1 6 History Units of Length began by

1

6 History: Units of Length began by using parts of the body: The English system began in… Cubityou length of a it: person’s forearm England …yup, guessed [earliest known measurement] Foot size of a person’s foot [usually the But, king’s measuring or ruler’s]things was Inch size of the before middle bone a person’s important long weofhad a pinky finger ‘system’

Units of volume began by filling objects with stuff and counting how many fit: Grain fill a space with wheat/oat seeds and count them Carat same as above, but using Carob seeds. Still used to measure diamonds today. 3
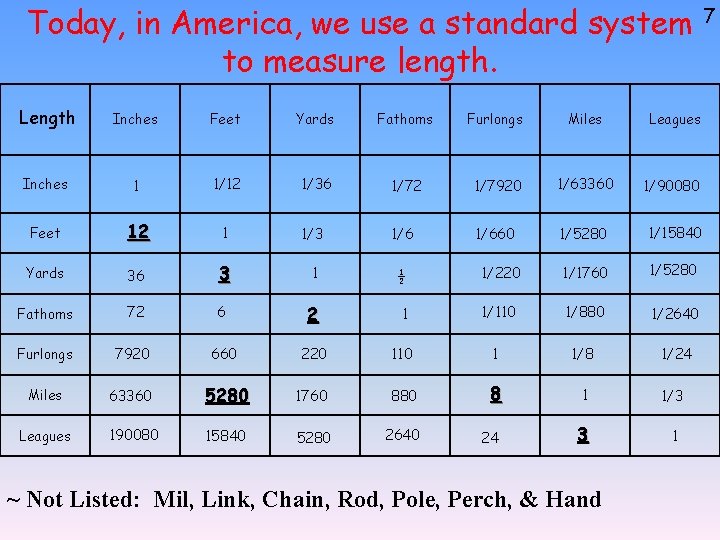
Today, in America, we use a standard system to measure length. Length Inches Feet Yards Fathoms Furlongs Miles Leagues Inches 1 1/12 1/36 1/72 1/7920 1/63360 1/90080 Feet 12 1 1/3 1/660 1/5280 1/15840 Yards 36 3 1 ½ 1/220 1/1760 1/5280 Fathoms 72 6 2 1 1/110 1/880 1/2640 Furlongs 7920 660 220 110 1 1/8 1/24 Miles 63360 5280 1760 880 8 1 1/3 Leagues 190080 15840 5280 2640 24 3 1 ~ Not Listed: Mil, Link, Chain, Rod, Pole, Perch, & Hand 7

If we were to use this ‘standard’ English 5 We could be here all day!!! This system in science class you would have to would beofcrazy to try to know what all those measurements were, plus you would and also need know all memorize use, toso of the measurements for the following: Power: Mass: Energy: Volume: Horsepower, inch-pound/second, foot. Pound, apothecary pound, calorie, Calorie, inch-pound, foot-pound, Gallon, liquid quart, dry ounce quart, liquid pound/second, yard-pound/second… apothecary ounce, dram, apothecary yard-pound, mile-pound… pint, dry pint, fluid ounce, teaspoon, dram, grain, carat, tablespoon, fluidscruple, dram, gill, peck, pennyweight, short long bushel, cubic inch, hundredweight, cubic foot, cubic hundredweight, short ton, long ton… yard, cubic fathom… Let’s Not Do That!

The Beauty of the Metric System You can measure all of the things listed above using the following units: Length: Meter (m) Volume: Liter (L) Mass: Gram (g) Energy: Joule (J) Power: Watt (W) 3

Metric Meanings & Measurements The system of measurement used by most people around the world is called the International System of Units (SI). It is the modern version of the Metric System. The Metric system was invented in 1670 by a French Astronomer & Mathematician The specific measurement “standards”named of the Gabriel Mouton. meter, & gram changed as science and meter liter, liter technology improved. Today there are very exact specifications for each. These amounts were agreed upon by many nations (including the US) at the Treaty of Meter in 1875. 2

