1 Alkanes and Cycloalkanes 1 Alkanes and Cycloalkanes
















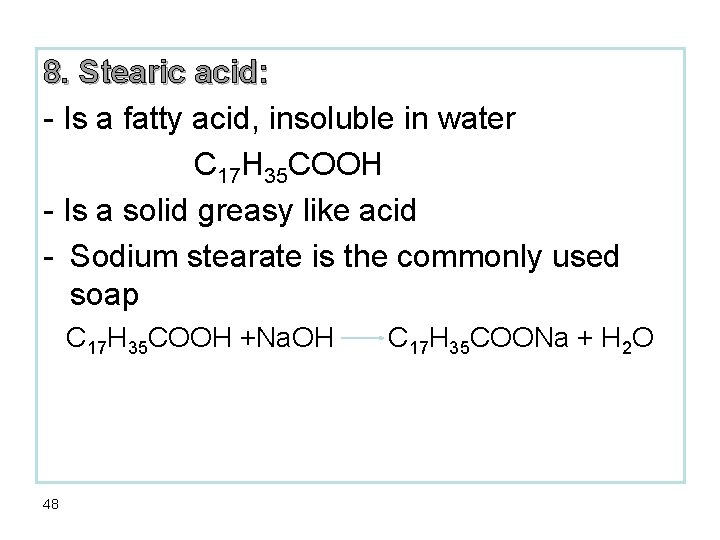

- Slides: 51

1. Alkanes and Cycloalkanes 1

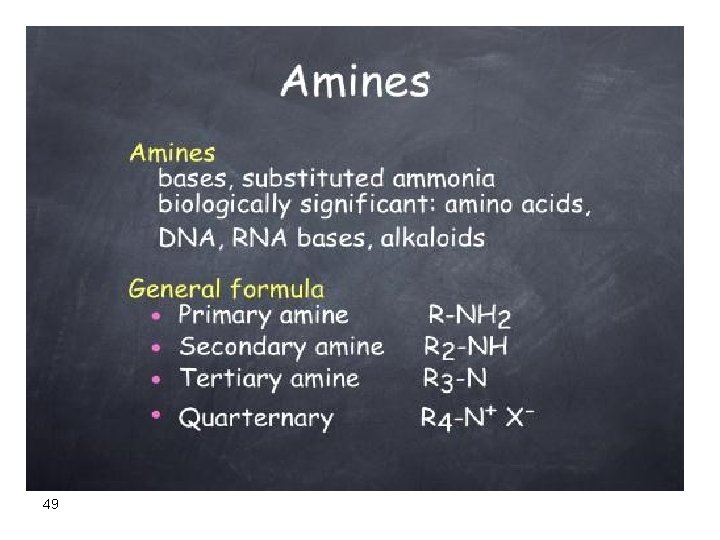
Alkanes and Cycloalkanes Hydrocarbons (contain only carbon and hydrogen) a) Saturated: (Contain only single bonds) Alkanes (Cn. H 2 N + 2 ) Cycloalkanes (Cn. H 2 N ) b) Unsaturated: contain Alkenes: double bonds (, , , Cn. H 2 N) Alkynes: triple bonds ((Cn. H 2 N - 2) Aromatic: benzene like compounds 2

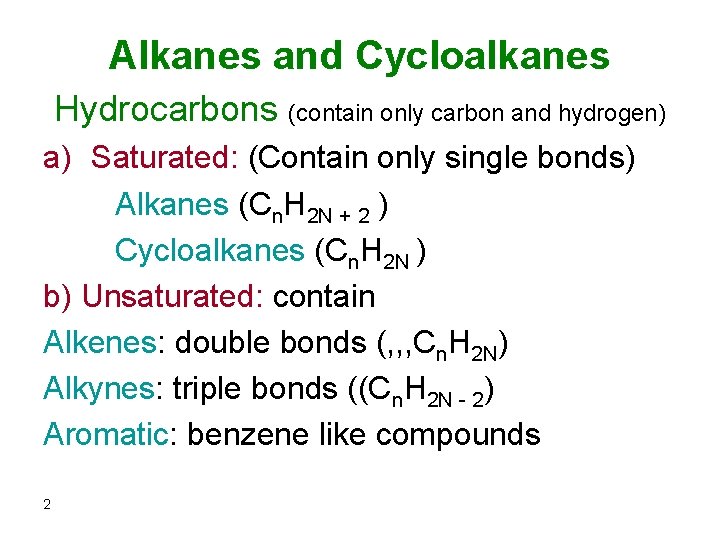
The structure of alkanes 3

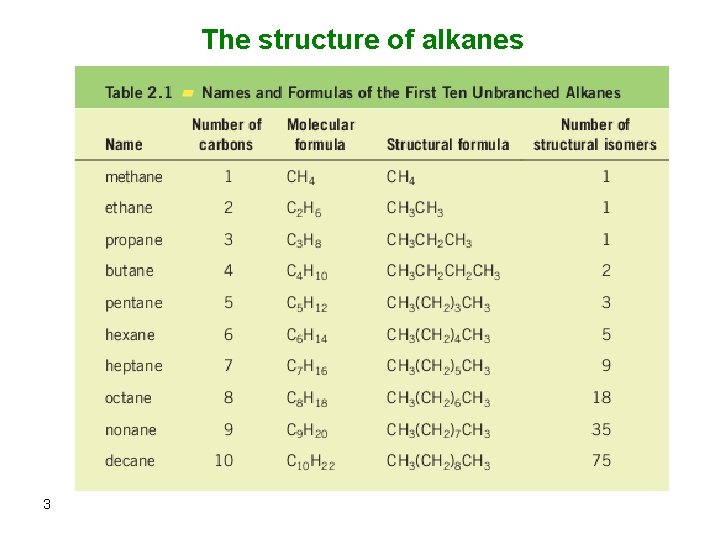
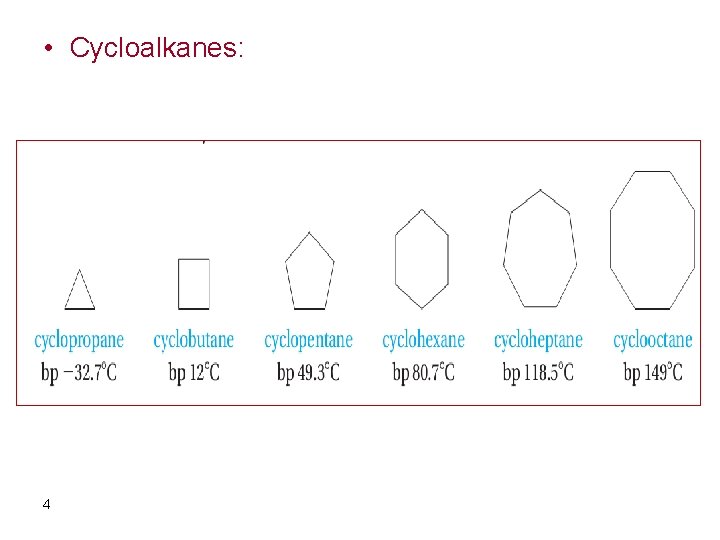
• Cycloalkanes: 4

