1 27 301 MicrostructureProperties Thermal Transport Properties Profs










![23 Effect of Impurities in Al. N [Slack] • Oxygen contents (in 1018 atoms. 23 Effect of Impurities in Al. N [Slack] • Oxygen contents (in 1018 atoms.](https://slidetodoc.com/presentation_image_h/a718897062f5a362f869fe89a54a0fef/image-23.jpg)











- Slides: 41

1 27 -301 Microstructure-Properties Thermal Transport Properties Profs. A. D. Rollett, M. De Graef Processing Performance Microstructure Properties Last modified: 16 th Dec. ‘ 15 Please acknowledge Carnegie Mellon if you make public use of these slides

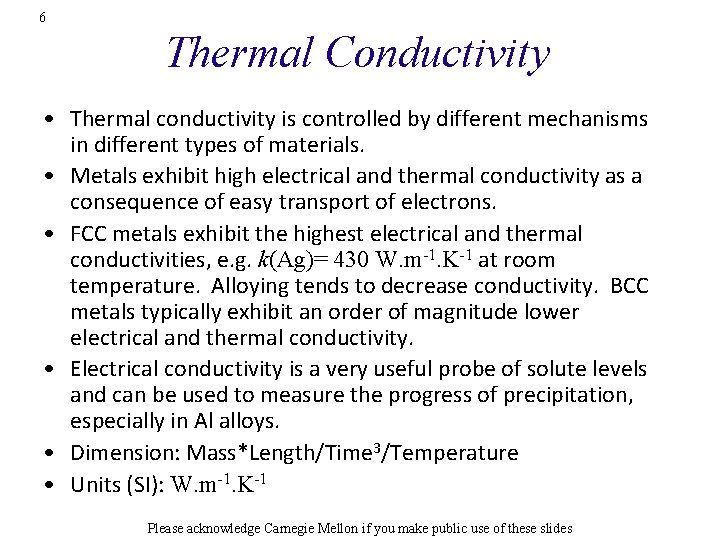
2 Objective • The objective of this lecture is to explain the relationship between thermal properties of materials and their microstructure. Also to point out the counter-intuitive notion that certain insulators have better thermal conductivity than all metals. http: //upload. wikimedia. org/wikipedia/commons/1/1 e/Thermal_conductivity. svg Please acknowledge Carnegie Mellon if you make public use of these slides

3 Questions 1. What is the basic Eq for thermal conductivity (k) in terms of e. g. specific heat? k = 1/3 CV v l 2. Practical importance of k? Many applications, e. g. microelectronic heat sinks. 3. What role do phonons play in k? In electrical insulators they are the (only) transport mechanism for heat. 4. Which materials have high k, low k? Adamantine materials (and fcc metals) have high k; most others are low k. 5. Typical dependence of k on temperature? Rises as T 3 at low temperatures and falls as 1/T at high T. 6. What Eq. relates k to lattice properties e. g. Debye temp. ? k = B<M>W 1/3 Q 3/(T 2). 7. What effect does specimen size, grain structure, phase structure have on k? Any defect including surfaces, interfaces, scatters phonons and decreases k. 8. What processing steps affect k? In ceramics, sintering (for example) raises density which improves k. 9. If we want anisotropy in k, what can we do? Align grain boundaries such that the long direction of the grains is parallel to the desired high k direction. 10. What other property correlates with high thermal conductivity? Hardness correlates: diamond is the hardest known material. Please acknowledge Carnegie Mellon if you make public use of these slides

4 Notation • • • k or k is thermal conductivity <M> is the average atomic mass W is the atomic volume B is a constant QD is the Debye temperature T is the (absolute) temperature is the Grüneisen constant v is the speed of sound l is the mean free path of phonons C is the specific heat r is the density. Please acknowledge Carnegie Mellon if you make public use of these slides

5 Relevance of Thermal Conductivity • Building materials; here low conductivity (allied with low cost) is generally useful for insulation† in particular. • Heat sinks (e. g. keeping electronics cool). • Thermoelectric materials; here low conductivity helps the efficiency. • Clothes; for outdoor clothing, low conductivity (good insulation) is useful. • Thermal barrier coatings for turbine blades, the space shuttle. • Heat conduction in nuclear fuel (UO 2) is poor and limits fuel life. †Around most of the world, R-values are given in SI units, typically m²·K/W (or equivalently, m²·°C/W). In the United States customary units, R-values are given in units of ft²·°F·h/Btu. It is particularly easy to confuse SI and US R-values, because R-values both in the US and elsewhere are often cited without their units, e. g. , R-3. 5. Usually, however, the correct units can be inferred from the context and from the magnitudes of the values. United States R-values are approximately six times SI R-values. [http: //en. wikipedia. org/wiki/R-value_%28 insulation%29] Please acknowledge Carnegie Mellon if you make public use of these slides

