1 4 Units of Measurement Advanced Chemistry Using




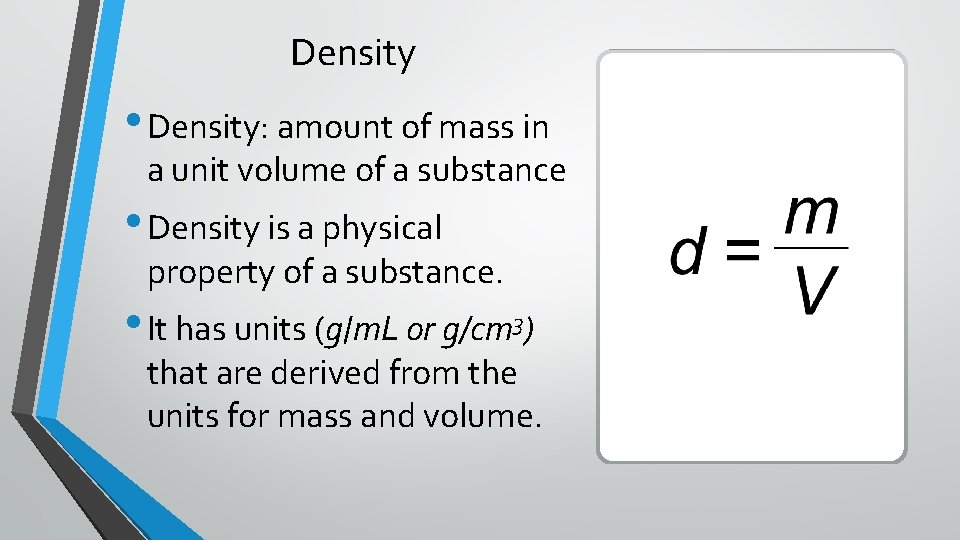






- Slides: 20

1. 4 Units of Measurement Advanced Chemistry

Using Units • Many properties of matter are quantitative, associated with numbers. • Units of a quantity must be specified. • Ex: Saying the length of a pencil is 17. 5 is meaningless.

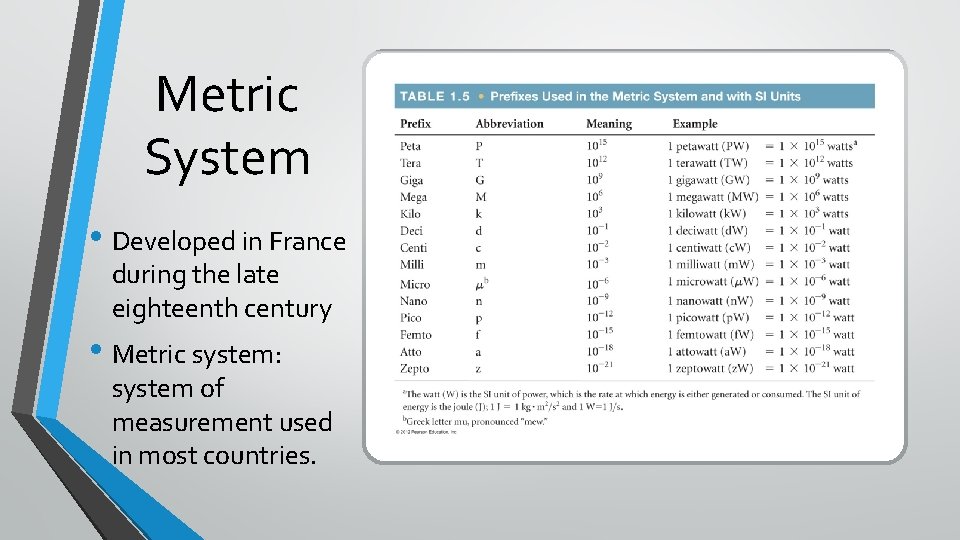
Metric System • Developed in France during the late eighteenth century • Metric system: system of measurement used in most countries.

