1 5 a Learning Outcomes define oxidation number

1. 5 a Learning Outcomes • define oxidation number, oxidation state • define oxidation and reduction in terms of change of oxidation numbers • define oxidising agent and reducing agent • state the rules for oxidation numbers (exclude peroxides, except for hydrogen peroxide) • calculate oxidation numbers of transition metals in their compounds and of other elements • use oxidation numbers in nomenclature of transition metal compounds

Learning Outcomes • arrange the electrochemical series of metals in order of their ease of oxidation (reactions, other than displacement reactions, not required)

1. 5 Oxidation & Reduction Also known as Redox Means something in terms of: -Addition or Removal Oxygen / Hydrogen -Electrons - Oxidation Numbers Introduction to topic via three simple examples
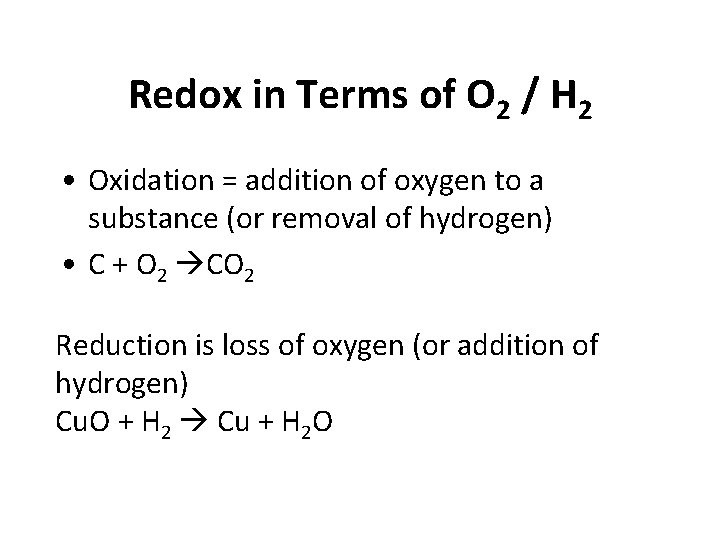
Redox in Terms of O 2 / H 2 • Oxidation = addition of oxygen to a substance (or removal of hydrogen) • C + O 2 CO 2 Reduction is loss of oxygen (or addition of hydrogen) Cu. O + H 2 Cu + H 2 O
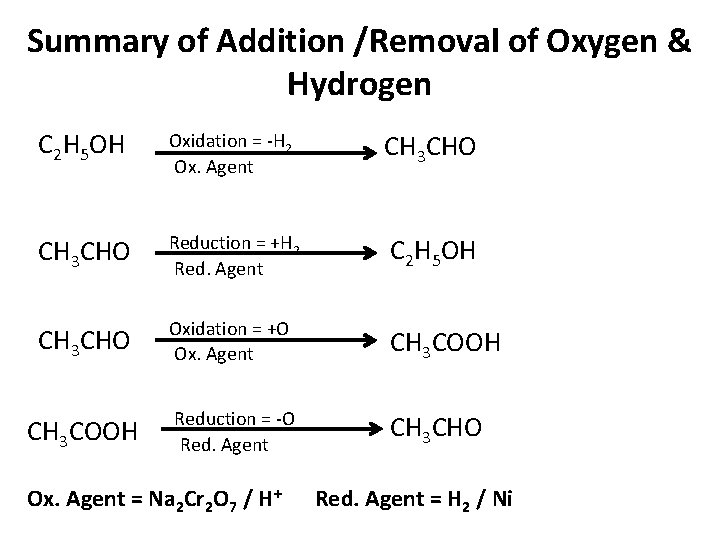