6 Thermal Conductivity • Thermal conductivity is controlled by different mechanisms in different types of materials. • Metals exhibit high electrical and thermal conductivity as a consequence of easy transport of electrons. • FCC metals exhibit the highest electrical and thermal conductivities, e. g. k(Ag)= 430 W. m-1. K-1 at room temperature. Alloying tends to decrease conductivity. BCC metals typically exhibit an order of magnitude lower electrical and thermal conductivity. • Electrical conductivity is a very useful probe of solute levels and can be used to measure the progress of precipitation, especially in Al alloys. • Dimension: Mass*Length/Time 3/Temperature • Units (SI): W. m-1. K-1 Please acknowledge Carnegie Mellon if you make public use of these slides

7 Thermal Conductivity: Ceramics • Most ionically and covalently bonded materials exhibit very low conductivities (electrical and thermal). This is a result of the large band gap between the valence and conduction bands. • Covalently bonded solids tend to have higher thermal conductivities (and higher melting points) than ionically bonded solids. • Insulating materials with high thermal conductivity are useful, however, for electronic applications where they are used to conduct heat away from components with high heat dissipation requirements. • Adamantine materials (diamond-like) have (very high) thermal conductivities comparable to those of fcc metals. • Aerogels have extremely low values of thermal conductivity. • Thermal waves can be used to measure k, e. g. by irradiating a sample with a laser pulse and observing the change in temperature on the other side. • Do not be misled by sources that say that covalently bonded substances have low melting points (compared to, say ionically bonded substances). They are referring to compounds, whereas the above remarks apply to solids. Please acknowledge Carnegie Mellon if you make public use of these slides

8 Microelectronic Packaging • All microelectronic circuits are placed on an insulating substrate. In recent times, the power dissipation has become a very important issue and high thermal conductivity is useful. Basic package: D. Frear, JOM (1999), 51, 22 -27 Proposed advanced technology: scholar. lib. vt. edu/theses/available/ etd-0106100 -113320/unrestricted/Chp. I. pdf Please acknowledge Carnegie Mellon if you make public use of these slides

9 Heat Sinks for Microelectronics Please acknowledge Carnegie Mellon if you make public use of these slides

10 Applications • Susceptors, rings for Rapid Thermal Processing/Annealing • Plasma-etch components • RF-heated [radio frequency] susceptors • Optical components in synchrotrons and high lower lasers • Electronics, especially LSI and VLSI (large scale integration) • Wear resistant components Please acknowledge Carnegie Mellon if you make public use of these slides

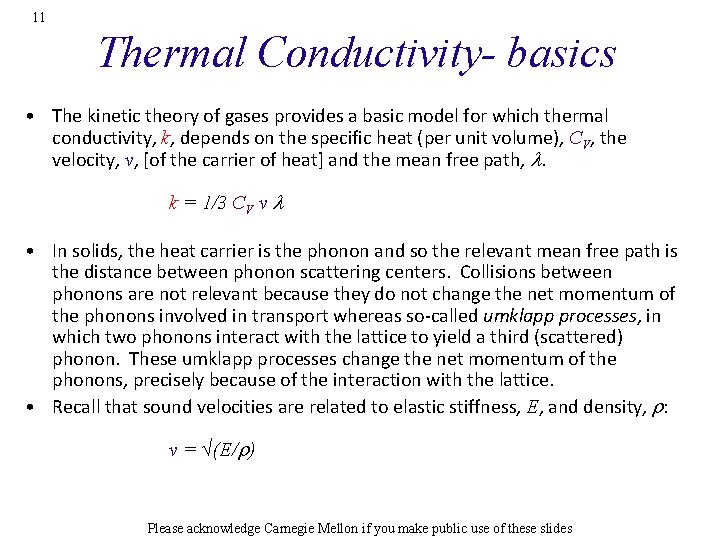
11 Thermal Conductivity- basics • The kinetic theory of gases provides a basic model for which thermal conductivity, k, depends on the specific heat (per unit volume), CV, the velocity, v, [of the carrier of heat] and the mean free path, l. k = 1/3 CV v l • In solids, the heat carrier is the phonon and so the relevant mean free path is the distance between phonon scattering centers. Collisions between phonons are not relevant because they do not change the net momentum of the phonons involved in transport whereas so-called umklapp processes, in which two phonons interact with the lattice to yield a third (scattered) phonon. These umklapp processes change the net momentum of the phonons, precisely because of the interaction with the lattice. • Recall that sound velocities are related to elastic stiffness, E, and density, r: v = √(E/r) Please acknowledge Carnegie Mellon if you make public use of these slides

