1 4 Units of Measurement v Unit is






- Slides: 14

1. 4 Units of Measurement v Unit is the smallest component of a whole v The units used for scientific measurements are those of the metric system v Measurement is used to measure quantities v Quantity is something that has magnitude, size, or amount (volume, length).

v Length: “How far? ” question involves being able to measure the distance between two points. 10 (meaningless) 10 cm or inch v Time: To answer the question, “How long did it take? ” v When a number represents a measured quantity, the units of that quantity must always be specified

v. Scientists all over the world use (since 1960) a single measurement system called SI units (Système International ) The SI consists of v Base units v Derived units v. The set of base units comprises an irreducible set of units for measuring all physical variables v. The derived units can be expressed in terms of the base units

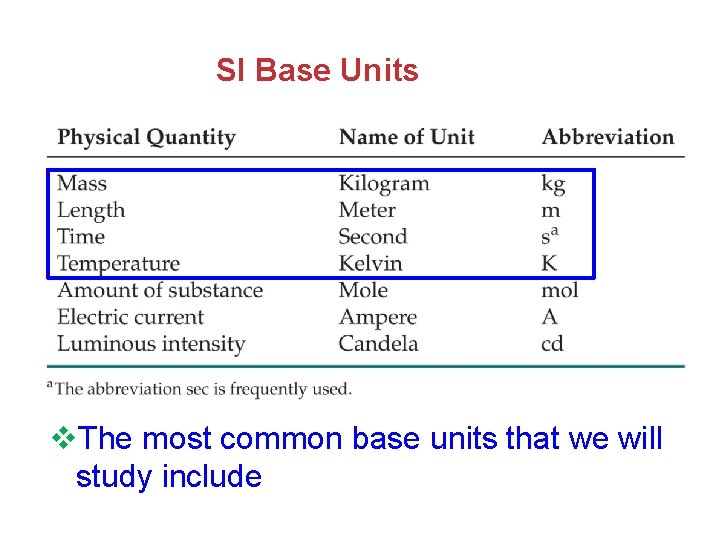
SI Base Units v. The most common base units that we will study include

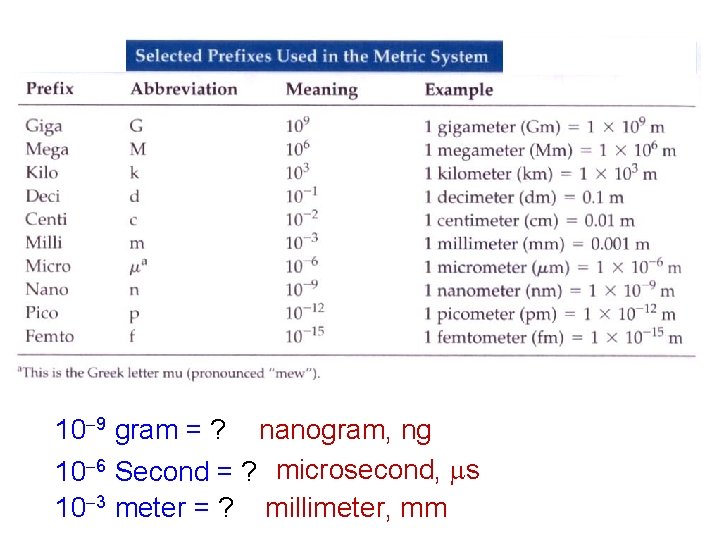
10 9 gram = ? nanogram, ng 10 6 Second = ? microsecond, s 10 3 meter = ? millimeter, mm

Length v. The SI standard unit for length is the meter (m). v. A distance of 1 m is about the width of an average doorway. v. To express longer distances, the kilometer (km) is used. One kilometer is equal to 1000 m. v. To express shorter distances, the centimeter (cm) is used. One centimeter is equal to 1/100 of a meter. v. Length can be measured using a meter stick or rulers.

Mass v. Mass is a measure of the quantity of matter. The standard unit for mass is the kilogram (kg). v. This base unit is unusable because it uses a prefix, kilo- instead of the word gram alone. Other units for mass are obtained by adding prefixes to the world gram v. The gram (g), which is 1/1000 of a kg is used for measuring masses of small objects.

Time v. The standard unit of measurement for time is the second (s) v. Larger amounts of time are measured in minutes and hours v. There are 60 seconds in one minute. There are 60 minutes in one hour. Given that there are 24 hours in one day

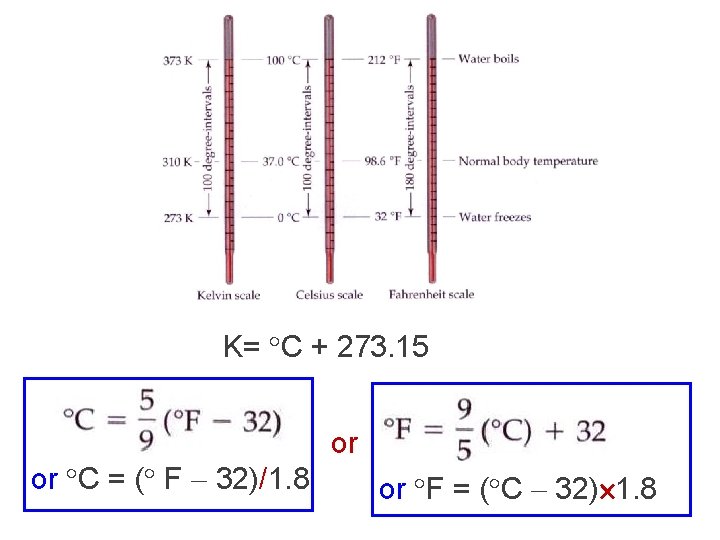
Temperature v. Temperature is a measure of the hotness or coldness of an object. v. The standard unit of measurement for temperature is degrees Kelvin (K). v. Temperature can also be measured in degrees Celsius ( C) and degrees Fahrenheit ( F).