Summary of Addition /Removal of Oxygen & Hydrogen C 2 H 5 OH Oxidation = -H 2 Ox. Agent CH 3 CHO Reduction = +H 2 Red. Agent C 2 H 5 OH CH 3 CHO Oxidation = +O Ox. Agent CH 3 COOH Reduction = -O Red. Agent CH 3 CHO Ox. Agent = Na 2 Cr 2 O 7 / H+ Red. Agent = H 2 / Ni

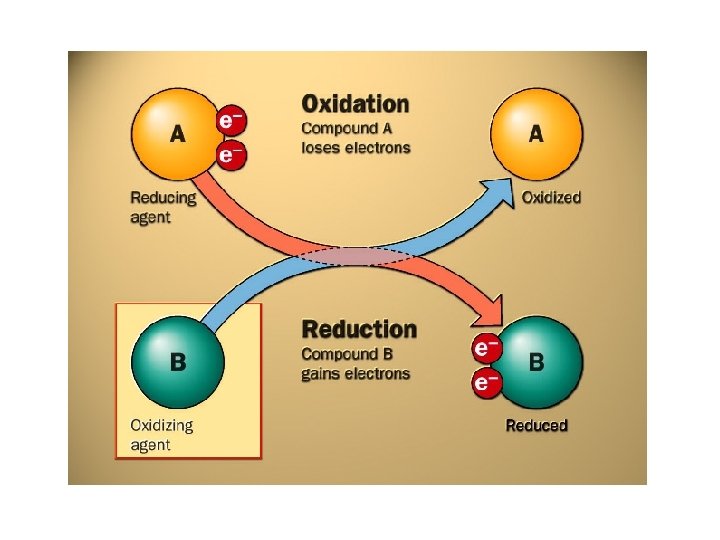
Oxidation in Terms of Electrons OILRIG Oxidation involves Loss of electrons Reduction Involves Gain of Electrons Species Oxidised is Reducing Agent Species Reduced is the Oxidising Agent
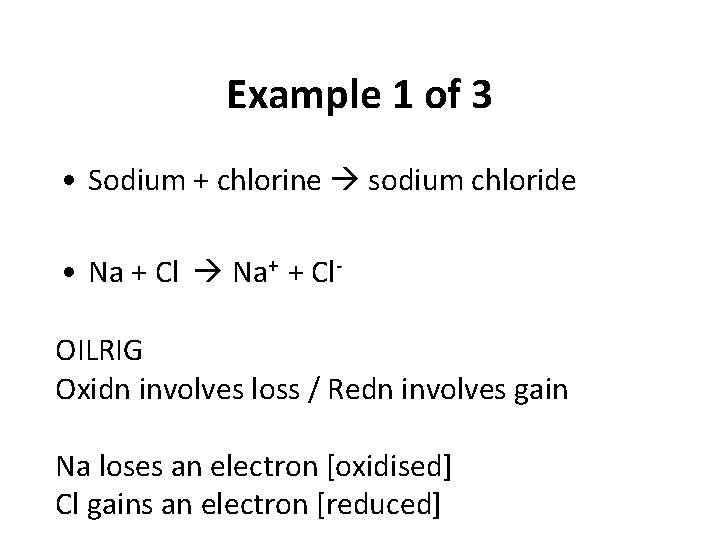
Example 1 of 3 • Sodium + chlorine sodium chloride • Na + Cl Na+ + Cl. OILRIG Oxidn involves loss / Redn involves gain Na loses an electron [oxidised] Cl gains an electron [reduced]

Example 2 of 3 • Magnesium + Oxygen magnesium oxide • Mg + O Mg. O • => Oxidn / Redn by inspection? Mg Mg+2 loses 2 electrons [oxidation] O O-2 gains 2 electrons [reduction]
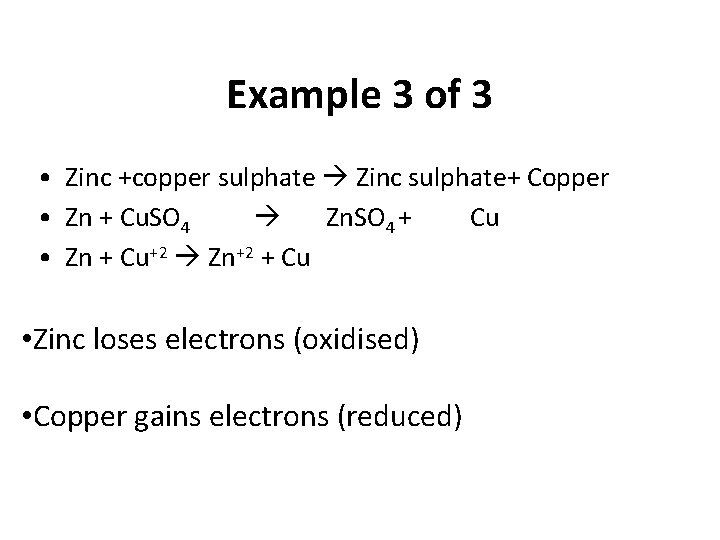
Example 3 of 3 • Zinc +copper sulphate Zinc sulphate+ Copper • Zn + Cu. SO 4 Zn. SO 4 + Cu • Zn + Cu+2 Zn+2 + Cu • Zinc loses electrons (oxidised) • Copper gains electrons (reduced)

Oxidising Agent • A substance that causes oxidation in another substance – the oxidising agent is itself reduced
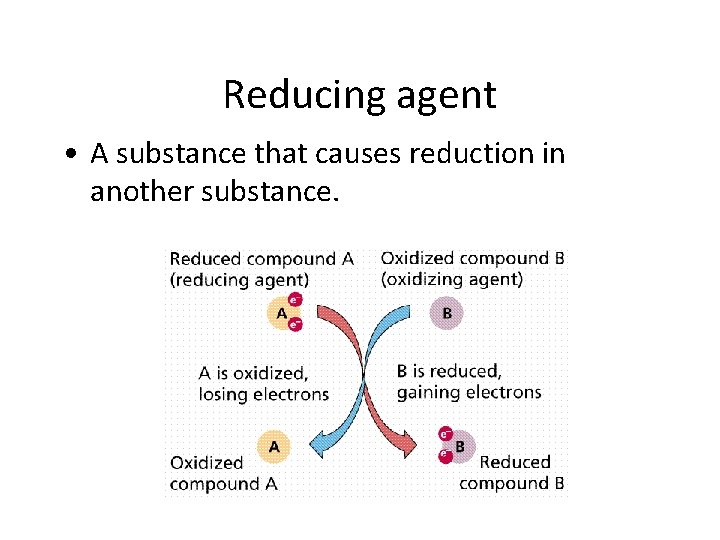
Reducing agent • A substance that causes reduction in another substance.
