1 Atomic Structure Objectives Describe properties of subatomic
1. Atomic Structure Objectives: Describe properties of subatomic particles Recall how element / nuclear symbols work Explain the existence of isotopes.

The atom model from GCSE:

The subatomic particles from GCSE: Subatomic particle Relative mass Proton Neutron Electron Relative charge
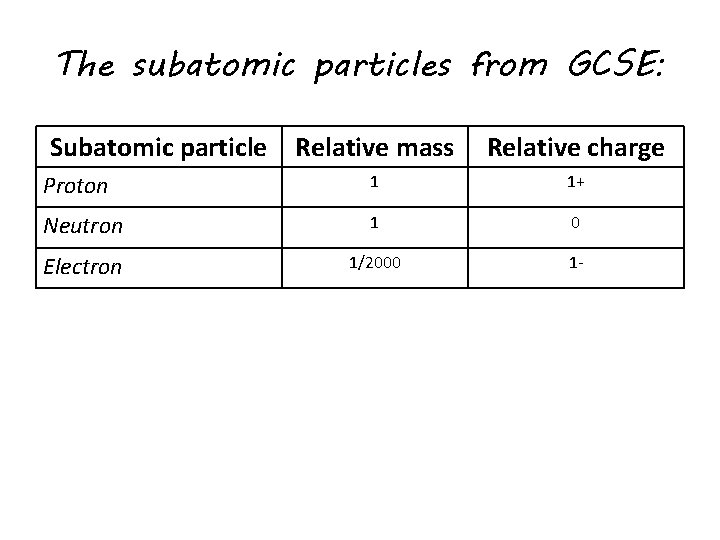
The subatomic particles from GCSE: Subatomic particle Relative mass Relative charge Proton 1 1+ Neutron 1 0 Electron 1/2000 1 -
THE STRUCTURE OF ATOMS Atoms consist of a number of fundamental particles, the most important are. . . Mass / kg PROTON NEUTRON ELECTRON Charge / C Relative mass Relative charge
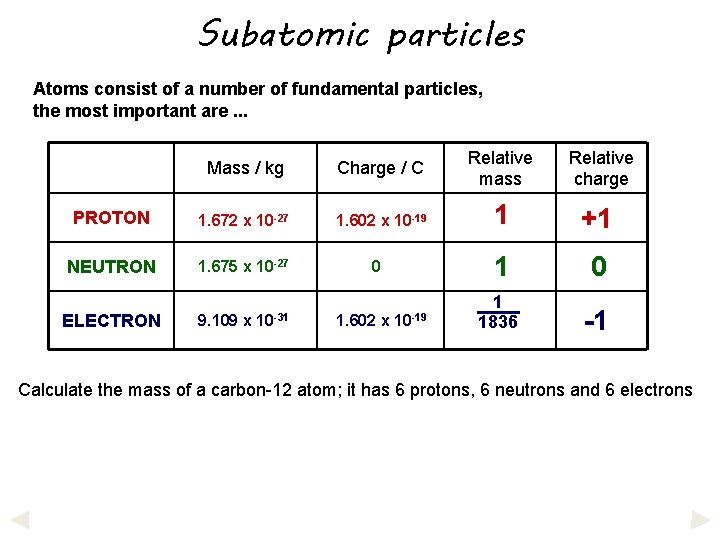
Subatomic particles Atoms consist of a number of fundamental particles, the most important are. . . Mass / kg Charge / C Relative mass Relative charge PROTON 1. 672 x 10 -27 1. 602 x 10 -19 1 +1 NEUTRON 1. 675 x 10 -27 1 0 1 1836 -1 ELECTRON 9. 109 x 10 -31 0 1. 602 x 10 -19 Calculate the mass of a carbon-12 atom; it has 6 protons, 6 neutrons and 6 electrons
