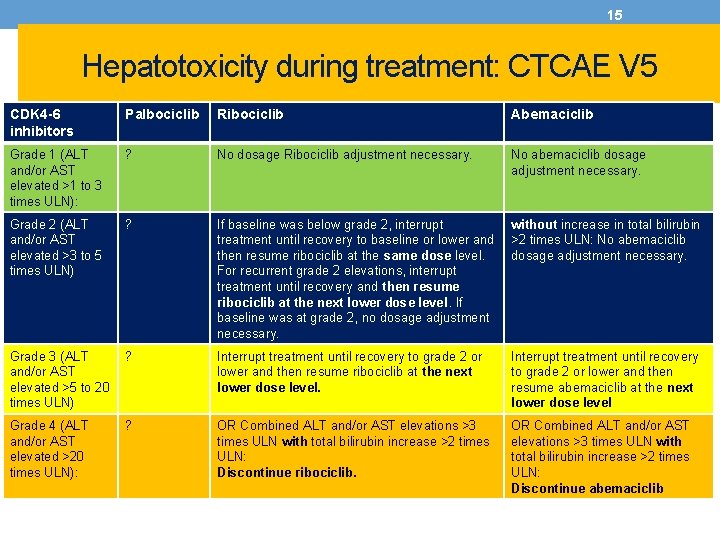
15 Hepatotoxicity during treatment: CTCAE V 5 CDK 4 -6 inhibitors Palbociclib Ribociclib Abemaciclib Grade 1 (ALT and/or AST elevated >1 to 3 times ULN): ? No dosage Ribociclib adjustment necessary. No abemaciclib dosage adjustment necessary. Grade 2 (ALT and/or AST elevated >3 to 5 times ULN) ? If baseline was below grade 2, interrupt treatment until recovery to baseline or lower and then resume ribociclib at the same dose level. For recurrent grade 2 elevations, interrupt treatment until recovery and then resume ribociclib at the next lower dose level. If baseline was at grade 2, no dosage adjustment necessary. without increase in total bilirubin >2 times ULN: No abemaciclib dosage adjustment necessary. Grade 3 (ALT and/or AST elevated >5 to 20 times ULN) ? Interrupt treatment until recovery to grade 2 or lower and then resume ribociclib at the next lower dose level. Interrupt treatment until recovery to grade 2 or lower and then resume abemaciclib at the next lower dose level Grade 4 (ALT and/or AST elevated >20 times ULN): ? OR Combined ALT and/or AST elevations >3 times ULN with total bilirubin increase >2 times ULN: Discontinue ribociclib. OR Combined ALT and/or AST elevations >3 times ULN with total bilirubin increase >2 times ULN: Discontinue abemaciclib
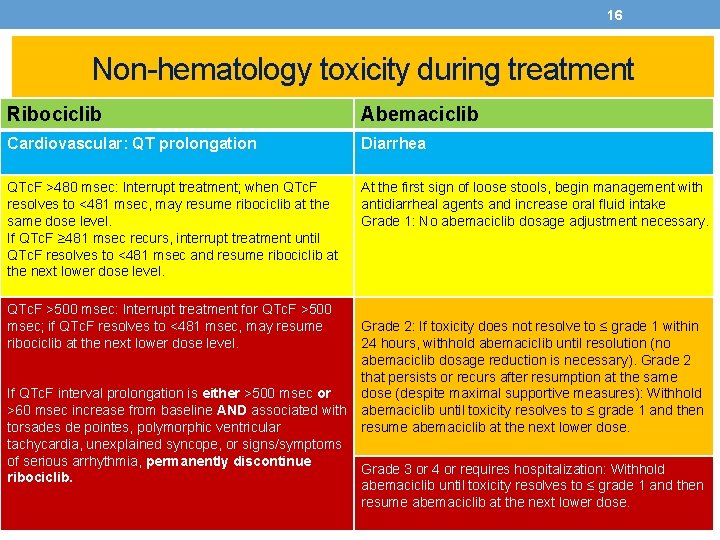
16 Non-hematology toxicity during treatment Ribociclib Abemaciclib Cardiovascular: QT prolongation Diarrhea QTc. F >480 msec: Interrupt treatment; when QTc. F resolves to <481 msec, may resume ribociclib at the same dose level. If QTc. F ≥ 481 msec recurs, interrupt treatment until QTc. F resolves to <481 msec and resume ribociclib at the next lower dose level. At the first sign of loose stools, begin management with antidiarrheal agents and increase oral fluid intake Grade 1: No abemaciclib dosage adjustment necessary. QTc. F >500 msec: Interrupt treatment for QTc. F >500 msec; if QTc. F resolves to <481 msec, may resume ribociclib at the next lower dose level. If QTc. F interval prolongation is either >500 msec or >60 msec increase from baseline AND associated with torsades de pointes, polymorphic ventricular tachycardia, unexplained syncope, or signs/symptoms of serious arrhythmia, permanently discontinue ribociclib. Grade 2: If toxicity does not resolve to ≤ grade 1 within 24 hours, withhold abemaciclib until resolution (no abemaciclib dosage reduction is necessary). Grade 2 that persists or recurs after resumption at the same dose (despite maximal supportive measures): Withhold abemaciclib until toxicity resolves to ≤ grade 1 and then resume abemaciclib at the next lower dose. Grade 3 or 4 or requires hospitalization: Withhold abemaciclib until toxicity resolves to ≤ grade 1 and then resume abemaciclib at the next lower dose.