Some applications of hydrocarbons in health field 5

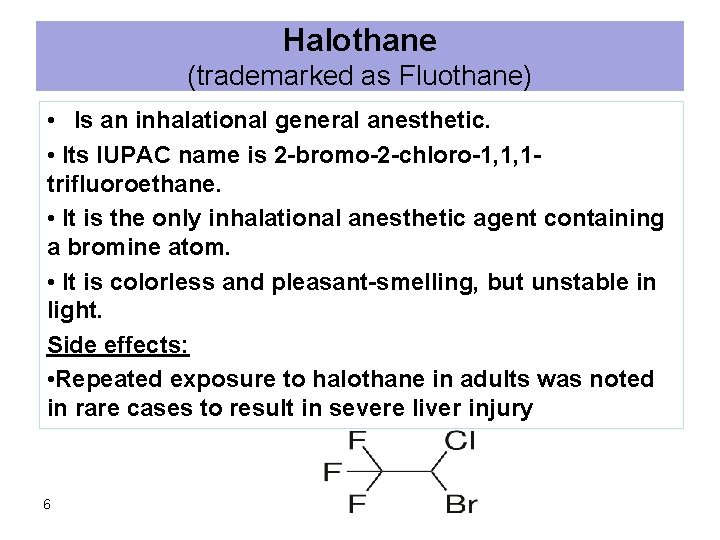
Halothane (trademarked as Fluothane) • Is an inhalational general anesthetic. • Its IUPAC name is 2 -bromo-2 -chloro-1, 1, 1 trifluoroethane. • It is the only inhalational anesthetic agent containing a bromine atom. • It is colorless and pleasant-smelling, but unstable in light. Side effects: • Repeated exposure to halothane in adults was noted in rare cases to result in severe liver injury 6

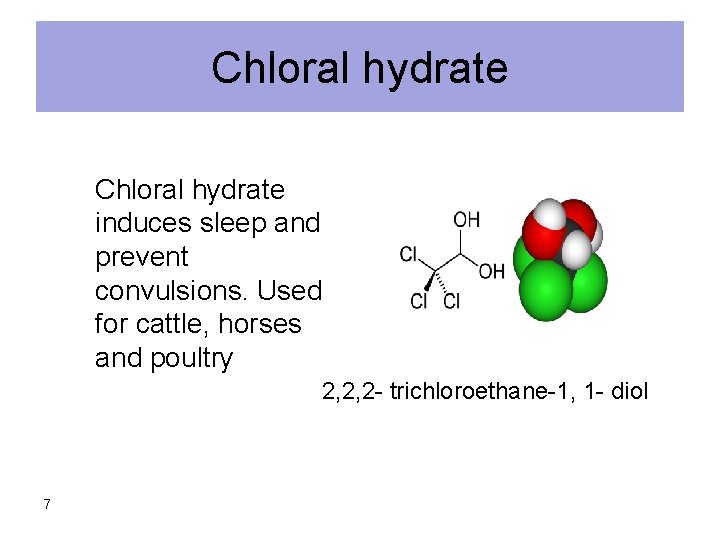
Chloral hydrate induces sleep and prevent convulsions. Used for cattle, horses and poultry 2, 2, 2 - trichloroethane-1, 1 - diol 7

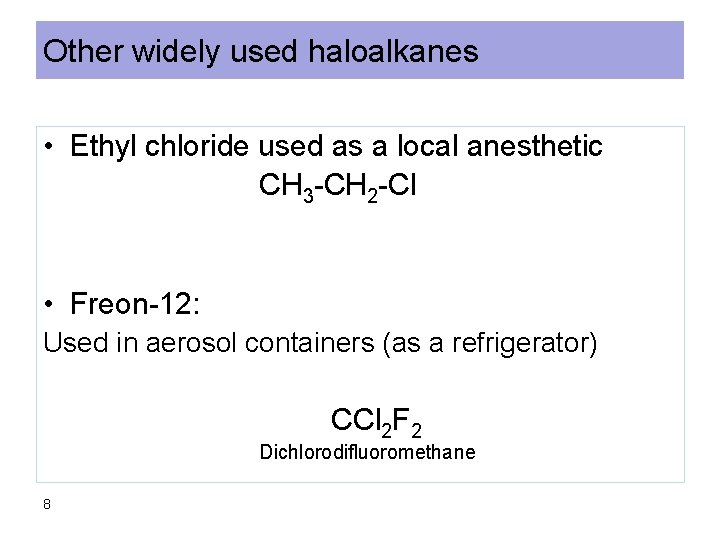
Other widely used haloalkanes • Ethyl chloride used as a local anesthetic CH 3 -CH 2 -Cl • Freon-12: Used in aerosol containers (as a refrigerator) CCl 2 F 2 Dichlorodifluoromethane 8