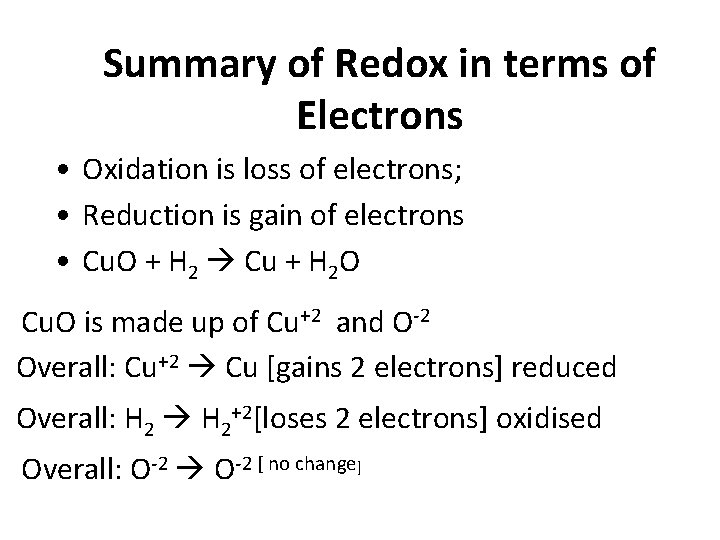
Summary of Redox in terms of Electrons • Oxidation is loss of electrons; • Reduction is gain of electrons • Cu. O + H 2 Cu + H 2 O Cu. O is made up of Cu+2 and O-2 Overall: Cu+2 Cu [gains 2 electrons] reduced Overall: H 2+2[loses 2 electrons] oxidised Overall: O-2 [ no change]

Summary of Redox in terms Oxidation numbers Oxidation Number: The charge that an atom has or appears to have assuming that the compound is ionic. Electrons always go to the most electronegative element • Oxidation is an increase on oxidation number • Reduction is a decrease in oxidation number.

Oxidation Number Rule 1 • • Elements on their own = 0 H 2 = 0 Zn = 0 Cl 2 = 0

Oxidation Number Rule 2 • • Ions = same as charge Cu +2 = +2 O-2 = -2 Cl-1 = -1
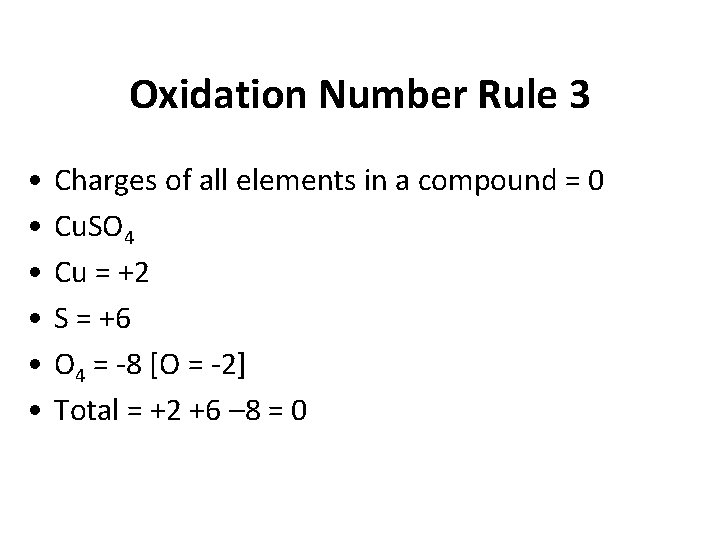
Oxidation Number Rule 3 • • • Charges of all elements in a compound = 0 Cu. SO 4 Cu = +2 S = +6 O 4 = -8 [O = -2] Total = +2 +6 – 8 = 0

Oxidation Number Rule 4 • Oxygen = -2 • Exceptions are • peroxides O = -1 [H 2 O 2, Na 2 O 2 ] • OF 2 O = +2, F = -1 (Oxygen diflouride)

Oxidation Number Rule 5 • Hydrogen = +1 • Exceptions are the metal hydrides • Na. H Na = +1, H = -1 (Sodium hydride) Mg. H 2 Mg = +2 each H = -1 Magnesium Hydride
![Oxidation Number Rule 6 • Halogens [ F, Cl, Br, I] are always – Oxidation Number Rule 6 • Halogens [ F, Cl, Br, I] are always –](http://slidetodoc.com/presentation_image_h2/1ce6d585fb7db891f82cb77ad4a3d23d/image-20.jpg)
Oxidation Number Rule 6 • Halogens [ F, Cl, Br, I] are always – 1 except when joined to a more electronegative element • Cl 2 O • Cl = +1, O = -2
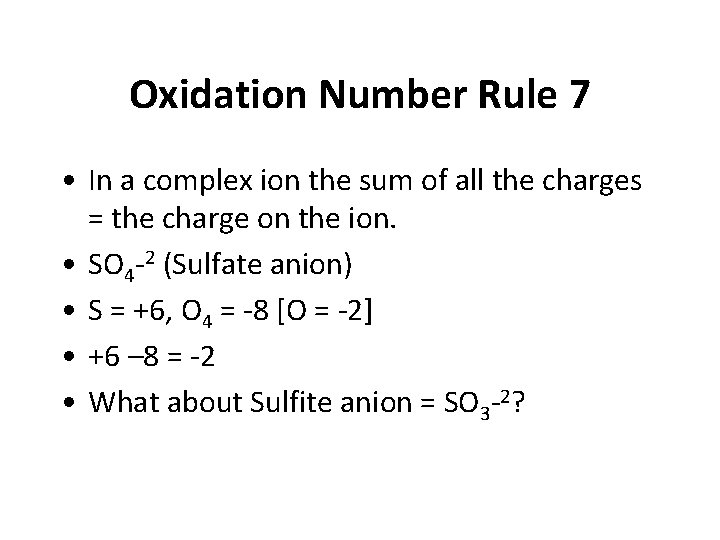
Oxidation Number Rule 7 • In a complex ion the sum of all the charges = the charge on the ion. • SO 4 -2 (Sulfate anion) • S = +6, O 4 = -8 [O = -2] • +6 – 8 = -2 • What about Sulfite anion = SO 3 -2?