12 Mean free path, specific heat • It turns out that at high temperatures, the number of phonons involved in such (umklapp) scattering is proportional to T, from which it follows that the mean free path goes as 1/T. • Also, as the temperature decreases and the mean free path goes up, eventually the length is limited by either the specimen size or the grain size. • At low enough temperatures, the specific heat varies strongly with temperature, C T 3. Thus thermal conductivity also varies as T 3. Please acknowledge Carnegie Mellon if you make public use of these slides

13 High k Ceramics • Phonons are elastic waves in the crystal lattice. Heat is mostly carried by phonons in the acoustic range, i. e. with frequencies below 20 k. Hz (or 1000 cm-1). • Srivastava gives an equation for thermal conductivity at high temperatures from three-phonon (scattering) interactions, where <M> is the average atomic mass, W is the atomic volume, B is a constant, QD is the Debye temperature (~516 K for Al. N), T is the temperature, and is the Grüneisen constant (~0. 77 for Al. N). k = B<M>W 1/3 Q 3/(T 2) • The Debye temperature correlates with the melting temperature; it varies more than the atomic mass and therefore dominates the conductivity (note the power of 3). Please acknowledge Carnegie Mellon if you make public use of these slides

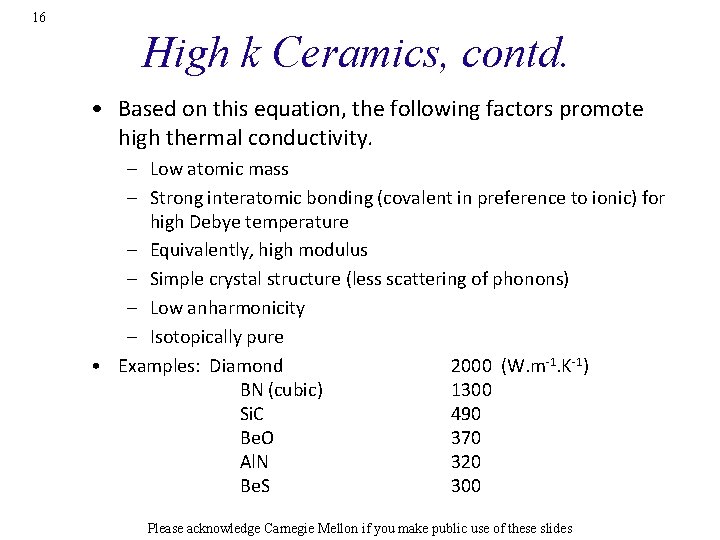
14 Example: Al. N Ag • Note the difference between single crystal conductivity and the (calculated) polycrystal behavior. • Conductivity first increases, then decreases with T. [Slack] Please acknowledge Carnegie Mellon if you make public use of these slides

15 Specimen Size • In sufficiently pure materials, even the sample size makes a difference because the phonons scatter off the sides of the sample! • Graph shown is for Li. F. After a lecture on thermal conductivity by AH Harker, UCL. Please acknowledge Carnegie Mellon if you make public use of these slides

16 High k Ceramics, contd. • Based on this equation, the following factors promote high thermal conductivity. – Low atomic mass – Strong interatomic bonding (covalent in preference to ionic) for high Debye temperature – Equivalently, high modulus – Simple crystal structure (less scattering of phonons) – Low anharmonicity – Isotopically pure • Examples: Diamond 2000 (W. m-1. K-1) BN (cubic) 1300 Si. C 490 Be. O 370 Al. N 320 Be. S 300 Please acknowledge Carnegie Mellon if you make public use of these slides