Alcohols, Thiols, Phenols, Ether 9

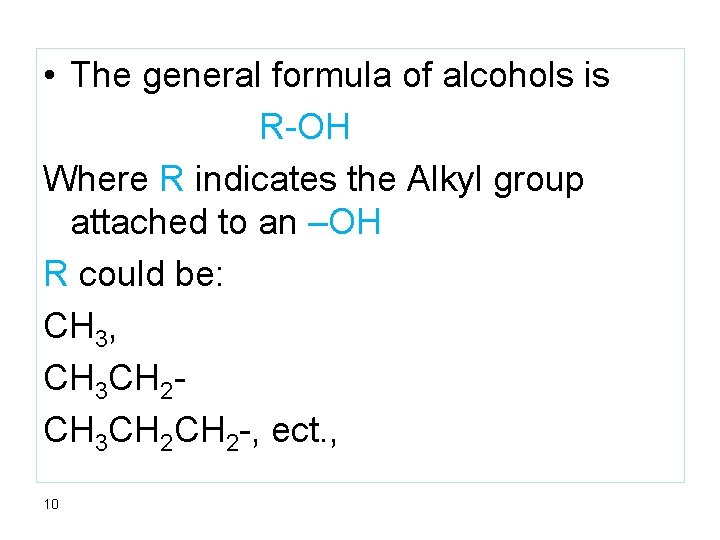
• The general formula of alcohols is R-OH Where R indicates the Alkyl group attached to an –OH R could be: CH 3, CH 3 CH 2 CH 2 -, ect. , 10

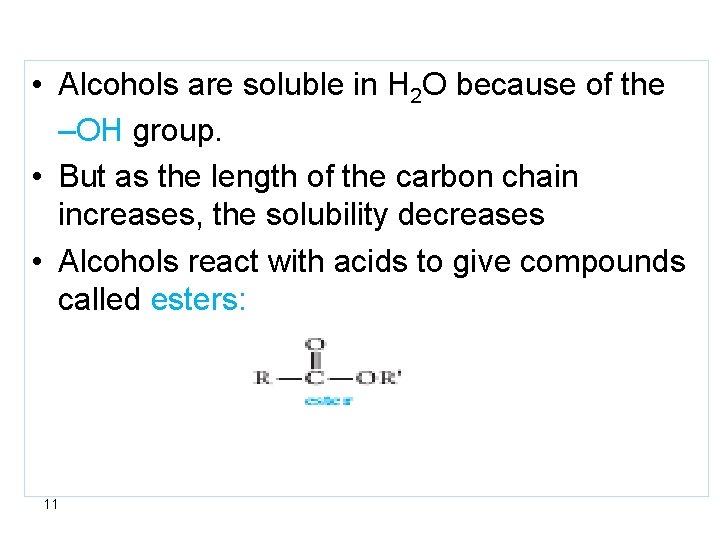
• Alcohols are soluble in H 2 O because of the –OH group. • But as the length of the carbon chain increases, the solubility decreases • Alcohols react with acids to give compounds called esters: 11

USES OF ALCOHOLS 1. Methyl • • alcohol (methanol) CH 3 OH Commonly Known as wood alcohol The U. S. production of methanol is 1. 4 billion gallons per year. Most of it is used to produce formaldehyde and other chemicals, Is also used as an anti-freeze. Used as a solvent in many industrial reactions. 12

• It should never be applied to the body neither should the vapors be inhaled because it can be absorbed both through the skin and through the respiratory tract. • Ingestion as little as 15 ml can cause blindness (it is oxidized to formaldehyde (CH 2=O), which binds to opsin, preventing formation of rhodopsin, the light-sensitive pigment needed for vision. • While 30 ml are enough to cause death. 13

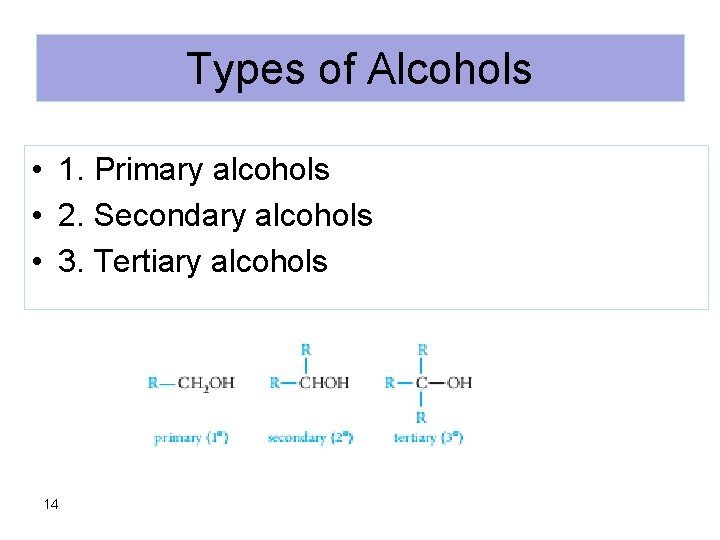
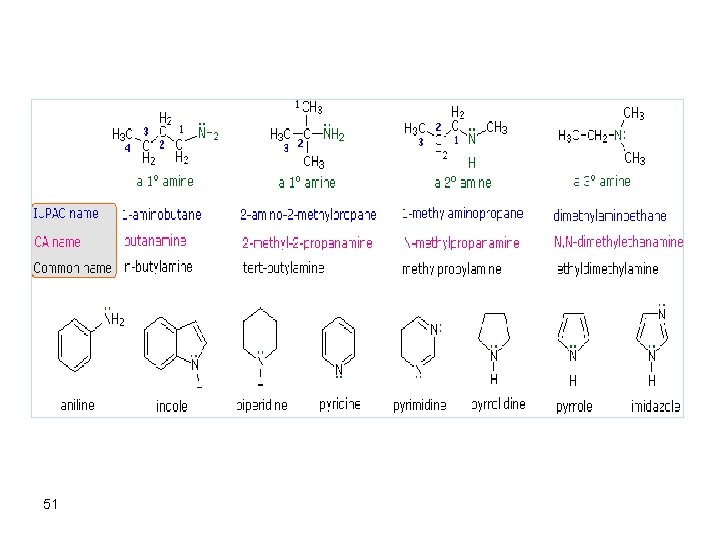
Types of Alcohols • 1. Primary alcohols • 2. Secondary alcohols • 3. Tertiary alcohols 14

Reactions of Alcohols • 1. Dehydration of alcohols: Alcohols can be dehydrated by heating them with a strong acid. For example, When ethanol is heated at 180°C with a small amount of concentrated sulfuric acid, a good yield of ethylene is obtained. 15