17 Clinical Efficacy of CDK 4 -6 inhibitors Landmark trials of CDK 4 -6 Inhibitors in 1 st and 2 nd line use
18 Clinical Efficacy of CDK 4 -6 inhibitors in 1 st line Palbociclib Ribociclib Abemaciclib PALOMA-2 Study P= Phase III double-blind RCT, 666 postmenopausal women with ER-positive, HER 2 negative breast cancer, who had not had prior treatment for advanced disease were randomly assigned, in a 2: 1 ratio, , to receive palbociclib 125 mg per day (on a 3 -weeks-on, 1 -weekoff schedule) plus letrozole or placebo plus letrozole MONALEEZA-2 Study P= Phase III double-blind RCT, 668 postmenopausal women with ER-positive, HER 2 negative breast cancer, who had not had prior treatment for advanced disease were randomly assigned, in a to receive ribociclib 600 mg per day (on a 3 -weeks-on, 1 week-off schedule) plus letrozole or placebo plus letrozole MONARCH-3 Study P= Double-blind, Phase III RCT study of abemaciclib or placebo plus a AI in 493 postmenopausal women with HR-positive, HER 2 negative advanced breast cancer who had no prior systemic therapy in the advanced setting. I= Ribociclib + AI O= The primary objective was PFS. Secondary objectives included response evaluation and safety. A planned interim analysis occurred after 189 events. I= Abemaciclib 150 mg BID + AI C= Placebo+ AI I= Palbociclib + AI C= Placebo+ AI O= The primary end point was PFS, secondary end points were overall survival, objective response, clinical benefit response, patient-reported outcomes, pharmacokinetic effects, and safety. O= The primary end point was PFS. Secondary end points included overall survival, overall response rate, and safety. PALOMA-2 Study, MONALEEZA-2 Study & MONARCH-3 Study
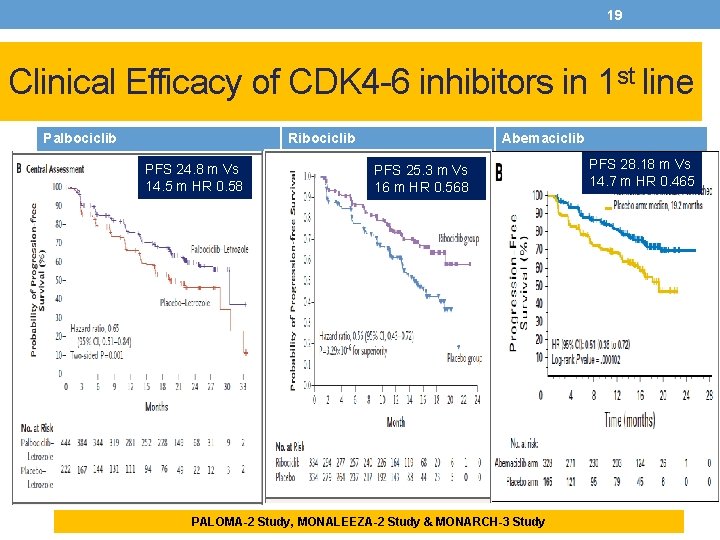
19 Clinical Efficacy of CDK 4 -6 inhibitors in 1 st line Palbociclib. Ribociclib PFS 24. 8 m Vs 14. 5 m HR 0. 58 Abemaciclib PFS 25. 3 m Vs 16 m HR 0. 568 PALOMA-2 Study, MONALEEZA-2 Study & MONARCH-3 Study PFS 28. 18 m Vs 14. 7 m HR 0. 465
20 Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. MONALEEZA 7 An earlier analysis of this phase 3 trial MONALEEZA 7 showed that the addition of a Ribociclib to endocrine therapy provided a greater benefit with regard to PFS than endocrine therapy alone in premenopausal or perimenopausal patients with advanced hormonereceptor–positive breast cancer. In this article, the results of a protocol-specified interim analysis of the key secondary end point of overall survival is described. The estimated overall survival at 42 months was 70. 2% (95% confidence interval [CI], 63. 5 to 76. 0) in the ribociclib group and 46. 0% (95% CI, 32. 0 to 58. 9) in the placebo group (hazard ratio for death, 0. 71; 95% CI, 0. 54 to 0. 95; P=0. 00973 by log-rank test). Im SA et al, Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N Engl J Med. 2019 Jul 25; 381(4): 307 -316.