Length Definition: Length is a measurement from here to there Tool: You measure length using a Meter Stick. 30 mm 16. 8 cm
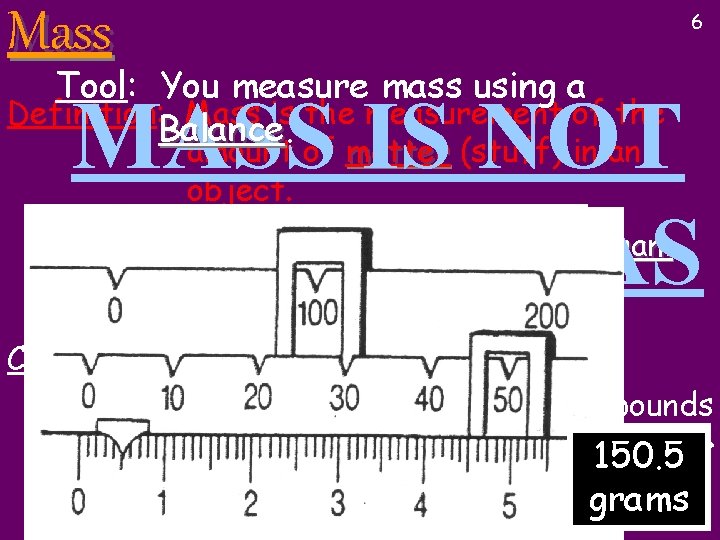
Mass 6 Tool: You measure mass using a Definition: Mass is the measurement of the Balance MASS IS NOT THE SAME AS WEIGHT amount of matter (stuff) in an object. The standard unit of mass is the Kilogram (grams are too tiny to be useful) Conversions: One Kilogram (Kg) is a little over two pounds {On 132. 5 EARTH} 150. 5 1 Kg = 2. 2 lbs grams

Weight 5 The moon’s gravity is 1/6 of that of the Earth’s. So, if you weighed 120 pounds on Definition: Weight is a measure of how Earth and traveled to the moon what gravity affects mass. would happen? (remember, Gravity is the Tool: You measure weight using a Scale force of attraction between any two objects) Weight: 20 lbs The standard unit for measuring weight is Mass: IT WOULD NOT CHANGE!!! the Newton

4 Area Definition: Area is the amount of flat Instructions: space inside a set of Measure the Length of the object = L units boundaries. Measure the Width of the object = W units To measure area you have to combine two units of Length 2 m Tools: the same for measuring length (meter stick) 3 m Multiply them together: 2 m x 3 m = 6 m 2 2 Area = ( L x W ) Units