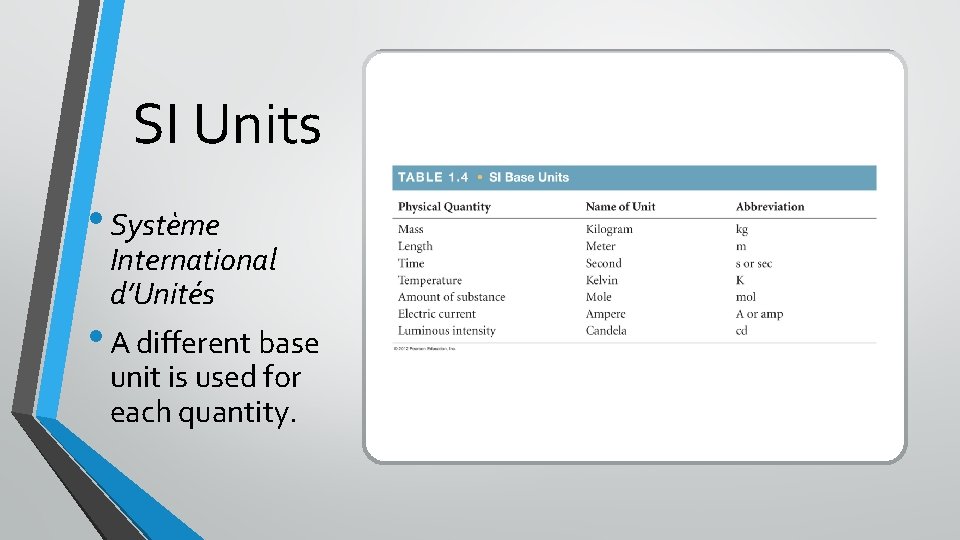
SI Units • Système International d’Unités • A different base unit is used for each quantity.

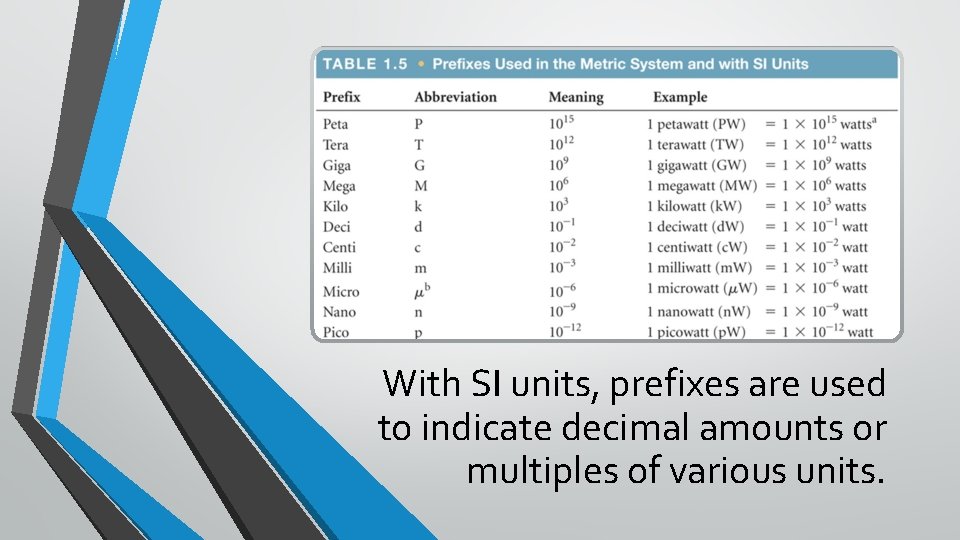
With SI units, prefixes are used to indicate decimal amounts or multiples of various units.

Length and Mass • The SI base unit of length is the meter slightly longer than a yard. • Mass is a measure of the amount of material in an object *Is NOT the same as weight • The SI base unit for mass is the kilogram equal to about 2. 2 pounds (lbs)

Temperature • Temperature: a measure of the hotness or coldness of an object • By definition temperature is a measure of the average kinetic energy of particles in a sample • Physical property • Heat always flows spontaneously form a substance at higher temperature to one with lower temperature.