22 Clinical Efficacy of CDK 4 -6 inhibitors in 2 nd line Palbociclib Ribociclib Abemaciclib PALOMA-3 Study P= Double-blind, Phase III RCT study of Palbociclib or placebo plus Fulvestrant in 521 postmenopausal women (2: 1) with HR-positive, HER 2 -negative advanced breast cancer who had progressed on prior endocrine therapy I= Palbociclib 125 mg daily (3 weeks on 1 week off) + Fulvestrant (2) C= Placebo+ Fulvestrant (1) O= The primary objective was PFS. secondary end points included overall survival, objective response rate (ORR), clinical benefit rate, patient reported outcomes, and safety. MONALEESA-3 Study P= Double-blind, Phase III RCT study of Ribociclib or placebo plus Fulvestrant in 484 postmenopausal women with HRpositive, HER 2 -negative advanced breast cancer who were treatment na¨ıve or had received up to one line of prior endocrine therapy in the advanced setting (Denovo=20%, Non-denovo 80%, DFI ≤ 12 m= 4. 5%, ≥ 12 m = 75. 5% were randomized 2: 1 ratio to. I= Ribociclib 600 mg daily (3 weeks on 1 week off) + Fulvestrant C= Placebo+ Fulvestrant O= The primary objective was PFS. secondary end points included overall survival, overall response rate and safety. MONARCH-2 Study P= Double-blind, Phase III RCT study of abemaciclib or placebo plus Fulvestrant in 669 postmenopausal women with HR -positive, HER 2 -negative advanced breast cancer who had progressed prior endocrine therapy (adjuvat, neoadjuvant (59%) Or or while receiving first-line (39%) ET for metastatic disease I= Abemaciclib 150 mg BID + Fulvestrant C= Placebo+ Fulvestrant O= The primary objective was PFS. secondary end points included OS, ORR) duration of response, clinical benefit rate, quality of life, and safety. PALOMA-3 Study, MONALEEZA-3 Study & MONARCH-2 Study