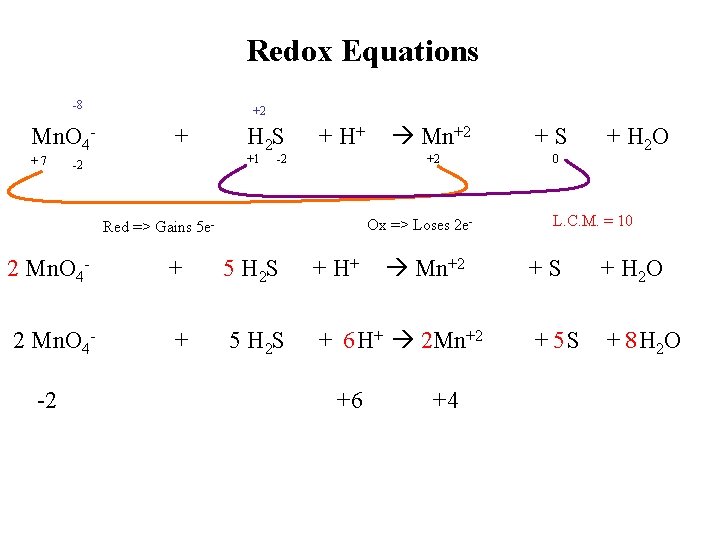
Redox Equations -8 Mn. O 4+7 +2 + H 2 S +1 -2 + H+ -2 Mn+2 +2 Ox => Loses 2 e- Red => Gains 5 e- Mn+2 2 Mn. O 4 - + 5 H 2 S + H+ 2 Mn. O 4 - + 5 H 2 S + 6 H+ 2 Mn+2 -2 +6 +4 +S 0 + H 2 O L. C. M. = 10 +S + H 2 O + 5 S + 8 H 2 O
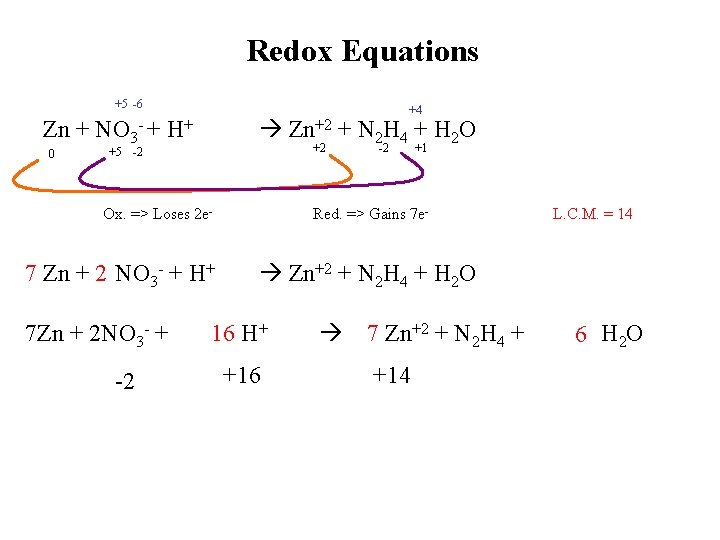
Redox Equations +5 -6 +4 Zn+2 + N 2 H 4 + H 2 O Zn + NO 3 - + H+ 0 +2 +5 -2 Ox. => Loses 2 e- 7 Zn + 2 NO 3 - + H+ 7 Zn + 2 NO 3 - + -2 -2 +1 Red. => Gains 7 e- L. C. M. = 14 Zn+2 + N 2 H 4 + H 2 O 16 H+ +16 7 Zn+2 + N 2 H 4 + +14 6 H 2 O
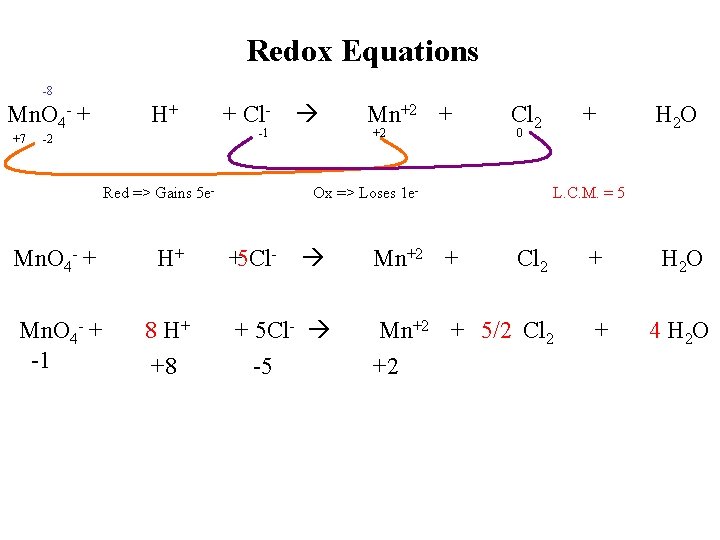
Redox Equations -8 Mn. O 4 - + +7 H+ -2 + Cl-1 Red => Gains 5 e- Mn. O 4 - + H+ Mn. O 4 - + -1 8 H+ +8 Mn+2 + +2 Cl 2 0 Ox => Loses 1 e- +5 Cl- + 5 Cl- -5 Mn+2 + + H 2 O L. C. M. = 5 Cl 2 + H 2 O Mn+2 + 5/2 Cl 2 +2 + 4 H 2 O
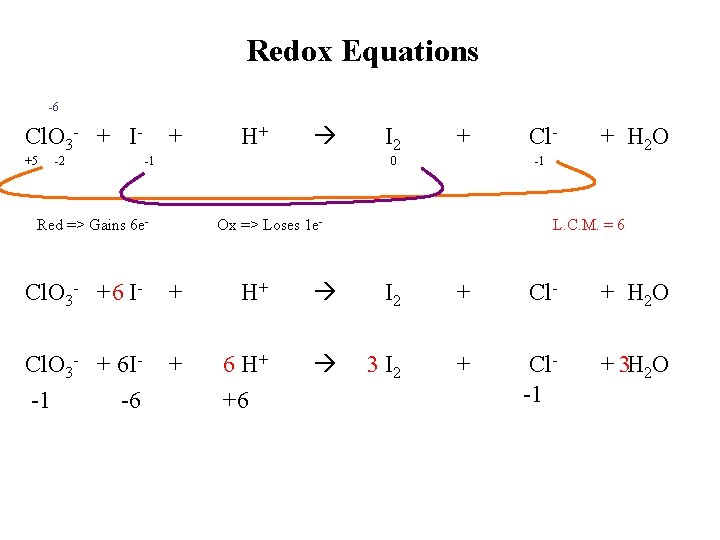
Redox Equations -6 Cl. O 3 - + I+5 -2 + H+ -1 I 2 + 0 Red => Gains 6 e- Cl- + H 2 O -1 Ox => Loses 1 e- L. C. M. = 6 Cl. O 3 - + 6 I- + H+ I 2 + Cl- + H 2 O Cl. O 3 - + 6 I-1 -6 + 6 H+ +6 3 I 2 + Cl-1 + 3 H 2 O
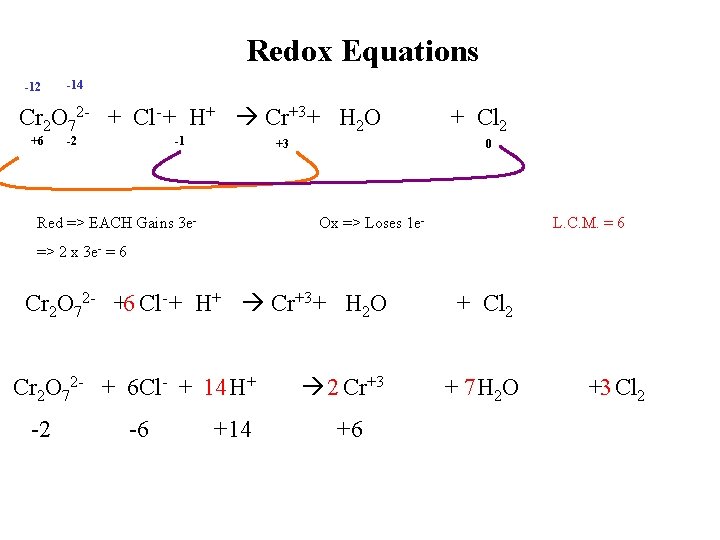
Redox Equations -12 -14 Cr 2 O 72 - + Cl- + H+ Cr+3 + H 2 O +6 -2 -1 +3 Red => EACH Gains 3 e- + Cl 2 0 Ox => Loses 1 e- L. C. M. = 6 => 2 x 3 e- = 6 Cr 2 O 72 - +6 Cl- + H+ Cr+3 + H 2 O + Cl 2 2 Cr+3 + 7 H 2 O Cr 2 O 72 - + 6 Cl- + 14 H+ -2 -6 +14 +6 +3 Cl 2