• 2. Formation of Ethers 16

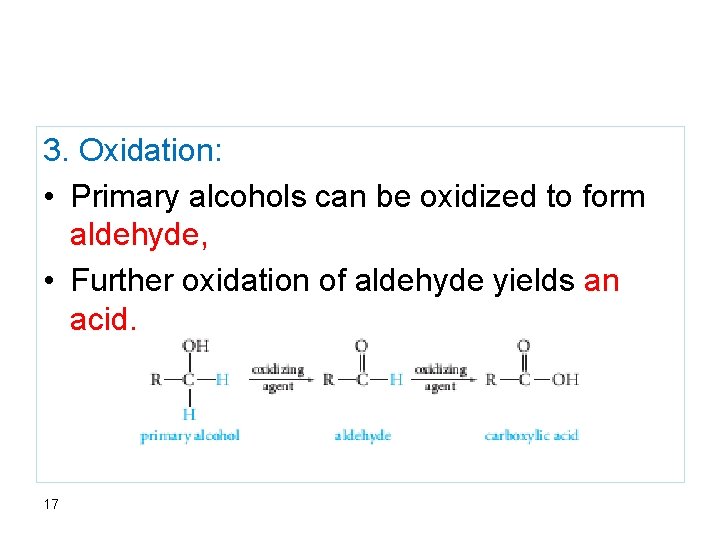
3. Oxidation: • Primary alcohols can be oxidized to form aldehyde, • Further oxidation of aldehyde yields an acid. 17

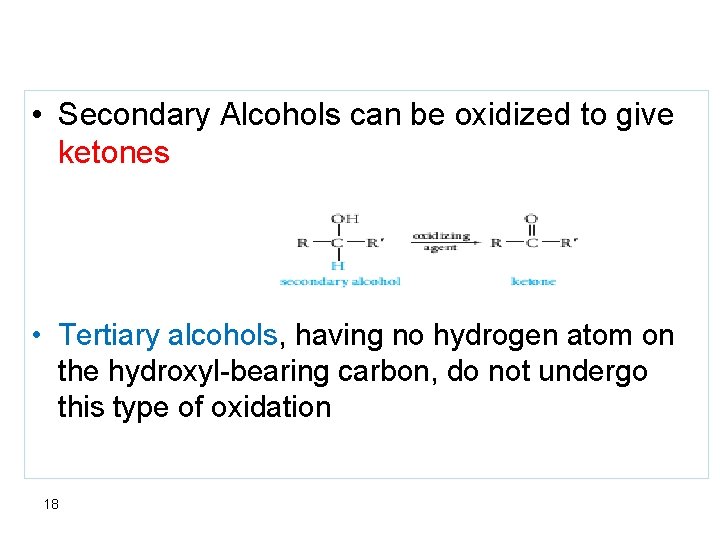
• Secondary Alcohols can be oxidized to give ketones • Tertiary alcohols, having no hydrogen atom on the hydroxyl-bearing carbon, do not undergo this type of oxidation 18

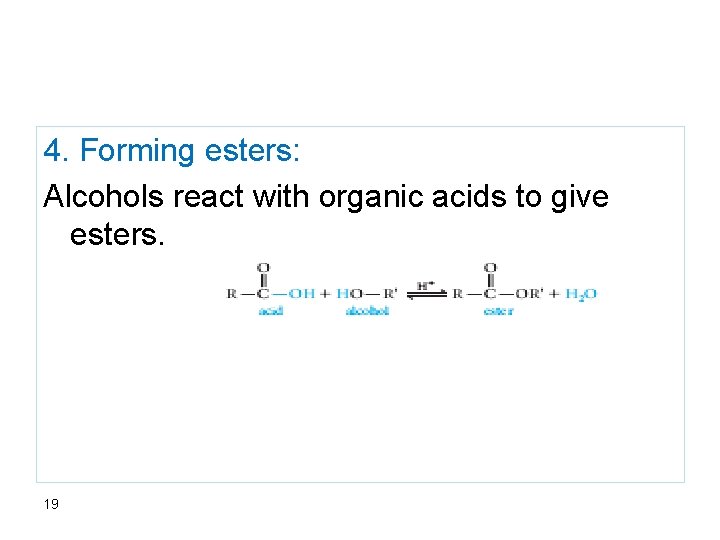
4. Forming esters: Alcohols react with organic acids to give esters. 19

2. Thiols • Are sulfur analogs of alcohols and contain an -SH functional group in place of an –OH group 20

Properties of thiols • Many thiols are found in nature. • They have unpleasant odor. • Onion when cut, releases 1 -propanethiol HS-CH 2 -CH 3 • The odor of garlic is due to thiols. • Skunk when attacked it releases thiols. • Thiols added to heating - & cooking- gas to detect gas leak. 21

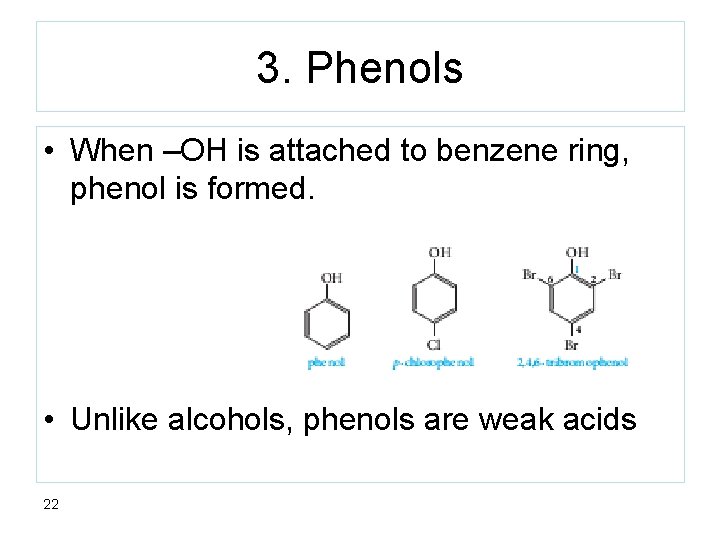
3. Phenols • When –OH is attached to benzene ring, phenol is formed. • Unlike alcohols, phenols are weak acids 22