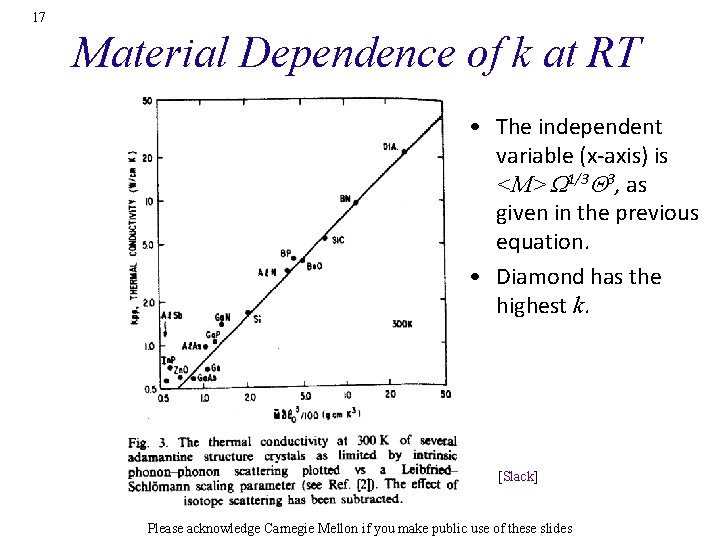
17 Material Dependence of k at RT • The independent variable (x-axis) is <M>W 1/3 Q 3, as given in the previous equation. • Diamond has the highest k. [Slack] Please acknowledge Carnegie Mellon if you make public use of these slides

18 Defects • Heat sinks are typically made by sintering powders together. • The important defects are the following. – – Point defects in the lattice Grain boundaries Pores Second phases from dopants, sintering aids • Grain size: grain boundaries scatter phonons. Therefore thermal conductivity goes down with decreasing grain size. See: http: //en. wikipedia. org/wiki/Thermal_properties_of_nanostructures http: //www. physics. ucf. edu/~schellin/research/interfaces. html Please acknowledge Carnegie Mellon if you make public use of these slides

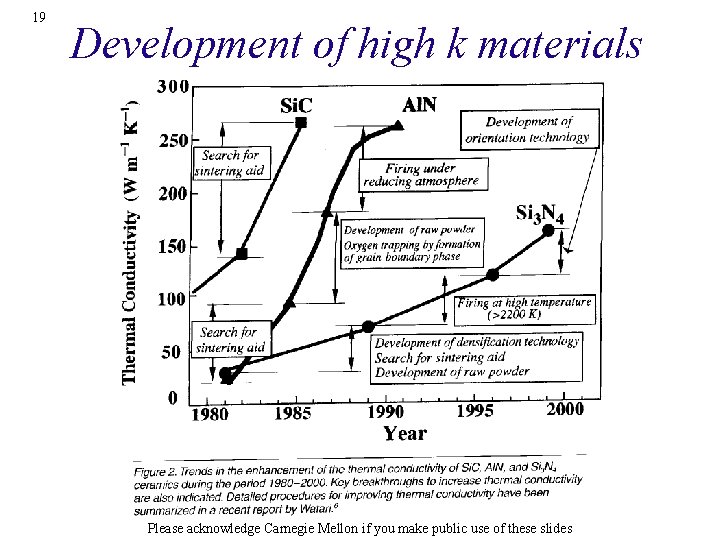
19 Development of high k materials Please acknowledge Carnegie Mellon if you make public use of these slides

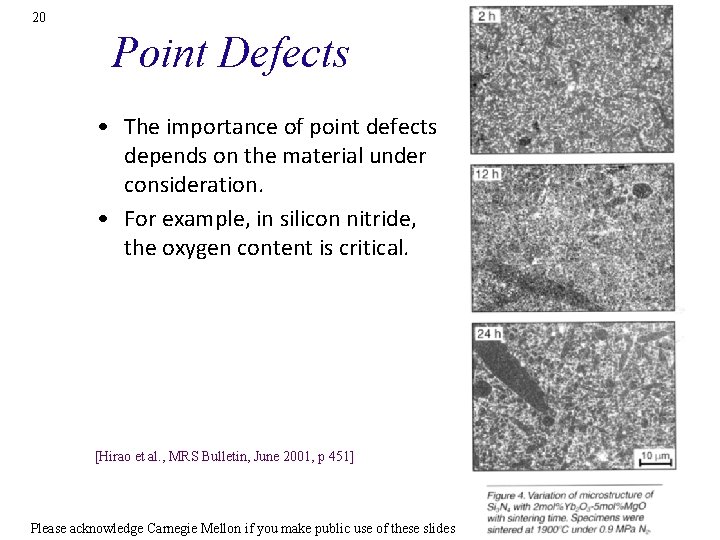
20 Point Defects • The importance of point defects depends on the material under consideration. • For example, in silicon nitride, the oxygen content is critical. [Hirao et al. , MRS Bulletin, June 2001, p 451] Please acknowledge Carnegie Mellon if you make public use of these slides