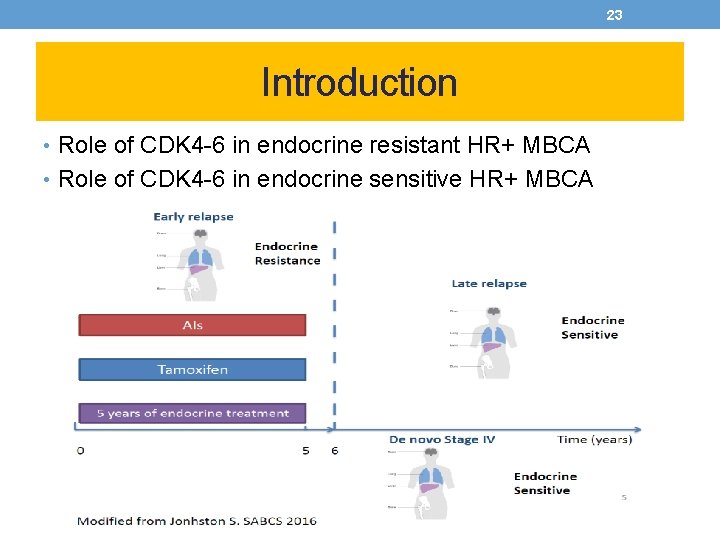
23 Introduction • Role of CDK 4 -6 in endocrine resistant HR+ MBCA • Role of CDK 4 -6 in endocrine sensitive HR+ MBCA
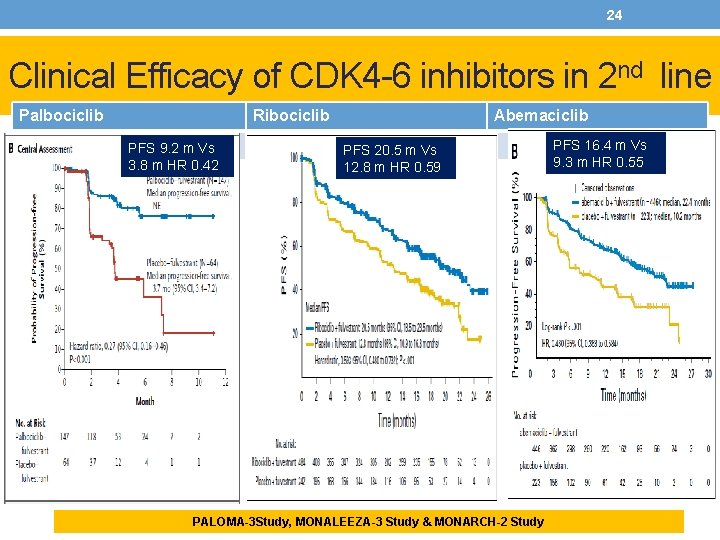
24 Clinical Efficacy of CDK 4 -6 inhibitors in 2 nd line Palbociclib Ribociclib PFS 9. 2 m Vs 3. 8 m HR 0. 42 Abemaciclib PFS 20. 5 m Vs 12. 8 m HR 0. 59 PALOMA-3 Study, MONALEEZA-3 Study & MONARCH-2 Study PFS 16. 4 m Vs 9. 3 m HR 0. 55
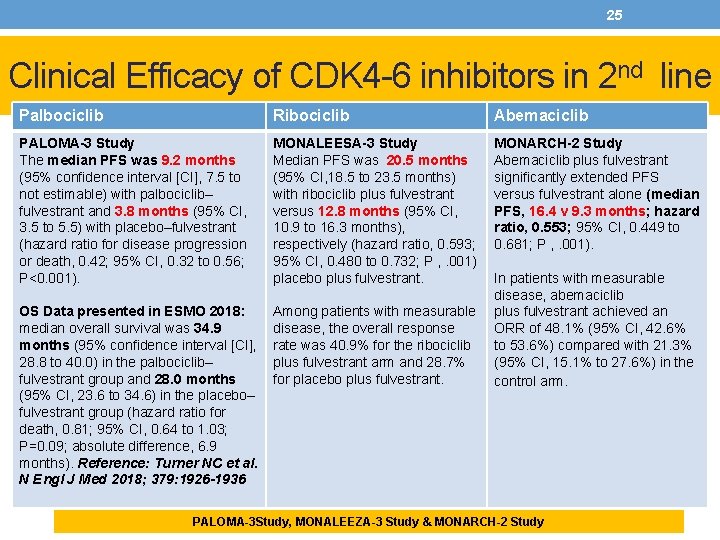
25 Clinical Efficacy of CDK 4 -6 inhibitors in 2 nd line Palbociclib Ribociclib Abemaciclib PALOMA-3 Study The median PFS was 9. 2 months (95% confidence interval [CI], 7. 5 to not estimable) with palbociclib– fulvestrant and 3. 8 months (95% CI, 3. 5 to 5. 5) with placebo–fulvestrant (hazard ratio for disease progression or death, 0. 42; 95% CI, 0. 32 to 0. 56; P<0. 001). MONALEESA-3 Study Median PFS was 20. 5 months (95% CI, 18. 5 to 23. 5 months) with ribociclib plus fulvestrant versus 12. 8 months (95% CI, 10. 9 to 16. 3 months), respectively (hazard ratio, 0. 593; 95% CI, 0. 480 to 0. 732; P , . 001) placebo plus fulvestrant. MONARCH-2 Study Abemaciclib plus fulvestrant significantly extended PFS versus fulvestrant alone (median PFS, 16. 4 v 9. 3 months; hazard ratio, 0. 553; 95% CI, 0. 449 to 0. 681; P , . 001). OS Data presented in ESMO 2018: median overall survival was 34. 9 months (95% confidence interval [CI], 28. 8 to 40. 0) in the palbociclib– fulvestrant group and 28. 