Volume 4 Definition: Volume is the amount of 3 D Tools: space an object takes up. For Solids: use the same tools you would for length (meter To measure volume: stick) unless using Water For solids: combine three units length Displacement (seeoflater notes) (L, W, H) or For Liquids: use a Flask, Flask Beaker, Beaker or For Liquids: use Liters Graduated Cylinder
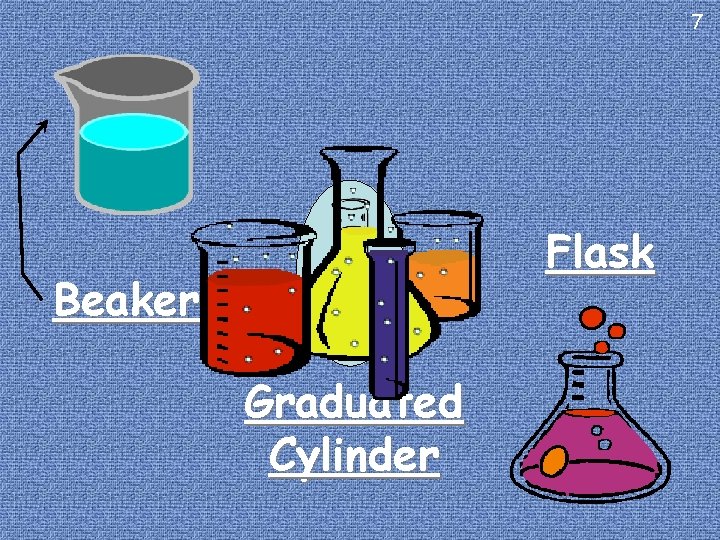
7 Flask Beaker Graduated Cylinder
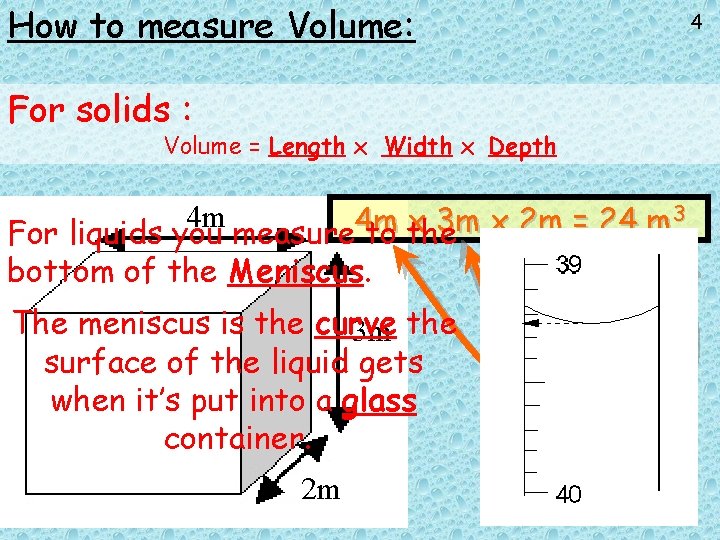
How to measure Volume: For solids : Volume = Length x Width x Depth 3 4 m 4 m x 3 m x 2 m = 24 m For liquids you measure to the bottom of the Meniscus. The meniscus is the curve 3 m the surface of the liquid gets when it’s put into a glass container. 2 m 4
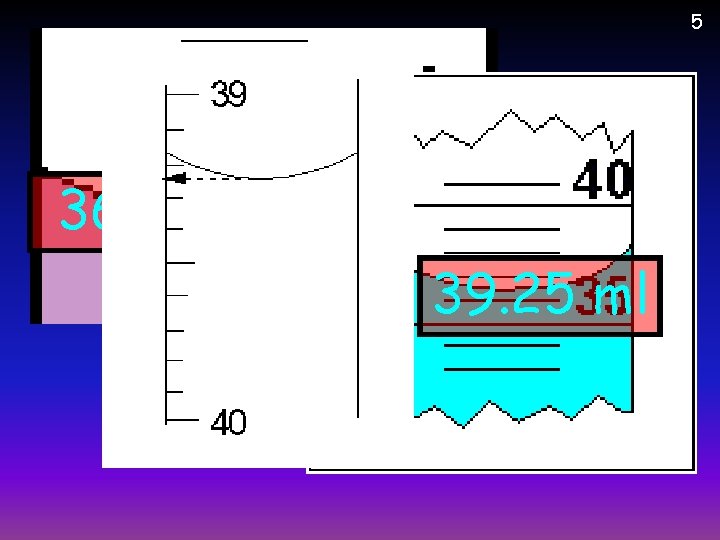
5 36. 5 ml 39. 25 ml 73 ml