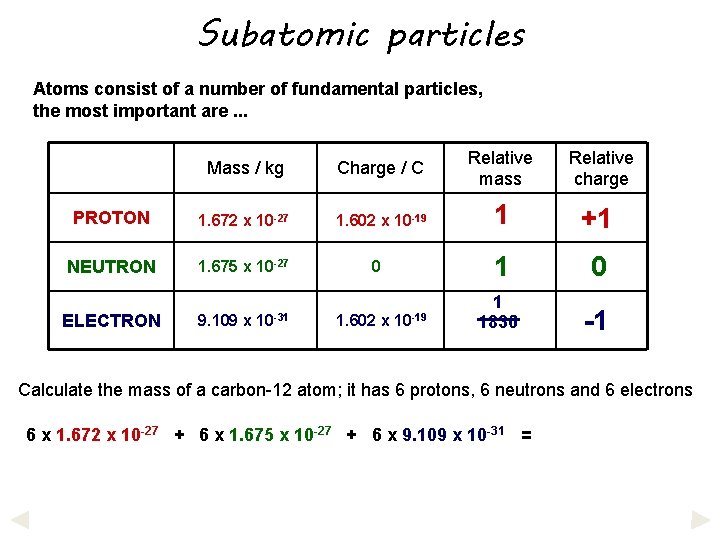
Subatomic particles Atoms consist of a number of fundamental particles, the most important are. . . Mass / kg Charge / C Relative mass Relative charge PROTON 1. 672 x 10 -27 1. 602 x 10 -19 1 +1 NEUTRON 1. 675 x 10 -27 1 0 1 1836 -1 ELECTRON 9. 109 x 10 -31 0 1. 602 x 10 -19 Calculate the mass of a carbon-12 atom; it has 6 protons, 6 neutrons and 6 electrons 6 x 1. 672 x 10 -27 + 6 x 1. 675 x 10 -27 + 6 x 9. 109 x 10 -31 =
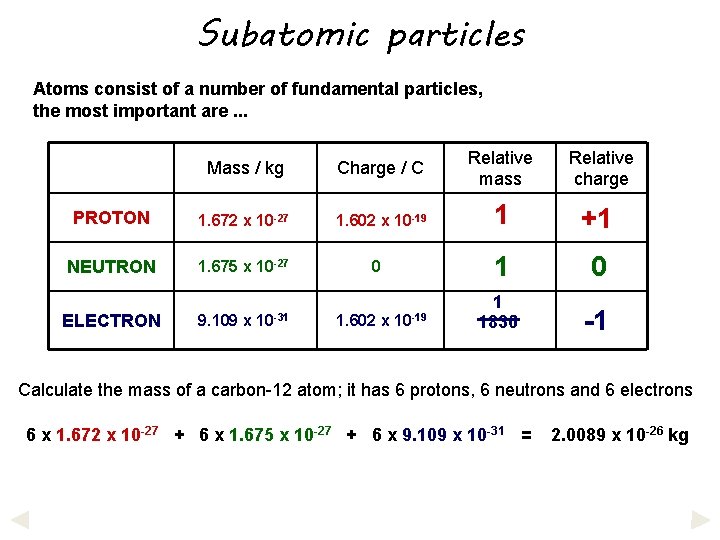
Subatomic particles Atoms consist of a number of fundamental particles, the most important are. . . Mass / kg Charge / C Relative mass Relative charge PROTON 1. 672 x 10 -27 1. 602 x 10 -19 1 +1 NEUTRON 1. 675 x 10 -27 1 0 1 1836 -1 ELECTRON 9. 109 x 10 -31 0 1. 602 x 10 -19 Calculate the mass of a carbon-12 atom; it has 6 protons, 6 neutrons and 6 electrons 6 x 1. 672 x 10 -27 + 6 x 1. 675 x 10 -27 + 6 x 9. 109 x 10 -31 = 2. 0089 x 10 -26 kg