21 Oxygen content • Oxygen content is minimized by sintering under a nitrogen atmosphere. Please acknowledge Carnegie Mellon if you make public use of these slides

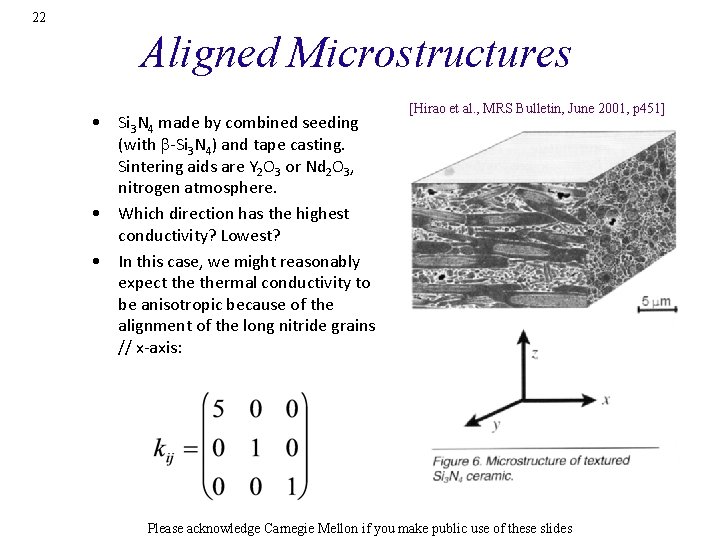
22 Aligned Microstructures • Si 3 N 4 made by combined seeding (with b-Si 3 N 4) and tape casting. Sintering aids are Y 2 O 3 or Nd 2 O 3, nitrogen atmosphere. • Which direction has the highest conductivity? Lowest? • In this case, we might reasonably expect thermal conductivity to be anisotropic because of the alignment of the long nitride grains // x-axis: [Hirao et al. , MRS Bulletin, June 2001, p 451] Please acknowledge Carnegie Mellon if you make public use of these slides
![23 Effect of Impurities in Al N Slack Oxygen contents in 1018 atoms 23 Effect of Impurities in Al. N [Slack] • Oxygen contents (in 1018 atoms.](https://slidetodoc.com/presentation_image_h/a718897062f5a362f869fe89a54a0fef/image-23.jpg)
23 Effect of Impurities in Al. N [Slack] • Oxygen contents (in 1018 atoms. cm-3): W 201: 42. R 47 (Si): 1 R 162: 300 • Conductivity goes down with increasing oxygen content • Substitutional impurities are strong scatterers of phonons Please acknowledge Carnegie Mellon if you make public use of these slides

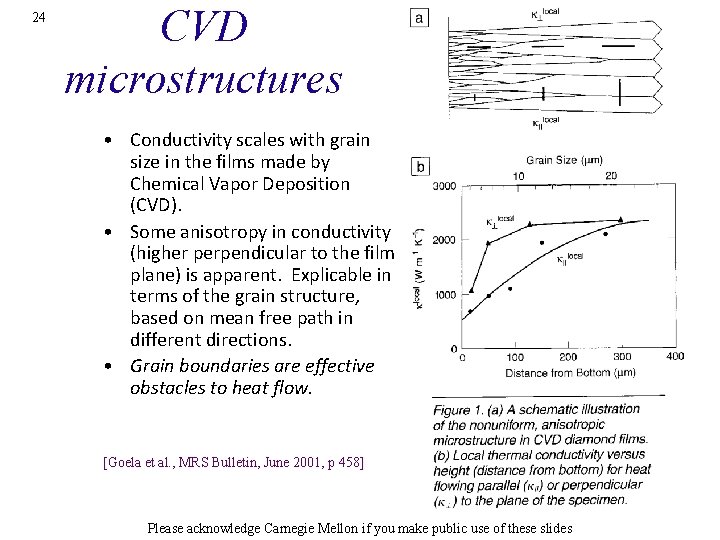
24 CVD microstructures • Conductivity scales with grain size in the films made by Chemical Vapor Deposition (CVD). • Some anisotropy in conductivity (higher perpendicular to the film plane) is apparent. Explicable in terms of the grain structure, based on mean free path in different directions. • Grain boundaries are effective obstacles to heat flow. [Goela et al. , MRS Bulletin, June 2001, p 458] Please acknowledge Carnegie Mellon if you make public use of these slides