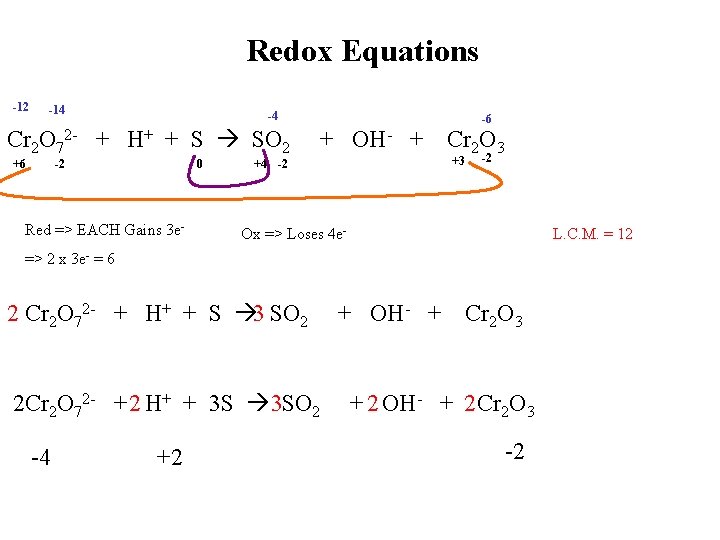
Redox Equations -12 -14 -4 Cr 2 O 72 - + H+ + S SO 2 +6 -2 0 Red => EACH Gains 3 e- + OH- + -6 Cr 2 O 3 +3 +4 -2 -2 Ox => Loses 4 e- L. C. M. = 12 => 2 x 3 e- = 6 2 Cr 2 O 72 - + H+ + S 3 SO 2 2 Cr 2 O 72 - + 2 H+ + 3 S 3 SO 2 -4 +2 + OH- + Cr 2 O 3 + 2 OH- + 2 Cr 2 O 3 -2
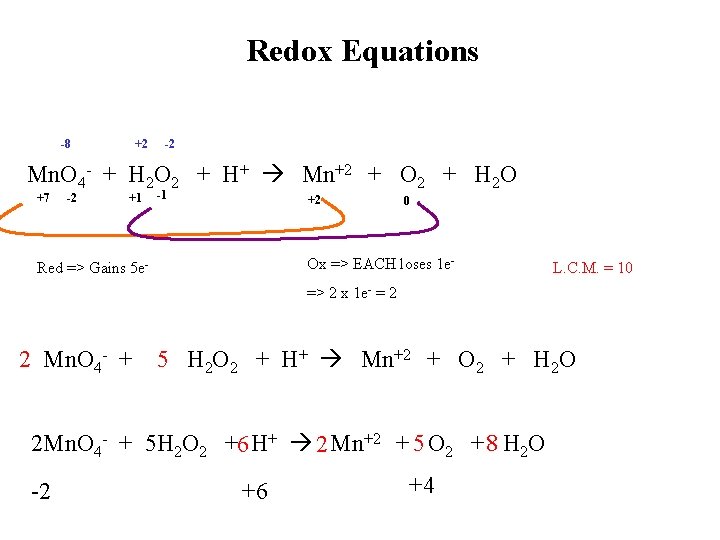
Redox Equations -8 +2 -2 Mn. O 4 - + H 2 O 2 + H+ Mn+2 + O 2 + H 2 O +7 -2 +1 -1 +2 0 Ox => EACH loses 1 e- Red => Gains 5 e- L. C. M. = 10 => 2 x 1 e- = 2 2 Mn. O 4 - + 5 H 2 O 2 + H+ Mn+2 + O 2 + H 2 O 2 Mn. O 4 - + 5 H 2 O 2 +6 H+ 2 Mn+2 + 5 O 2 + 8 H 2 O -2 +6 +4
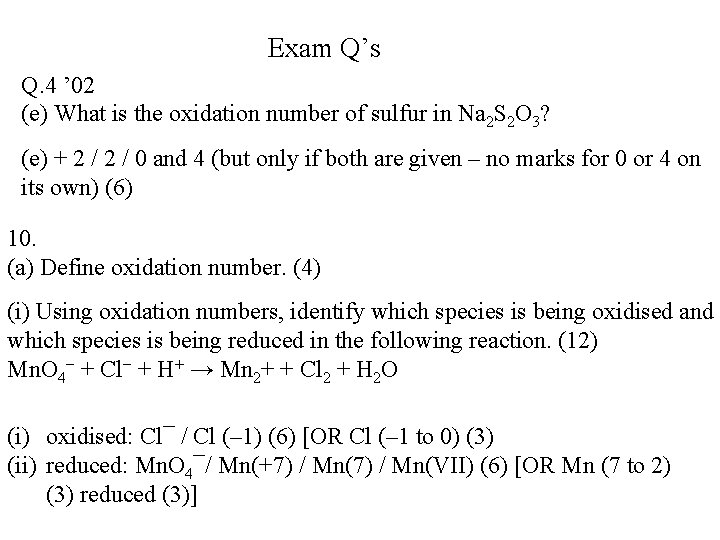
Exam Q’s Q. 4 ’ 02 (e) What is the oxidation number of sulfur in Na 2 S 2 O 3? (e) + 2 / 0 and 4 (but only if both are given – no marks for 0 or 4 on its own) (6) 10. (a) Define oxidation number. (4) (i) Using oxidation numbers, identify which species is being oxidised and which species is being reduced in the following reaction. (12) Mn. O 4− + Cl− + H+ → Mn 2+ + Cl 2 + H 2 O (i) oxidised: Cl¯ / Cl (– 1) (6) [OR Cl (– 1 to 0) (3) (ii) reduced: Mn. O 4¯/ Mn(+7) / Mn(VII) (6) [OR Mn (7 to 2) (3) reduced (3)]
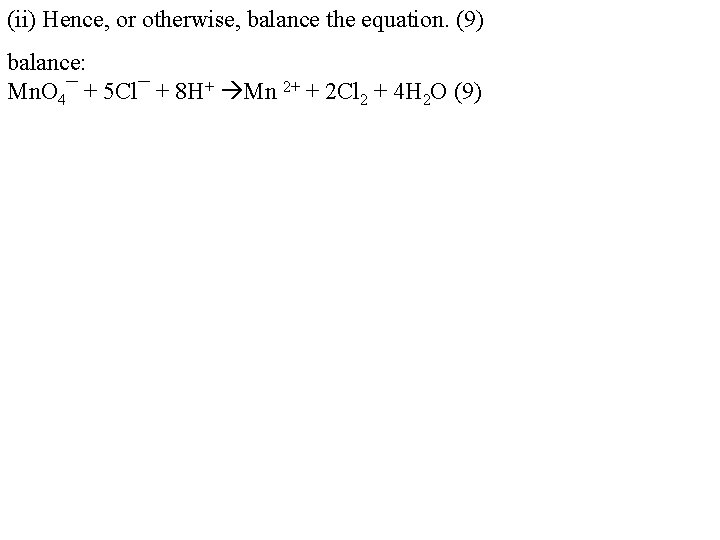
(ii) Hence, or otherwise, balance the equation. (9) balance: Mn. O 4¯ + 5 Cl¯ + 8 H+ Mn 2+ + 2 Cl 2 + 4 H 2 O (9)