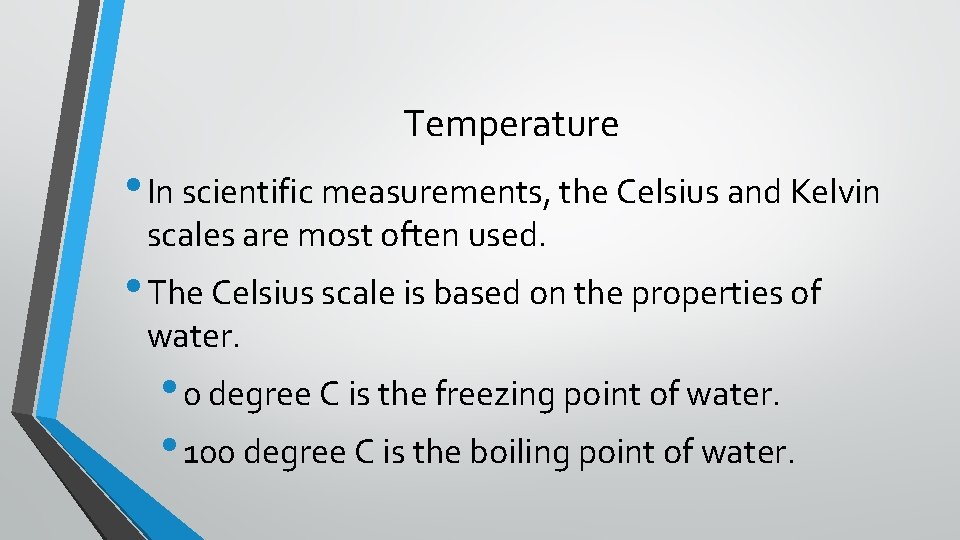
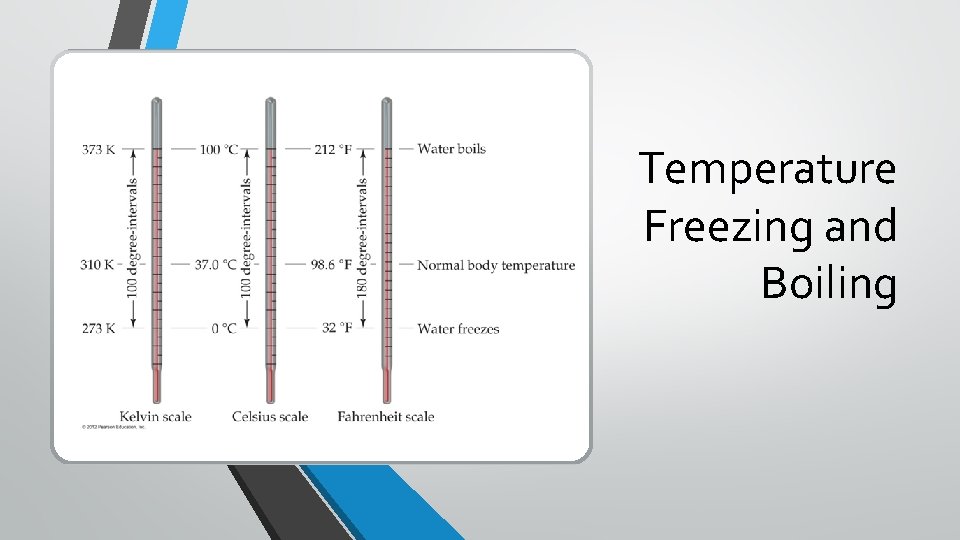
Temperature • In scientific measurements, the Celsius and Kelvin scales are most often used. • The Celsius scale is based on the properties of water. • 0 degree C is the freezing point of water. • 100 degree C is the boiling point of water.

Temperature • The kelvin is the SI unit of temperature. • It is based on the properties of gases. • There are no negative Kelvin temperatures. • Absolute zero • K = ºC + 273. 15