0 months (95% CI, 23. 6 to 34. 6) in the placebo– fulvestrant group (hazard ratio for death, 0. 81; 95% CI, 0. 64 to 1. 03; P=0. 09; absolute difference, 6. 9 months). Reference: Turner NC et al. N Engl J Med 2018; 379: 1926 -1936 Among patients with measurable disease, the overall response rate was 40. 9% for the ribociclib plus fulvestrant arm and 28. 7% for placebo plus fulvestrant. In patients with measurable disease, abemaciclib plus fulvestrant achieved an ORR of 48. 1% (95% CI, 42. 6% to 53. 6%) compared with 21. 3% (95% CI, 15. 1% to 27. 6%) in the control arm. PALOMA-3 Study, MONALEEZA-3 Study & MONARCH-2 Study
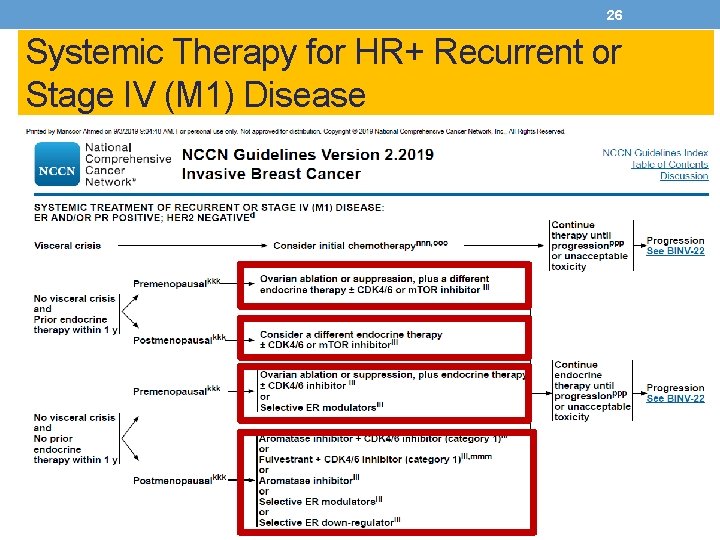
26 Systemic Therapy for HR+ Recurrent or Stage IV (M 1) Disease
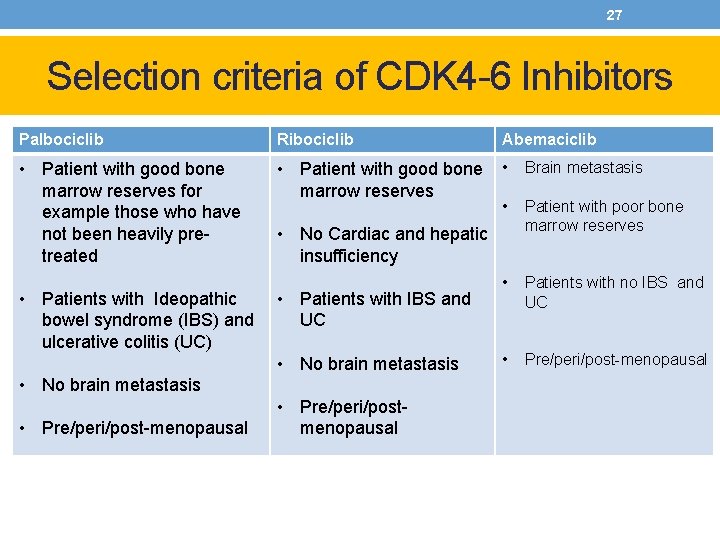
27 Selection criteria of CDK 4 -6 Inhibitors Palbociclib Ribociclib Abemaciclib • Patient with good bone marrow reserves for example those who have not been heavily pretreated • Patient with good bone marrow reserves • Brain metastasis • Patient with poor bone marrow reserves • Patients with no IBS and UC • Pre/peri/post-menopausal • Patients with Ideopathic bowel syndrome (IBS) and ulcerative colitis (UC) • No Cardiac and hepatic insufficiency • Patients with IBS and UC • No brain metastasis • Pre/peri/post-menopausal • Pre/peri/postmenopausal

28 THANK YOU Mansoor A Khan, BS, MS, BCOP Oncology/Hematology/BMT Clinical Pharmacist King Abdulaziz Medical City, Jeddah Ministry of National Guard Health Affairs Satellite Symposium: 2 nd Saudi Oncology Pharmacy Association (SOPA 2019) September 14, 2019, Riyadh, Saudi Arabia
- Slides: 27