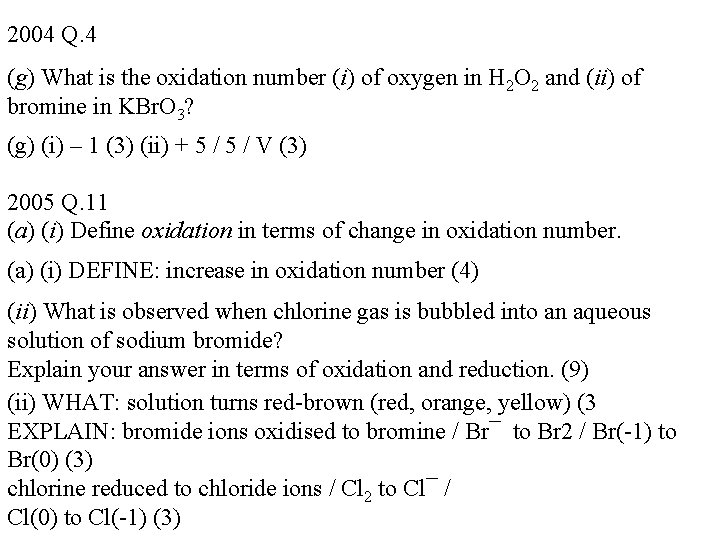
2004 Q. 4 (g) What is the oxidation number (i) of oxygen in H 2 O 2 and (ii) of bromine in KBr. O 3? (g) (i) – 1 (3) (ii) + 5 / V (3) 2005 Q. 11 (a) (i) Define oxidation in terms of change in oxidation number. (a) (i) DEFINE: increase in oxidation number (4) (ii) What is observed when chlorine gas is bubbled into an aqueous solution of sodium bromide? Explain your answer in terms of oxidation and reduction. (9) (ii) WHAT: solution turns red-brown (red, orange, yellow) (3 EXPLAIN: bromide ions oxidised to bromine / Br¯ to Br 2 / Br(-1) to Br(0) (3) chlorine reduced to chloride ions / Cl 2 to Cl¯ / Cl(0) to Cl(-1) (3)
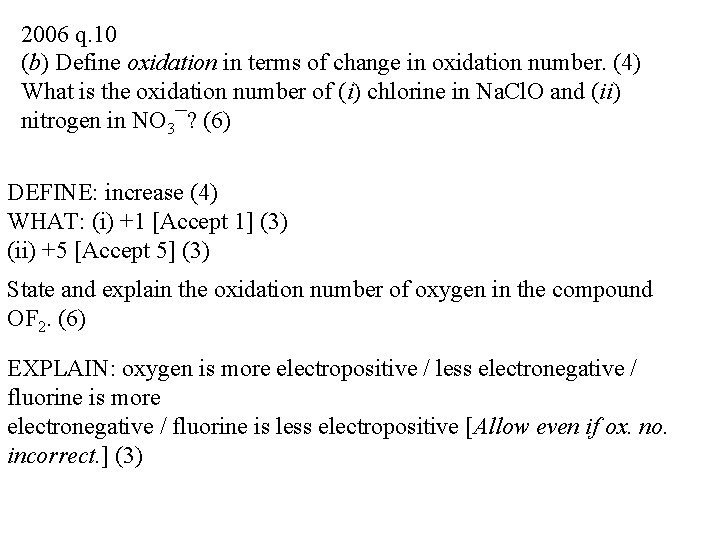
2006 q. 10 (b) Define oxidation in terms of change in oxidation number. (4) What is the oxidation number of (i) chlorine in Na. Cl. O and (ii) nitrogen in NO 3¯? (6) DEFINE: increase (4) WHAT: (i) +1 [Accept 1] (3) (ii) +5 [Accept 5] (3) State and explain the oxidation number of oxygen in the compound OF 2. (6) EXPLAIN: oxygen is more electropositive / less electronegative / fluorine is more electronegative / fluorine is less electropositive [Allow even if ox. no. incorrect. ] (3)
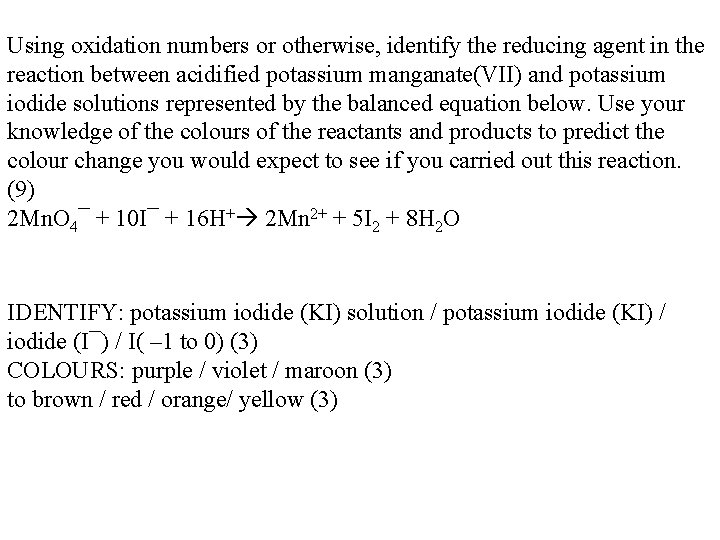