Mass number and atomic number Atomic Number (Z) Mass Number (A)

Mass number and atomic number Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus
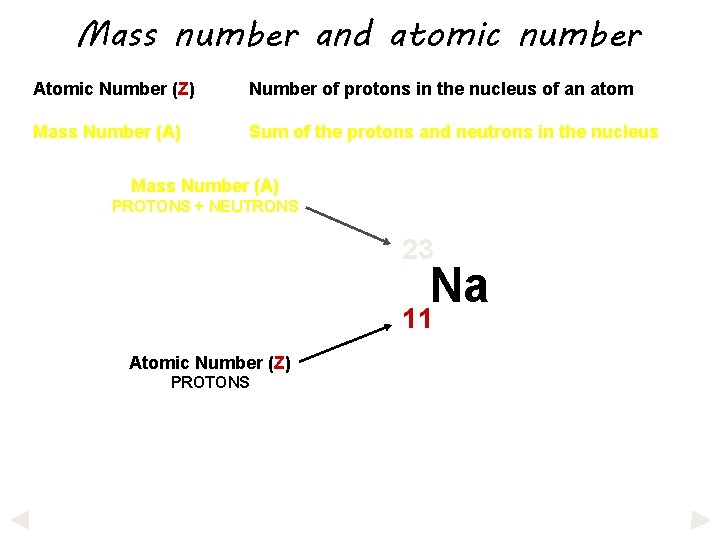
Mass number and atomic number Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus Mass Number (A) PROTONS + NEUTRONS 23 Na 11 Atomic Number (Z) PROTONS
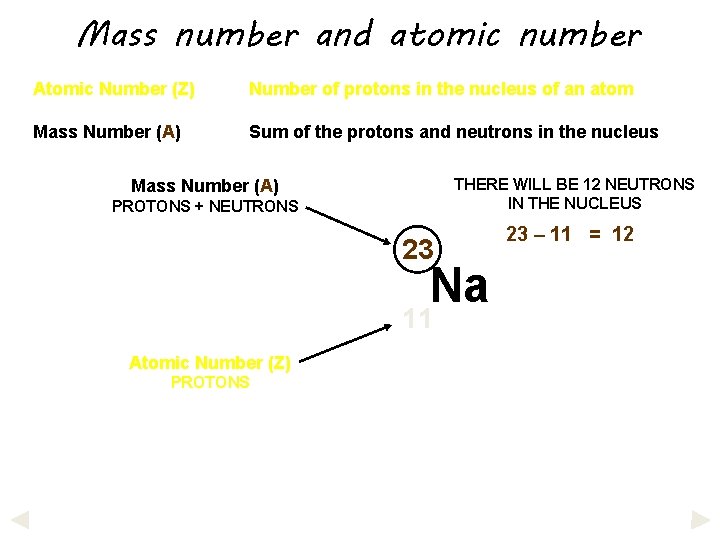
Mass number and atomic number Atomic Number (Z) Number of protons in the nucleus of an atom Mass Number (A) Sum of the protons and neutrons in the nucleus THERE WILL BE 12 NEUTRONS IN THE NUCLEUS Mass Number (A) PROTONS + NEUTRONS 23 Na 11 Atomic Number (Z) PROTONS 23 – 11 = 12

Atoms and Ions 1. 2. 3. 4. 5. What is an ion? How is a negative ion made? How is a positive ion made? Describe: Br. Describe Mg 2+
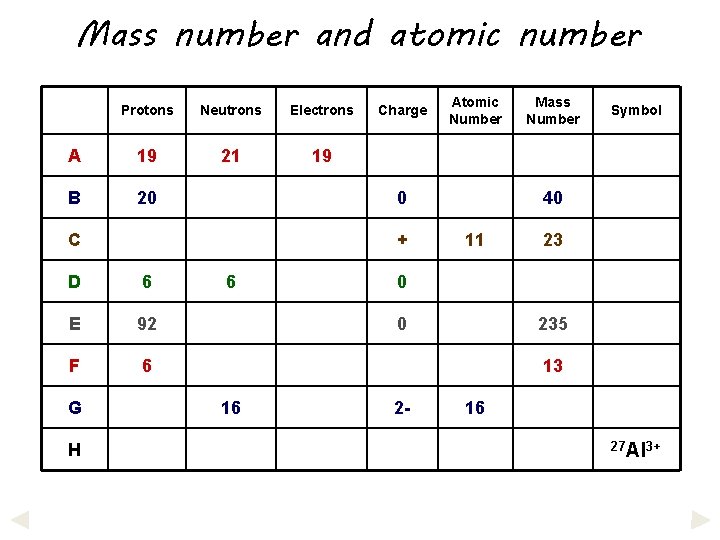
Mass number and atomic number Protons Neutrons Electrons A 19 21 19 B 20 + D 6 E 92 F 6 H Atomic Number 0 C G Charge 6 Mass Number Symbol 40 11 23 0 0 235 13 16 2 - 16 27 Al 3+
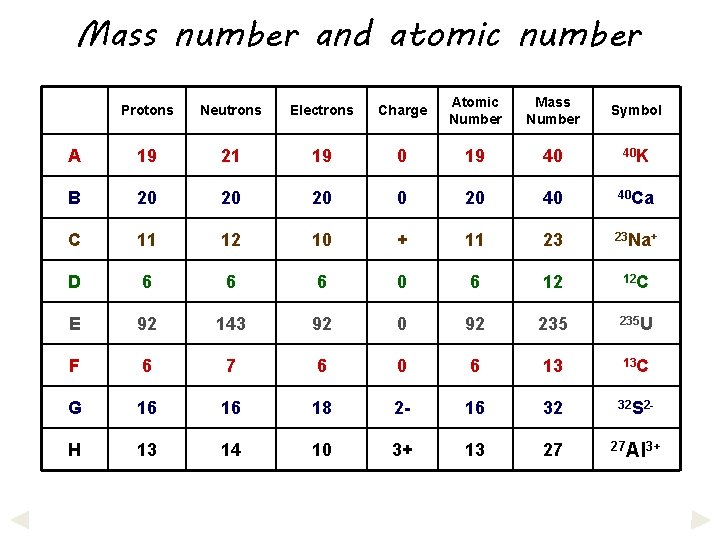
Mass number and atomic number Protons Neutrons Electrons Charge Atomic Number Mass Number Symbol A 19 21 19 0 19 40 40 K B 20 20 20 40 40 Ca C 11 12 10 + 11 23 23 Na+ D 6 6 6 0 6 12 12 C E 92 143 92 0 92 235 U F 6 7 6 0 6 13 13 C G 16 16 18 2 - 16 32 32 S 2 - H 13 14 10 3+ 13 27 27 Al 3+