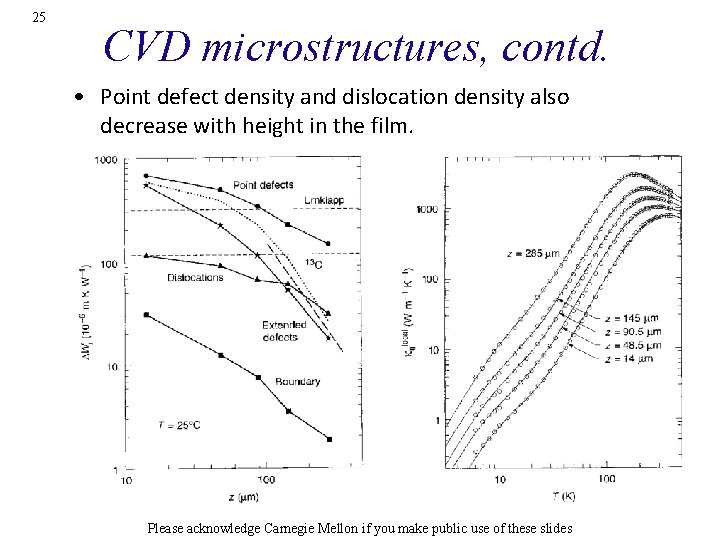
25 CVD microstructures, contd. • Point defect density and dislocation density also decrease with height in the film. Please acknowledge Carnegie Mellon if you make public use of these slides

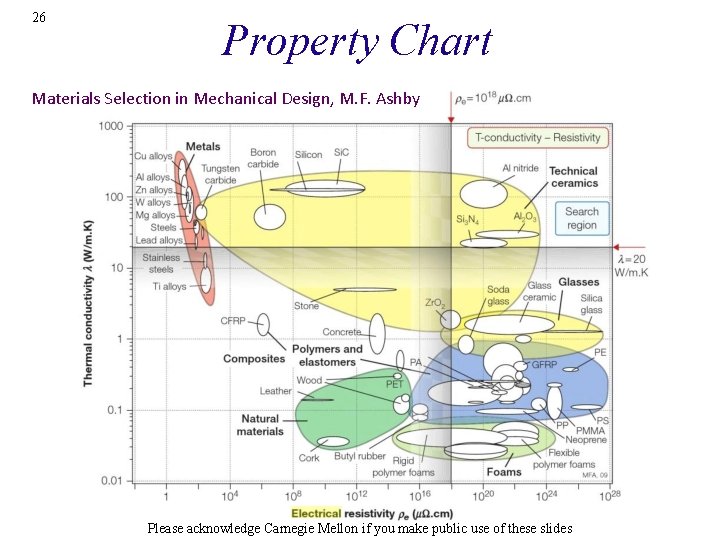
26 Property Chart Materials Selection in Mechanical Design, M. F. Ashby Please acknowledge Carnegie Mellon if you make public use of these slides