Measuring the volume of an Irregularly shaped solid object: Let’s face it; very few solid objects are perfect cubes. There is an extremely accurate method, though, of measuring the volume of nearly any solid object, regardless of shape. It fact, it was invented by Archimedes during the Greek Empire, circa 1500 BCE. This is known as Water Displacement. 3
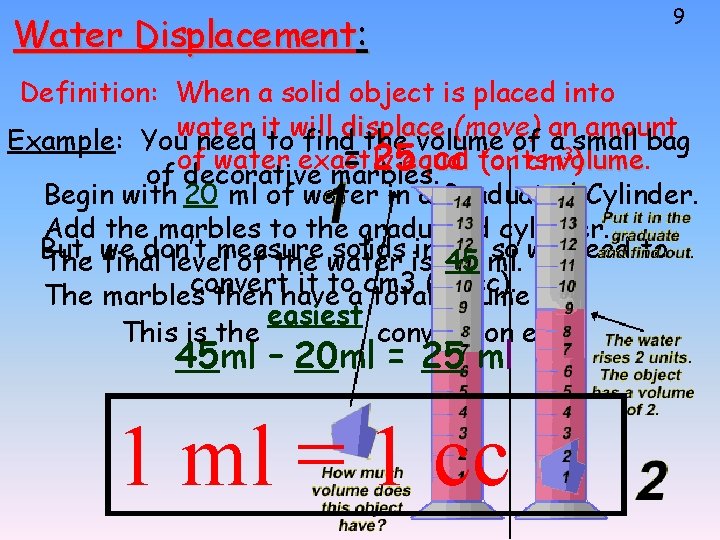
Water Displacement: 9 Definition: When a solid object is placed into water it will displace (move) an amount Example: You need to find the volume of a 3 small bag of water exactly = 25 equal cc to (orits cmvolume. ) volume of decorative marbles. Begin with 20 ml of water in a Graduated Cylinder. Add the marbles to the graduated cylinder. But, don’t solids is in 45 ml, ml. so we need to The we final levelmeasure of the water convert it to cm 3 (or cc). The marbles then have a total volume of: easiest This is the conversion ever. 45 ml – 20 ml = 25 ml 1 ml = 1 cc
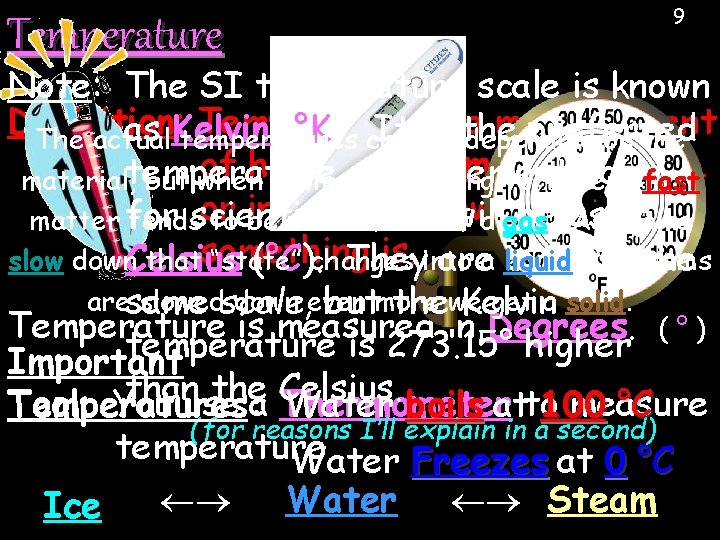
Temperature 9 Note: The SI temperature scale is known Definition: Temperature is a measurement as Kelvin (°K). It is the preferred The actual temperatures change depending on the of how fast are very moving, measurement scale material, temperature but when atoms are atoms moving very, fast or in in general, hot or atoms cold Wehow will be using matter for tendsscientists. to be the form of a gas. As is. into slow down. Celsius thatsomething “state” If atoms (°C). changes They area liquid. exactly the aresame slowedscale, down even get a solid. butmore thewe Kelvin Temperature is measured in Degrees. ( ° ) temperature is 273. 15° higher Important than the Celsius. Tool: You use a measure Temperatures: Thermometer Water boils atto 100 °C (for reasons I’ll explain in a second) temperature. Water Freezes at 0 °C Water Steam Ice
Temperature There is a theoretical temperature where atoms STOP moving. It is called Absolute Zero They know what the temperature is, but have never been able to reach it. No one knows what would happen to matter at this temperature. 1
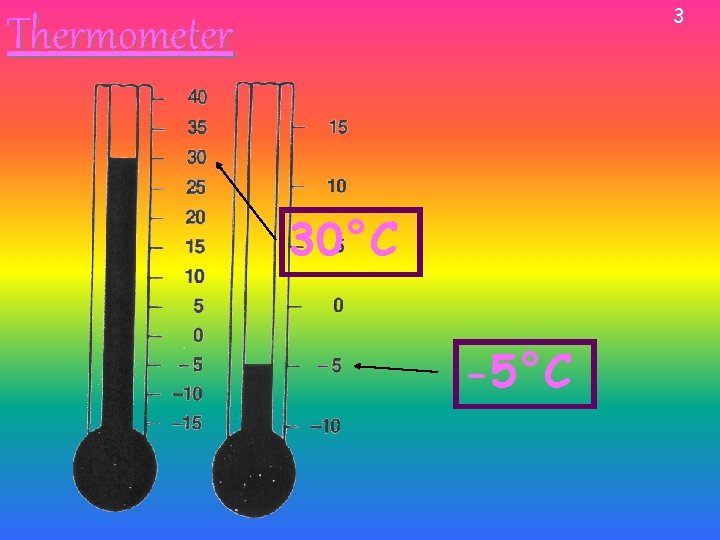
3 Thermometer 30°C -5°C
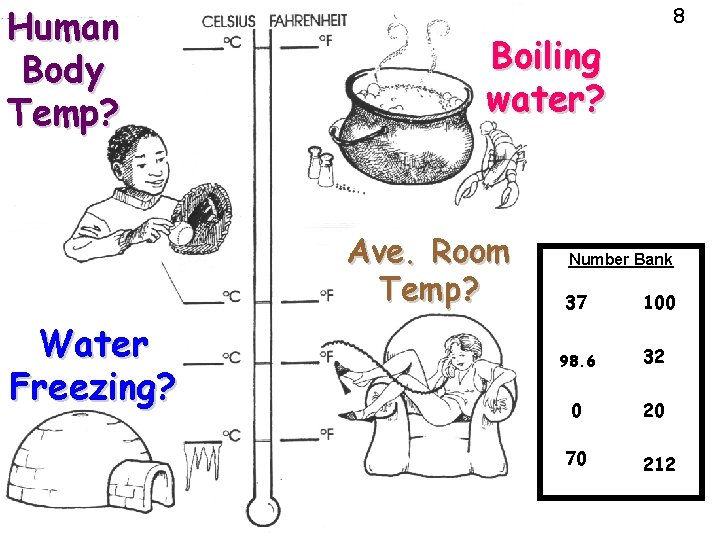
Human Body Temp? Boiling water? Ave. Room Temp? Water Freezing? 8 Number Bank 37 100 98. 6 32 0 20 70 212