K= C + 273. 15 or C = ( F 32)/1. 8 or or F = ( C 32) 1. 8

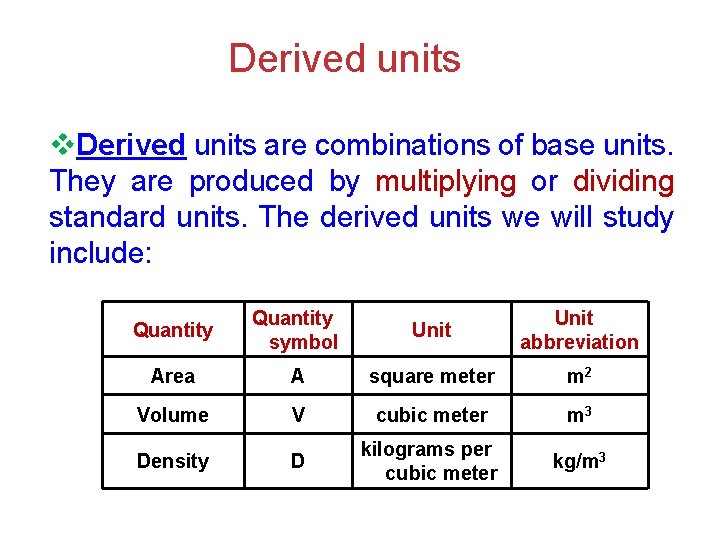
Derived units v. Derived units are combinations of base units. They are produced by multiplying or dividing standard units. The derived units we will study include: Quantity symbol Unit abbreviation Area A square meter m 2 Volume V cubic meter m 3 Density D kilograms per cubic meter kg/m 3

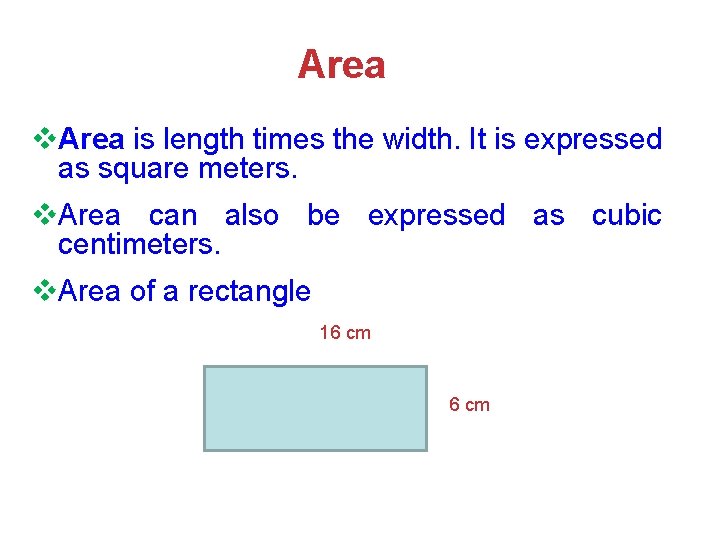
Area v. Area is length times the width. It is expressed as square meters. v. Area can also be expressed as cubic centimeters. v. Area of a rectangle 16 cm » 1111 6 cm

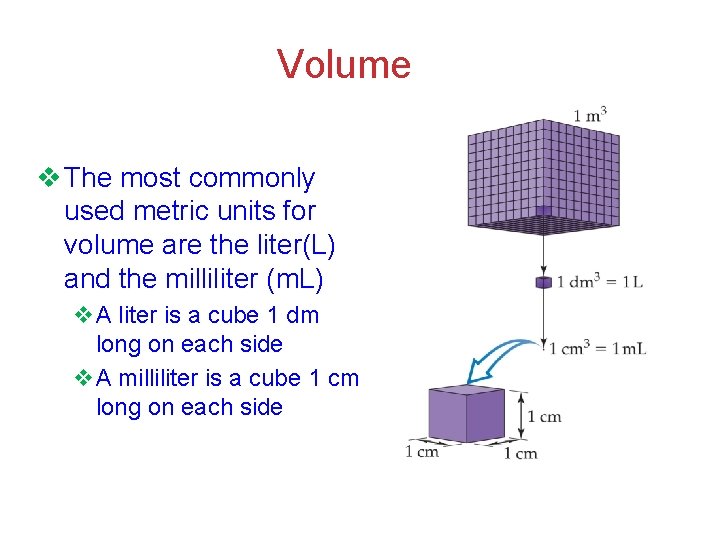
Volume v The most commonly used metric units for volume are the liter(L) and the milliliter (m. L) v. A liter is a cube 1 dm long on each side v. A milliliter is a cube 1 cm long on each side

density v. Density is the ratio of mass to volume, or mass divided by volume. It can be written: density = mass/volume or d = m/V v. Density does not depend on the size of the sample. As the mass of an object increases, its volume increases.