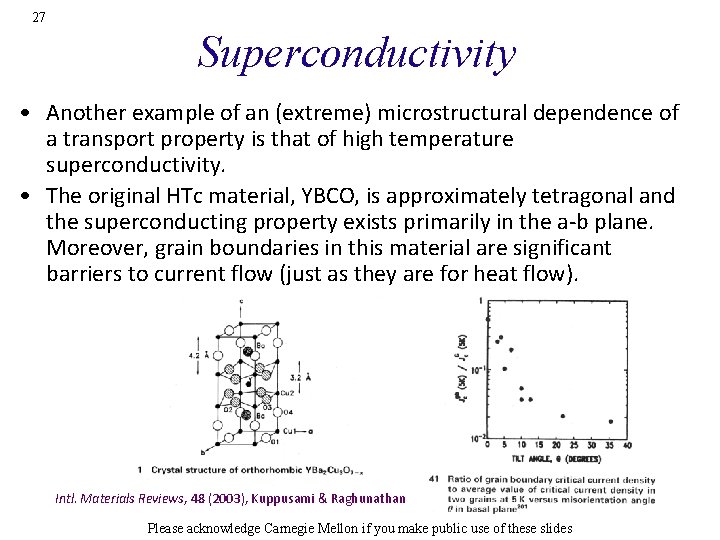
27 Superconductivity • Another example of an (extreme) microstructural dependence of a transport property is that of high temperature superconductivity. • The original HTc material, YBCO, is approximately tetragonal and the superconducting property exists primarily in the a-b plane. Moreover, grain boundaries in this material are significant barriers to current flow (just as they are for heat flow). Intl. Materials Reviews, 48 (2003), Kuppusami & Raghunathan Please acknowledge Carnegie Mellon if you make public use of these slides

28 Superconductivity, contd. • Consequently, in order to make long lengths of superconductor (for transmission cables, for example) enormous effort has gone into devising ways of making highly oriented thin films. • The most successful method involves producing a highly textured metal tape (e. g. Ni), depositing various buffer layers of oxides (e. g. Ce. O 2) on the metal, and then finally the superconductor. “High-performance YBCO-coated superconductor wires”, Paranthaman and Izumi, MRS Bulletin, 29 p 533 (2004) Please acknowledge Carnegie Mellon if you make public use of these slides

29 Summary • Thermal conductivity has been reviewed • High thermal conductivity insulators (ceramics) exist, such as diamond and Al. N. • Thermal conductivity in insulators is dependent on phonons: high conductivity requires minimization of phonon scattering. • Thermal conductivity is grain size dependent, provided that other defects (point defects, dislocations) do not limit it. • Practical heat sinks (for microelectronics) require sintering from powders. Therefore the properties of the sintered product is not as good as the single crystal properties. Please acknowledge Carnegie Mellon if you make public use of these slides

30 References • Physical Ceramics (1997), Y. -T. Chiang, D. P. Birnie III, W. D. Kingery, Wiley, New York, 0 -471 -59873 -9; pp. 474 -477. • MRS Bulletin, June 2001, vol. 26, No. 6, “High Thermal Conductivity Materials. ” • G. A. Slack, R. A. Tanzilli, R. O. Pohl & J. W. Vandersande, “The Intrinsic Thermal Conductivity of Al. N”, J. Phys. Chem. Solids, 48, 641 -647 (1987). • JOM June 1998: special topic on “Thermal Management Materials for Electronic Applications”, pp 46 -72 (several articles). • Electrons and Phonons, J. M. Ziman, Oxford, 1960. • Therm. Tile Ceramic Heat Sinks and Electrical Insulators From Coors. Tek, www. azom. com/details. asp? Article. ID=3729 • http: //www. customdicing. com/aln-links. htm • Heat Sink Materials and Components from WCu and Mo. Cu. Al. N Ceramic: marketech-heatsinks. com/pages/aln. html • Fun video about space shuttle tile: http: //www. youtube. com/watch? v=Pp 9 Yax 8 UNo. M Please acknowledge Carnegie Mellon if you make public use of these slides

31 Supplemental Slides Please acknowledge Carnegie Mellon if you make public use of these slides

32 Ceramic Microstructure • In order to understand the microstructures that we are dealing with, it is necessary to delve into ceramic processing briefly (but see the course on Ceramic Processing). • Although sintering of powders can be performed on single-phase material, it is common to use sintering aids that substantially modify the composition and microstructure of the final product. Please acknowledge Carnegie Mellon if you make public use of these slides

33 Sintering • Sintering is the name given to the process by which powder compacts agglomerate when exposed to high temperature. The process is driven by reduction in surface area. [Physical Ceramics] Please acknowledge Carnegie Mellon if you make public use of these slides

34 Geometry of Sintering • Three stages of geometrical evolution: Initial/ neck formation Intermediate/ "channel" formation i. e. continuous, tubular porosity Late Stage/ closed, isolated porosity • Estimate the relative densities at which the switch occurs from one stage to another based on monosize particles: channels exist once the edge length (all triple edges are pores) is >> pore diameter. This suggests a transition at 89% dense for simple cubic packing (if the pore channel diameter is less than the edge length divided by 3). The transition to late stage comes when pore channel diameter is so small that the channel breaks up into spherical pores. This is harder to estimate because kinetically determined. Please acknowledge Carnegie Mellon if you make public use of these slides