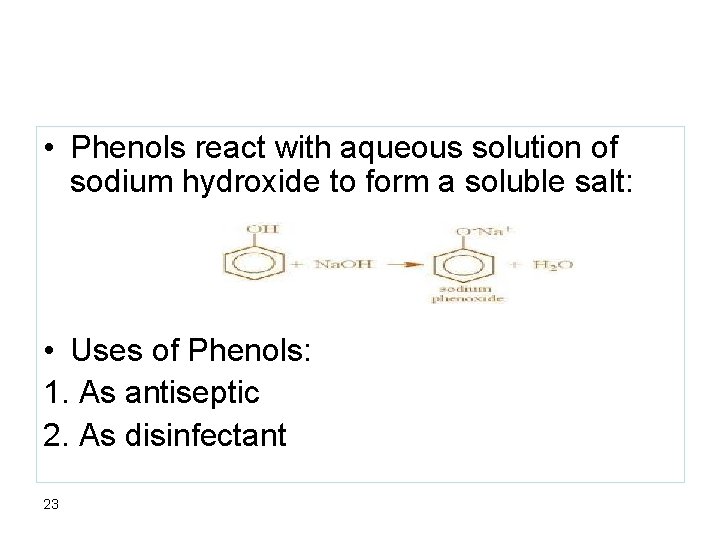
• Phenols react with aqueous solution of sodium hydroxide to form a soluble salt: • Uses of Phenols: 1. As antiseptic 2. As disinfectant 23

4. ethers • All ethers are compounds in which two organic groups are connected to a single oxygen atom. • The general formula for an ether is R-O-R’ • where R and R’ may be identical or different, and they may be alkyl or aryl groups. • In the common anesthetic, both R and R’ are ethyl groups. CH 3 CH 2 -O-CH 2 CH 3 24

Properties of ethers • Low boiling point • They are good solvents because they do not react with solutions. • Ethyl ether is used to extract organic materials from naturally occurring substances. 25

Ether as anesthetic • Used as a general anesthetic Advantages: 1. Easy to administer 2. Excellent muscular relaxant 3. Little effect on respiration, blood pressure 26

Disadventage: 1. Very flammable 2. Irritating to membrane of respiratory tract • Today ether as anesthetic has been replaced by: • Halothane • Nitrous oxide • Enflurane 27

Aldehydes, Ketones, Carboxylic Acids, And Amines 28

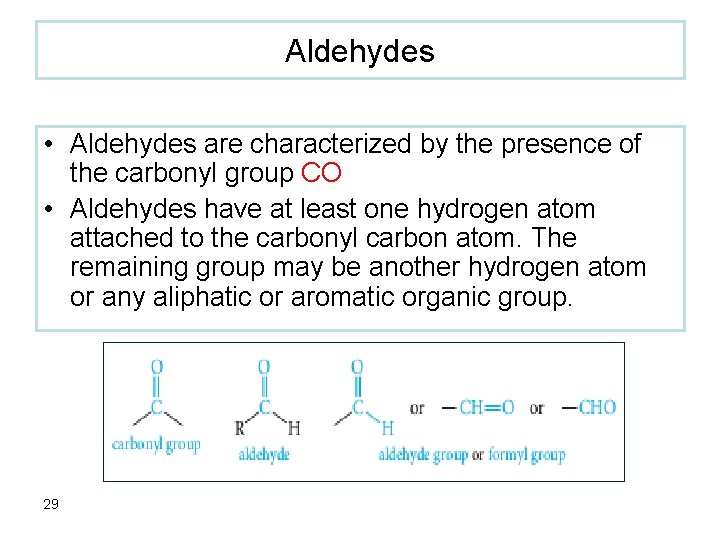
Aldehydes • Aldehydes are characterized by the presence of the carbonyl group CO • Aldehydes have at least one hydrogen atom attached to the carbonyl carbon atom. The remaining group may be another hydrogen atom or any aliphatic or aromatic organic group. 29

Uses of Aldehydes 1. Formalin is used as a disinfectant and preservative (used to preserve biologic specimens). 2. Formaldehyde is mostly used in the manufacture of plastics, building insulation. 3. Fumes of formaldehyde solutions are very irritating, so should not be used directly on a patient skin 30

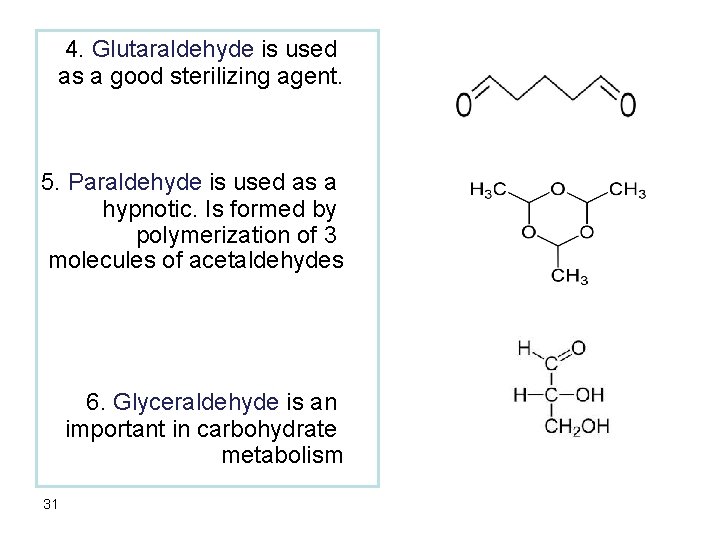
4. Glutaraldehyde is used as a good sterilizing agent. 5. Paraldehyde is used as a hypnotic. Is formed by polymerization of 3 molecules of acetaldehydes 6. Glyceraldehyde is an important in carbohydrate metabolism 31

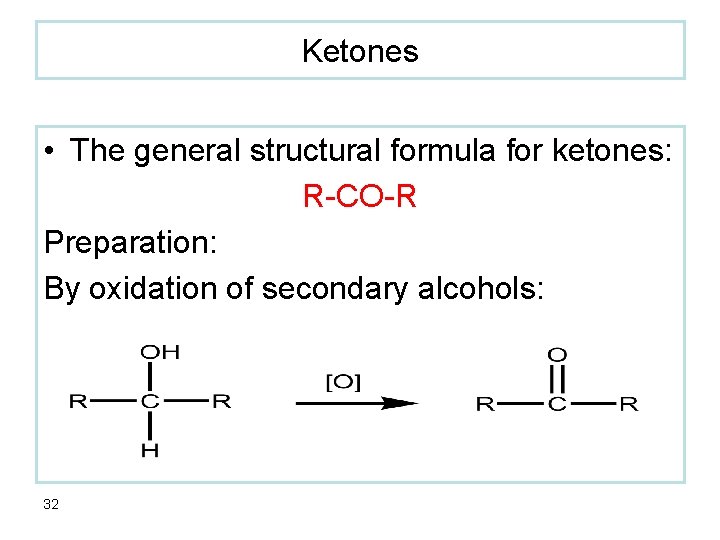
Ketones • The general structural formula for ketones: R-CO-R Preparation: By oxidation of secondary alcohols: 32