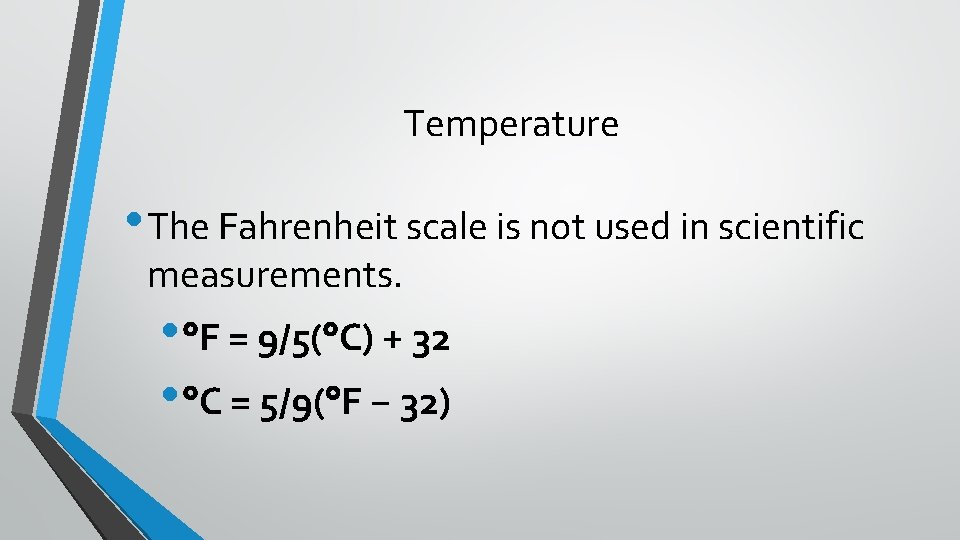
Temperature • The Fahrenheit scale is not used in scientific measurements. • F = 9/5( C) + 32 • C = 5/9( F − 32)

Temperature Freezing and Boiling

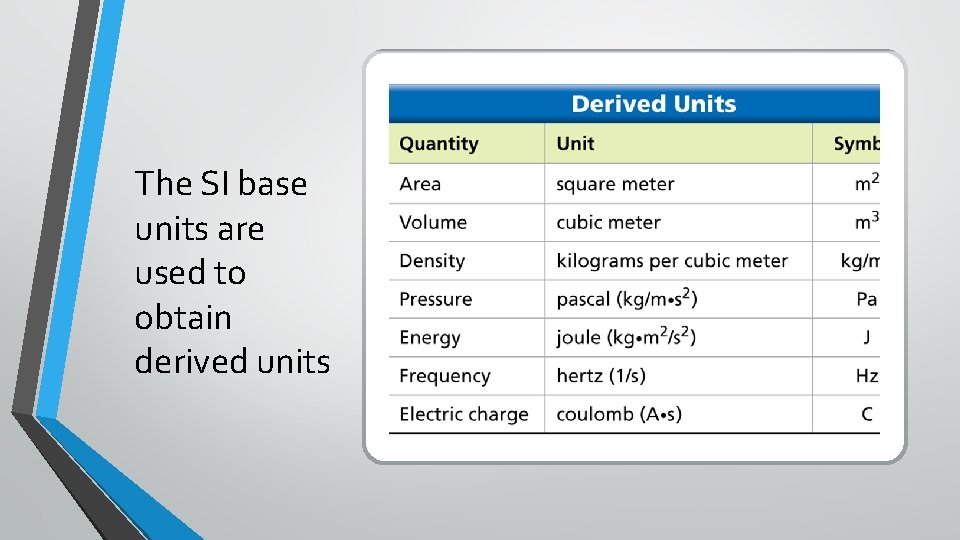
The SI base units are used to obtain derived units