Density 12 Definition: amount of Mass a VERY HARDThe QUESTION: material in a/ certain Equation: =has Mass Volume Would a. Density rectangular block measuring Volume 10 meters x 2 meters x 1 meter, Tool: You use the same tools for A cube that is 3 centimeters with a mass of 1000 Kg float? on all measuring volume & mass. sides has a mass of 45 grams. Explain. What is. Density: its density? Important The density of liquid 3 3 Density = 45 grams / 9 cm 3 water Density = 1000 Kg / 20 m = 1. 0 g/cm So, Mass anything = 1000 with Kg a= density Density 5 g/cm 3 smaller than 3 would float. Anything with 1. 0 g/cm a Volume m x 2 using m x the 1 msame = 20 units m 3 for Density =is 10 measured 3 would 3 sink. 3 density larger than 1. 0 g/cm mass & volume; usually g/cm or kg/m. Density = 50 Kg/m 3

Lets think about this… 3 Density of object = 50 Kg/m Volume = 10 m x 2 m x 100 3 Density of water = 1 g/cm Mass = 1000 Volume = 1000 Kgcm x 200 cm x 1000 3 Volume = 20, 000 cm The Units don’t match!!! Mass Density = 1, 000, 000 g g / 20, 000 cm 3 3 this… We=need rethink Density. 05 tog/cm How many cm are in a m? IT FLOATS! How many g are in a kg? So well, in fact, it could hold another 40, 000 lbs before sinking.
- Slides: 23