Uses of ketones 1. Acetone is a good solvent for fats and oils 2. Acetone is used in fingernail polish and polish removal 3. Acetone is normally present in small amounts in blood & urine. But in diabetes mellitus it is present in higher concentration. 4. Dihydroxyacetone is an intermediate in carbohydrate metabolism HO-CH 2 -CO-CH 2 OH 33

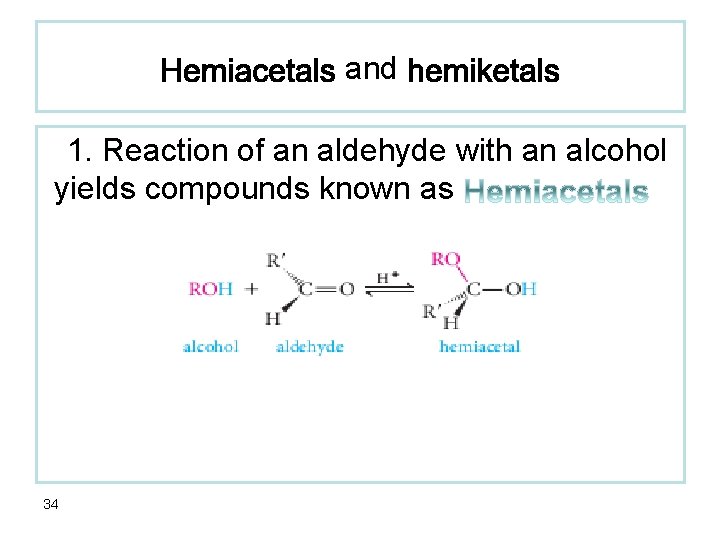
and 1. Reaction of an aldehyde with an alcohol yields compounds known as 34

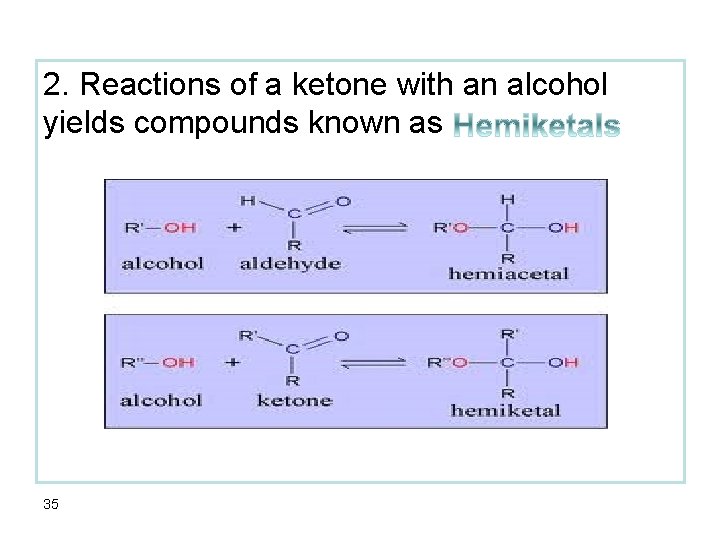
2. Reactions of a ketone with an alcohol yields compounds known as 35

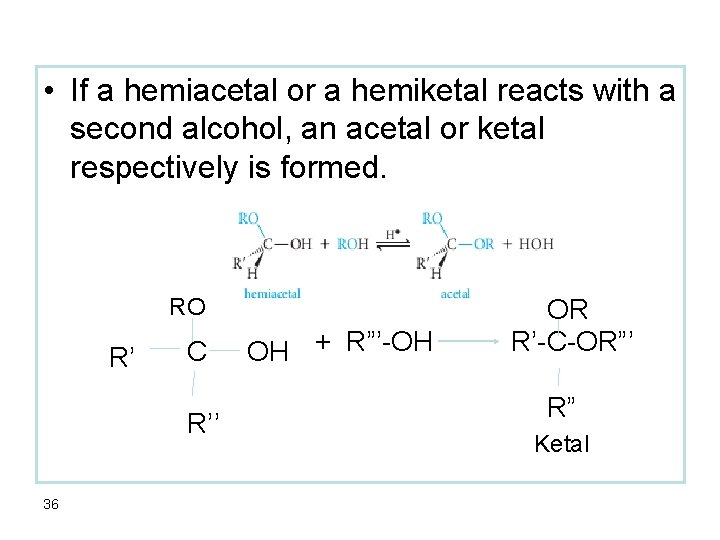
• If a hemiacetal or a hemiketal reacts with a second alcohol, an acetal or ketal respectively is formed. RO R’ C R’’ 36 OH + R”’-OH OR R’-C-OR”’ R” Ketal

• The Hemiacetals/Acetals & Hemiketal/Ketals are important in understanding the structures of monosaccharides 37

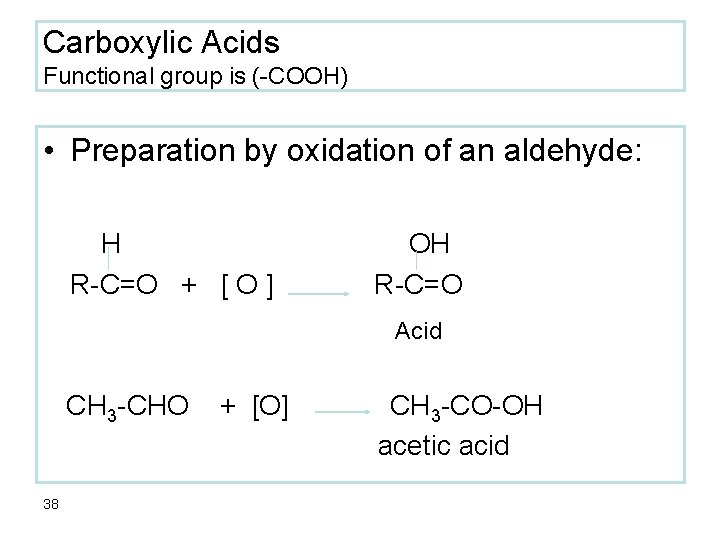
Carboxylic Acids Functional group is (-COOH) • Preparation by oxidation of an aldehyde: H R-C=O + [ O ] OH R-C=O Acid CH 3 -CHO 38 + [O] CH 3 -CO-OH acetic acid