35 Sintering Mechanisms • We identify the five mass transport mechanisms, w. r. t. initial stage (neck formation). 1. Vapor transfer 2. Boundary diffusion (grain boundary) 3. Surface diffusion 4. Bulk diffusion (note difference in sources of material) 5. Dislocation diffusion Please acknowledge Carnegie Mellon if you make public use of these slides

36 Bond versus Shrink • We also need to identify the effect of each mechanism because each one has a very different consequence if densification is desired, see fig. 5. 40 in Physical Ceramics. Clearly vapor phase transport is useful if a porous body is needed: Vapor transfer Bonding, no shrinkage Surface diffusion Bonding, no shrinkage Bulk diffusion Bonding if from surface " " Shrinkage if from boundary Grain Boundary diffusion Shrinkage Dislocation diffusion Shrinkage Please acknowledge Carnegie Mellon if you make public use of these slides

37 Intermediate Stage Sintering • The intermediate stage of sintering is the most complicated and difficult to model rigorously. Morphologically, it is the stage between development of necks and the shrinking of isolated pores. When the particles have necked to the point that they are faceted, fig. 5. 42 in Physical Ceramics, then there are channels along the edge of each facet while the particles are joined at the facets. At this stage, non-local behavior can be very important. If plastic deformation, especially by creep, occurs at an appreciable rate, the particles can deform and slide past each other. Rearrangement in space of particles can achieve a significant amount of densification without any requirement for diffusion or vapor transport. Most texts gloss over this stage of sintering despite its importance. Please acknowledge Carnegie Mellon if you make public use of these slides

38 Late Stage Sintering • The driving pressure for late stage sintering is almost entirely due to vacancy diffusion away from voids. For late stage sintering in particular, the temperatures typically used are such that grain boundary diffusion is dominant. Therefore the critical feature of pore elimination is that the pores remain attached to grain boundaries. If the grain boundaries succeed in detaching from the pores, densification will essentially cease. In other words, it is possible for grain coarsening to take place rapidly enough that boundaries become detached from pores, leaving them behind within grains. Those isolated pores do not lose volume as quickly as those on boundaries. Please acknowledge Carnegie Mellon if you make public use of these slides

39 Liquid Phase Sintering • Liquid phase sintering is effective because it accelerates mass transport enormously. We need a small angle for liquid phase sintering in order that wetting occur efficiently and quickly. It is critical that the liquid phase wet the solid phase, so that wherever contacts have developed between particles, so that a grain boundary is present, the liquid can penetrate and replace the g. b. with liquid. The criterion for this is simply that 2 sl < gb. • Liquids also allow for particle rearrangement by effectively lubricating the particles so that they can slide past one another. • Elimination of voids is accelerated if the solid can dissolve in the liquid phase, to a limited extent (obviously we do not want complete dissolution). Diffusion rates in liquids are typically three to four orders of magnitude faster than in solids. Please acknowledge Carnegie Mellon if you make public use of these slides

40 Grain Growth vs. Densification • The elevated temperatures used for sintering are high enough to allow grain growth to occur. This is generally undesirable in the sintering process since pores attached to grain boundaries can shed vacancies and shrink whereas pores isolated in the grain interiors depend on lattice (bulk) diffusion. At the temperatures typical of sintering, lattice diffusion is slow compared to boundary diffusion. Therefore it is important that grain growth not occur so fast the boundaries can separate from the pores. Recall that grain growth is also a thermally activated process described by the following classical description of the kinetics due to Hillert (1965), where R is the grain size (diameter), M(T) is the grain boundary mobility as a function of temperature, t is time, and g is the grain boundary energy: R 2 - R 02 = M(T) t/8 Please acknowledge Carnegie Mellon if you make public use of these slides

41 High Conductivity Ceramics • The previous slides summarized the situation in standard ceramics processing in which high density must be combined with small grain size. In the case of high conductivity ceramics, however, we need large grain size in order to minimize both grain boundary scattering, and the effect of grain boundary phases. • Therefore sintering aids to provide a liquid phase during sintering are commonly used. For silicon nitride, Yb 2 O 3 and Mg. O are used, for example. Please acknowledge Carnegie Mellon if you make public use of these slides