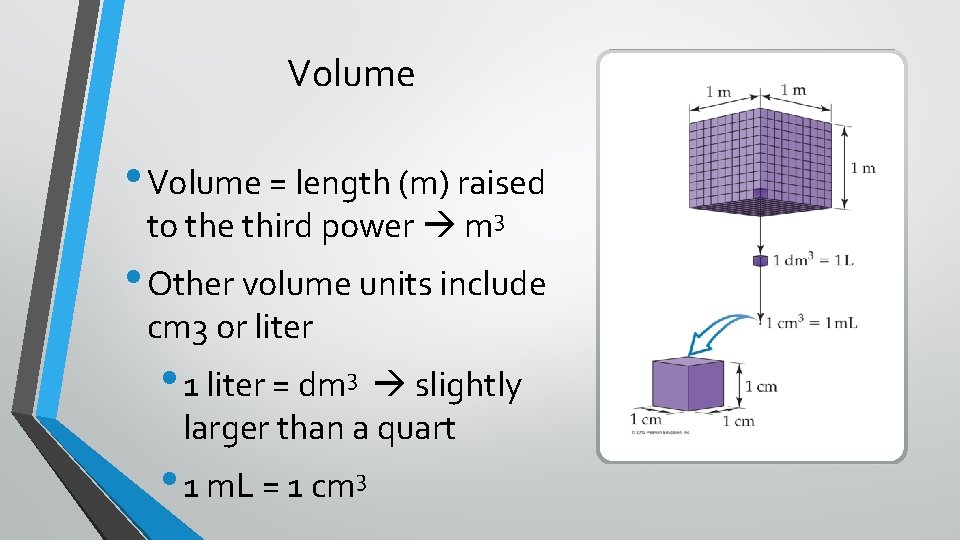
Volume • Volume = length (m) raised to the third power m 3 • Other volume units include cm 3 or liter • 1 liter = dm 3 slightly larger than a quart • 1 m. L = 1 cm 3
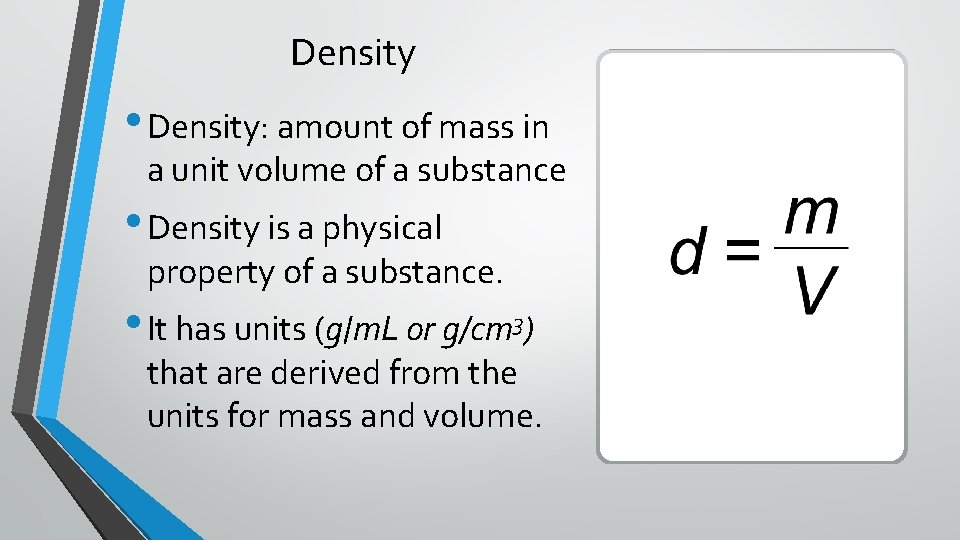
Density • Density: amount of mass in a unit volume of a substance • Density is a physical property of a substance. • It has units (g/m. L or g/cm 3) that are derived from the units for mass and volume.

Density Cont. • Density of Water is 1. 00 g/m. L • Densities are temperature dependent • If no temperature specified, we assume 25 degree C (room temperature)

Scientific Notation Review • Scientists often work with very large or very small numbers. • Scientific notation is a way of expressing a value as the product of a number between 1 and 10 and a power of 10.

Scientific Notation For numbers less than 1 that are written in scientific notation, the exponent is negative. For example, an average snail’s pace is 0. 00086 meters per second. In scientific notation, that speed is 8. 6 × 10 -4 m/s. The negative exponent tells you how many decimals places there are to the left of the 8. 6.

Scientific Notation • For numbers more than 1 that are written in scientific notation, the exponent is positive. • The speed of light is about 300, 000 meters per second. In scientific notation, that speed is 3. 0 × 108 m/s. • The positive exponent tells you how many decimals places there are to the right of the 3. 0.

Multiplying Numbers in Scientific Notation • To multiply numbers written in scientific notation, you multiply the numbers that appear before the multiplication signs and add the exponents.

Dividing Numbers in Scientific Notation • When dividing numbers written in scientific notation, you divide the numbers that appear before the exponential terms and subtract the exponents.