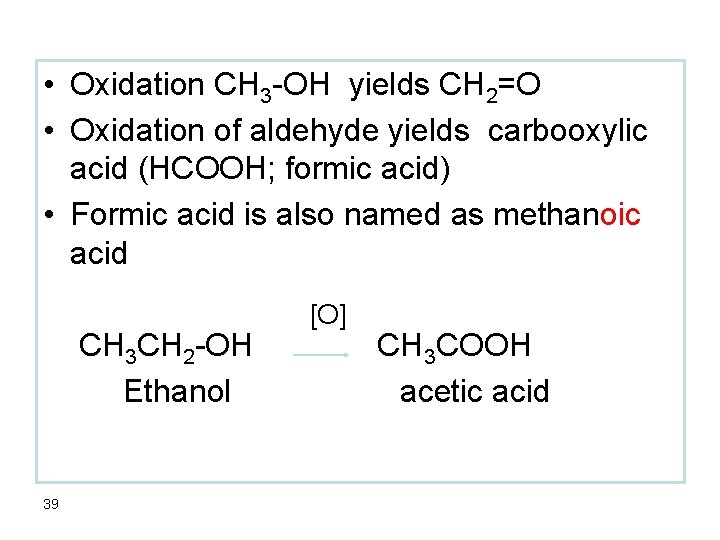
• Oxidation CH 3 -OH yields CH 2=O • Oxidation of aldehyde yields carbooxylic acid (HCOOH; formic acid) • Formic acid is also named as methanoic acid CH 3 CH 2 -OH Ethanol 39 [O] CH 3 COOH acetic acid

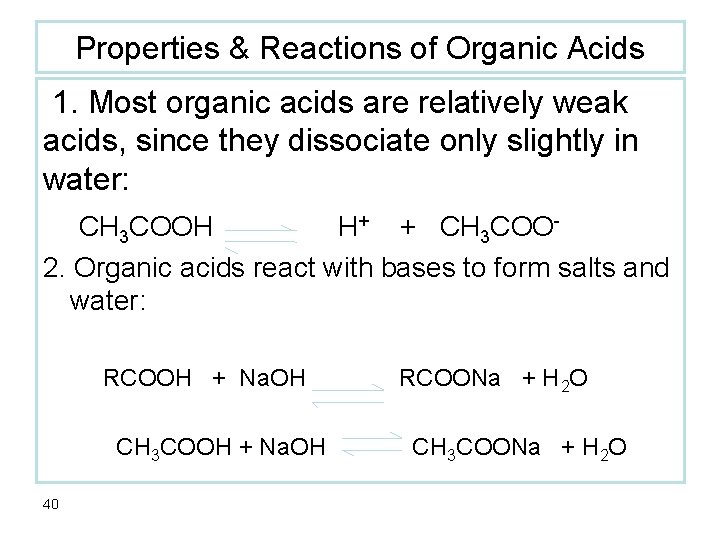
Properties & Reactions of Organic Acids 1. Most organic acids are relatively weak acids, since they dissociate only slightly in water: CH 3 COOH H+ + CH 3 COO 2. Organic acids react with bases to form salts and water: RCOOH + Na. OH CH 3 COOH + Na. OH 40 RCOONa + H 2 O CH 3 COONa + H 2 O

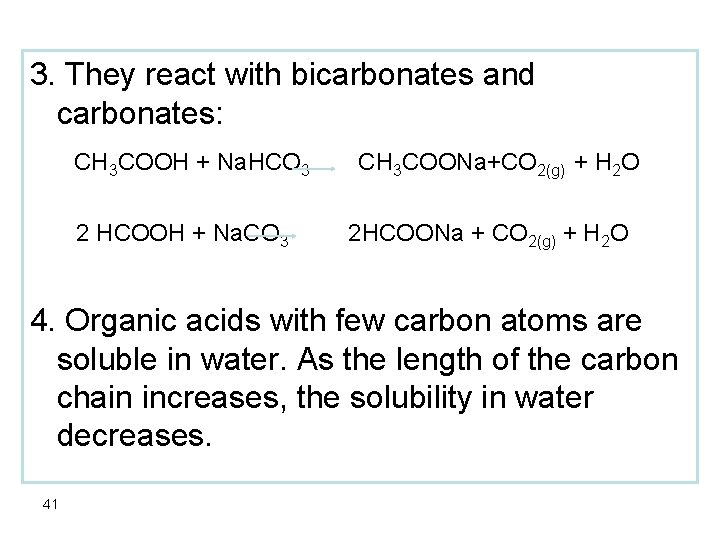
3. They react with bicarbonates and carbonates: CH 3 COOH + Na. HCO 3 2 HCOOH + Na. CO 3 CH 3 COONa+CO 2(g) + H 2 O 2 HCOONa + CO 2(g) + H 2 O 4. Organic acids with few carbon atoms are soluble in water. As the length of the carbon chain increases, the solubility in water decreases. 41

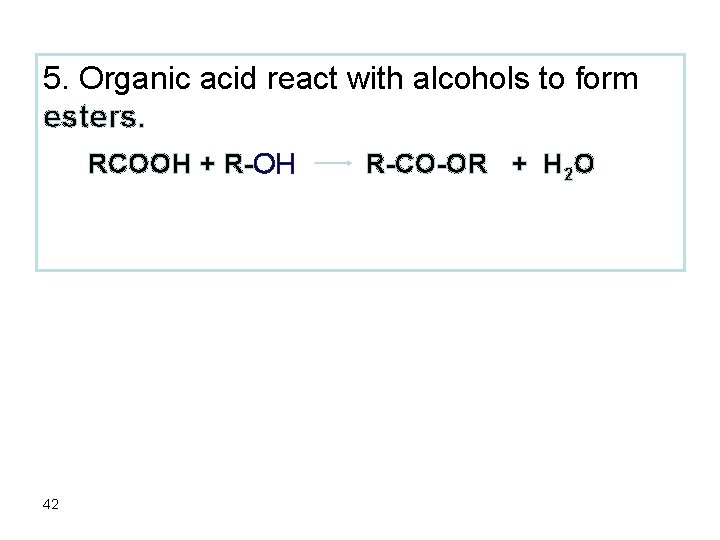
5. Organic acid react with alcohols to form esters. RCOOH + R-OH 42 R-CO-OR + H 2 O