Using oxidation numbers or otherwise, identify the reducing agent in the reaction between acidified potassium manganate(VII) and potassium iodide solutions represented by the balanced equation below. Use your knowledge of the colours of the reactants and products to predict the colour change you would expect to see if you carried out this reaction. (9) 2 Mn. O 4¯ + 10 I¯ + 16 H+ 2 Mn 2+ + 5 I 2 + 8 H 2 O IDENTIFY: potassium iodide (KI) solution / potassium iodide (KI) / iodide (I¯) / I( – 1 to 0) (3) COLOURS: purple / violet / maroon (3) to brown / red / orange/ yellow (3)

2007 Q. 10 (c) The halogens are good oxidising agents. (i) How does the oxidation number of the oxidising agent change during a redox reaction? (4) (c) (i) HOW: it decreases (4) (ii) Assign oxidation numbers in each of the following equations to show clearly that the halogen is the oxidising agent in each case. (12) Br 2 + 2 Fe 2+ → 2 Br– + 2 Fe 3+ oxidation number of Br in Br 2 = 0 (3) oxidation number of Br in Br¯ = – 1 (3) …reduced => ox. agent Cl 2 + SO 32– + H 2 O → Cl– + SO 4 2– + H+ oxidation number of Cl in Cl 2 = 0 (3) oxidation number of Cl in Cl¯ = – 1 (3) …reduced => ox. agent
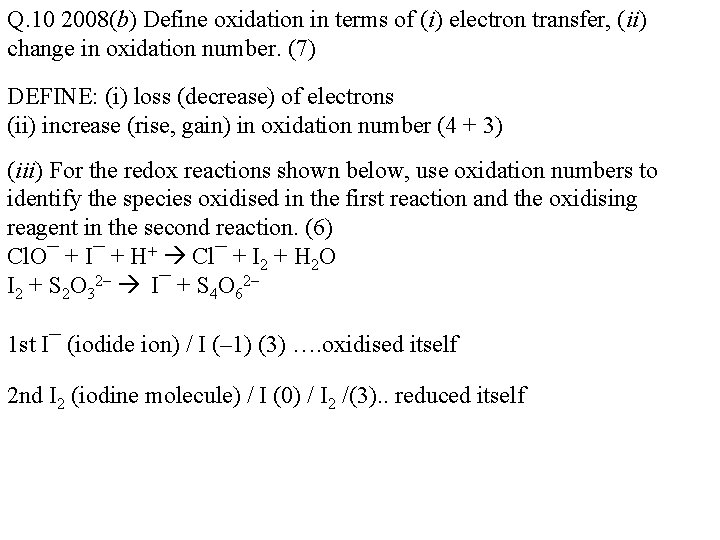