Isotopes Definition:
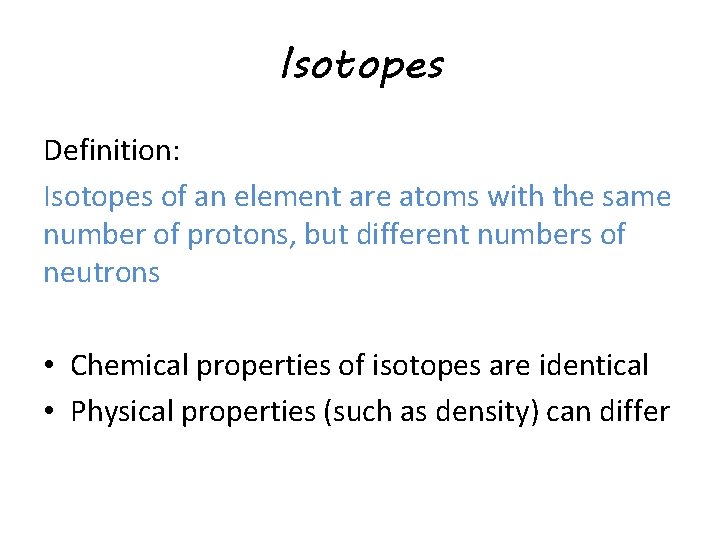
Isotopes Definition: Isotopes of an element are atoms with the same number of protons, but different numbers of neutrons • Chemical properties of isotopes are identical • Physical properties (such as density) can differ

Isotopes Example: Chlorine-35 and Chlorine-37
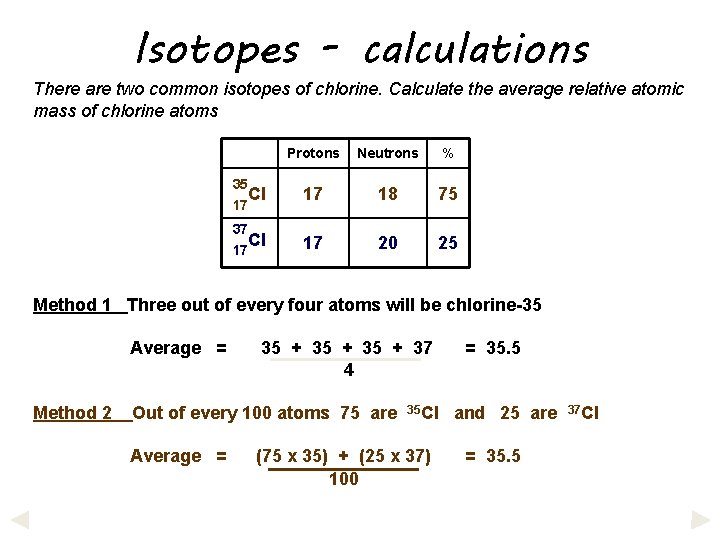
Isotopes - calculations There are two common isotopes of chlorine. Calculate the average relative atomic mass of chlorine atoms 35 17 37 17 Protons Neutrons % Cl 17 18 75 Cl 17 20 25 Method 1 Three out of every four atoms will be chlorine-35 Average = Method 2 35 + 37 4 Out of every 100 atoms 75 are Average = 35 Cl (75 x 35) + (25 x 37) 100 = 35. 5 and 25 are = 35. 5 37 Cl
Questions (actual exam ones) 1. a) b) c) 2. 3. Hydrogen, deuterium and tritium are all isotopes of each other. Identify one similarity and one difference between these isotopes. Deuterium can be written as 2 H. Determine the number of protons, neutrons and electrons in a neutral deuterium atom. Write a nuclear symbol for tritium, given that it has 2 neutrons. A certain atom X has one less proton and two more neutrons than K. What are its atomic number and mass number? Explain in terms of sub atom particles, why C-12 and C-14 are isotopes
- Slides: 20