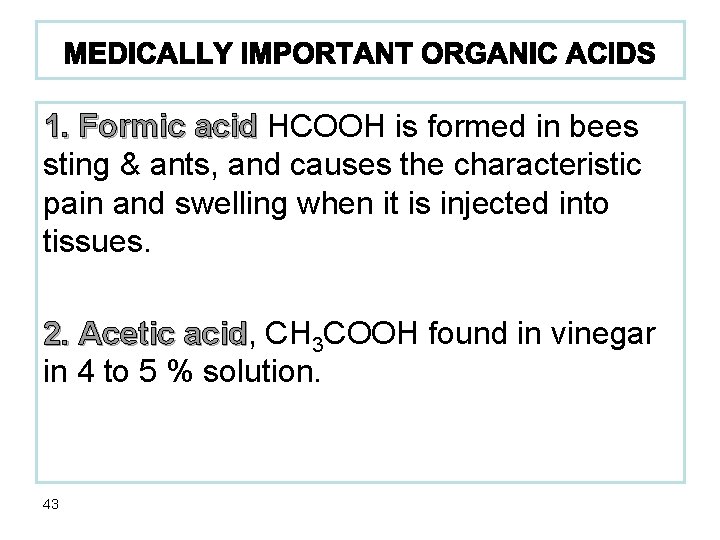
1. Formic acid HCOOH is formed in bees sting & ants, and causes the characteristic pain and swelling when it is injected into tissues. 2. Acetic acid, acid CH 3 COOH found in vinegar in 4 to 5 % solution. 43

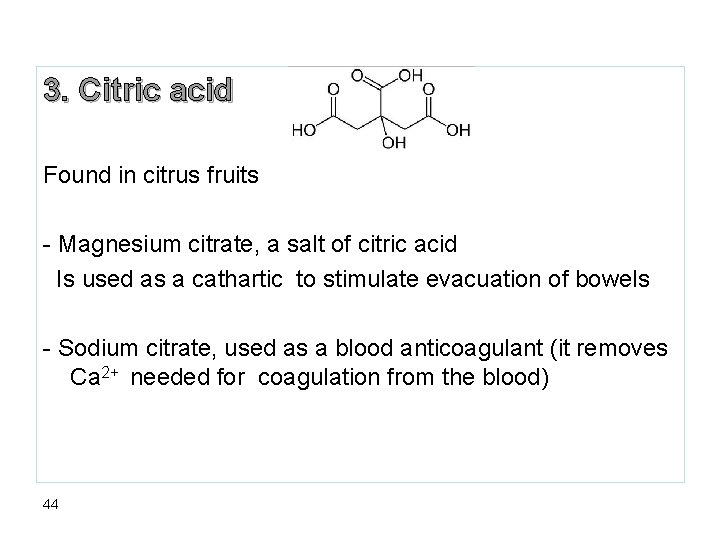
3. Citric acid Found in citrus fruits - Magnesium citrate, a salt of citric acid Is used as a cathartic to stimulate evacuation of bowels - Sodium citrate, used as a blood anticoagulant (it removes Ca 2+ needed for coagulation from the blood) 44

4. Lactic Acid CH 3 -CH(OH)-COOH • Found in sour milk. • It is formed whenever the body produces energy anaerobically 5. Oxalic acid HOOC-COOH • A strong naturally occurring acid • Used to remove stains from clothing • It is poisonous when taken internally 45

• Oxalate salts prevent clotting by chelating Ca 2+ From blood (however it should not be applied directly on the blood due to it’s toxicity) 6. Pyruvic acid - Produced during glycolysis. - In muscle pyruvate is reduced to lactic acid during anaerobic exercise - In tissues pyruvate is converted to acetyl. Coenzyme A, which enters the Krebs cycle. 46

7. Tartaric acid • It is both an acid & alcohol HOOC-CH-CH-COOH OH OH 2, 3 -dihydroxysuccinic acid Found in several fruits such as grapes and bananas Potassium hydrogen tartarate is used in making baking powder. Rochelle salt or potassium sodium tartarate used as a mild cathartic. 47
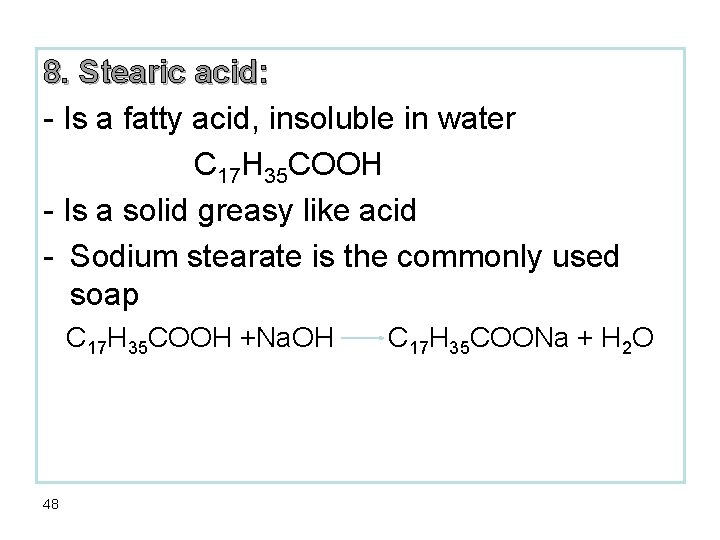
8. Stearic acid: - Is a fatty acid, insoluble in water C 17 H 35 COOH - Is a solid greasy like acid - Sodium stearate is the commonly used soap C 17 H 35 COOH +Na. OH 48 C 17 H 35 COONa + H 2 O

49

50

51