Q. 10 2008(b) Define oxidation in terms of (i) electron transfer, (ii) change in oxidation number. (7) DEFINE: (i) loss (decrease) of electrons (ii) increase (rise, gain) in oxidation number (4 + 3) (iii) For the redox reactions shown below, use oxidation numbers to identify the species oxidised in the first reaction and the oxidising reagent in the second reaction. (6) Cl. O¯ + I¯ + H+ Cl¯ + I 2 + H 2 O I 2 + S 2 O 32– I¯ + S 4 O 62– 1 st I¯ (iodide ion) / I (– 1) (3) …. oxidised itself 2 nd I 2 (iodine molecule) / I (0) / I 2 /(3). . reduced itself
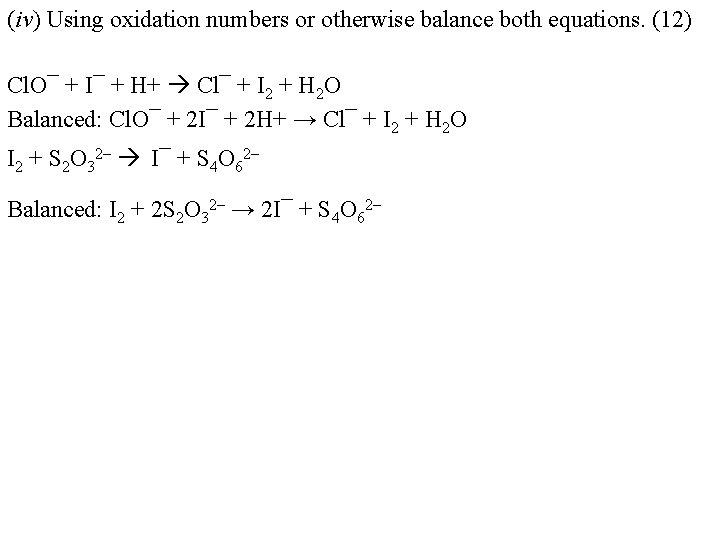
(iv) Using oxidation numbers or otherwise balance both equations. (12) Cl. O¯ + I¯ + H+ Cl¯ + I 2 + H 2 O Balanced: Cl. O¯ + 2 I¯ + 2 H+ → Cl¯ + I 2 + H 2 O I 2 + S 2 O 32– I¯ + S 4 O 62– Balanced: I 2 + 2 S 2 O 32– → 2 I¯ + S 4 O 62–

Electrochemical Series • Electrochemical Series – Metals listed in order of Decreasing ability as Reducing Agents (themselves oxidised) • Ca Ca+2 + 2 e- (Oxidation)
![Metals Electrochemical Series • • • [K] => Best Reducing Agent – becomes easily Metals Electrochemical Series • • • [K] => Best Reducing Agent – becomes easily](http://slidetodoc.com/presentation_image_h2/1ce6d585fb7db891f82cb77ad4a3d23d/image-38.jpg)
Metals Electrochemical Series • • • [K] => Best Reducing Agent – becomes easily oxidized [Na] [Ca] [Mg] [Al] [Zn] [Fe] [Sn] [Pb] [H] [Cu] [Ag] => Worst Reducing Agent – most difficult in list to oxidize
- Slides: 38