1 2 H He 3 4 5 6
































- Slides: 158

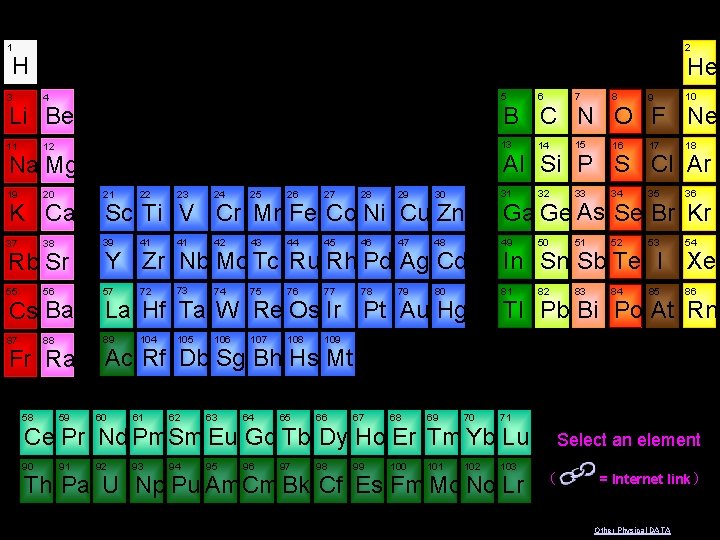
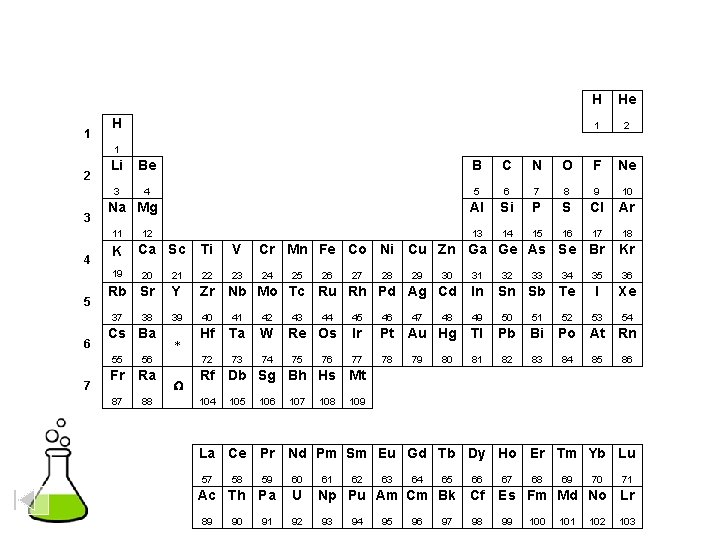
1 2 H He 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 41 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 104 105 106 107 108 109 B C N O F Ne Li Be Al Si P S Cl Ar Na Mg K Ca Rb Sr Cs Ba Fr Ra 58 59 Sc Ti V Cr Mn Fe Co Ni Cu Zn Y Zr Nb Mo Tc Ru Rh Pd Ag Cd La Hf Ta W Re Os Ir Pt Au Hg Ga Ge As Se Br Kr In Sn Sb Te I Xe Tl Pb Bi Po At Rn Ac Rf Db Sg Bh Hs Mt 60 61 62 63 64 65 66 67 68 69 70 71 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Select an element ( = Internet link ) Other Physical DATA

Printable Periodic Tables Click on the element symbol to download the PDF file. Click Here All of the following tables are in Acrobat PDF format. To view and print these files, you will need to install the free Adobe Acrobat Reader program on your computer. The program can be downloaded from the Adobe Website. http: //www. sciencegeek. net/index. html

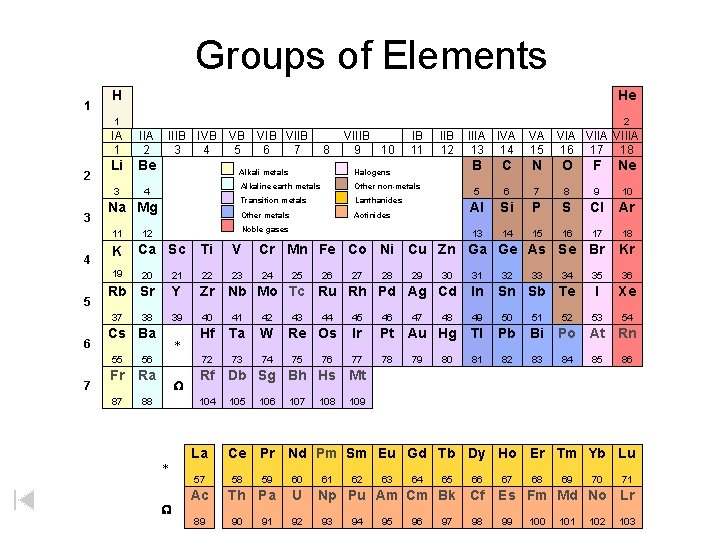
Groups of Elements 1 A 1 2 3 H 1 2 A Li Be 3 4 K 19 5 7 Nitrogen group 2 A Alkaline earth metals 6 A Oxygen group Transition metals 7 A Halogens 3 A Boron group 8 A Noble gases 4 A Carbon group 8 A He 3 A 4 A B C Hydrogen Inner transition metals 8 B 5 A 6 A 7 A 2 N O F Ne 5 6 7 8 9 10 Al Si P S Cl Ar 3 B 4 B 5 B 6 B 7 B 1 B 2 B 13 14 15 16 17 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 12 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 37 6 5 A Na Mg 11 4 1 A Alkali metals 38 Cs Ba 55 56 Fr Ra 87 88 * W La 57 W 24 25 43 26 44 Re Os 75 76 27 28 29 47 30 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 34 Sn Sb Te 45 79 48 31 52 Kr 35 36 I Xe 53 54 Po At Rn 84 85 86 Rf Db Sg Bh Hs Mt 104 * 23 18 Ac 89 105 106 107 108 109 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 58 59 60 Th Pa U 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103

Groups of Elements 1 H He 1 2 3 IIA 2 Li Be 3 4 K 19 5 7 VB 5 VIB VIIB 6 7 8 VIIIB 9 10 IB 11 Alkali metals Halogens Alkaline earth metals Other non-metals Transition metals Lanthanides Other metals Actinides IIB 12 Noble gases 12 Ca Sc IIIA IVA 13 14 VA 15 C N O F Ne 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 38 Cs Ba 55 56 Fr Ra 87 88 * W La 57 W 25 43 26 44 Re Os 75 76 27 28 29 47 30 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 34 Sn Sb Te 45 79 48 31 52 Po At Rn 84 85 86 Rf Db Sg Bh Hs Mt 104 * VIA VIIIA 16 17 18 B 20 37 6 IIIB IVB 3 4 Na Mg 11 4 2 IA 1 Ac 89 105 106 107 108 109 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 58 59 60 Th Pa U 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103

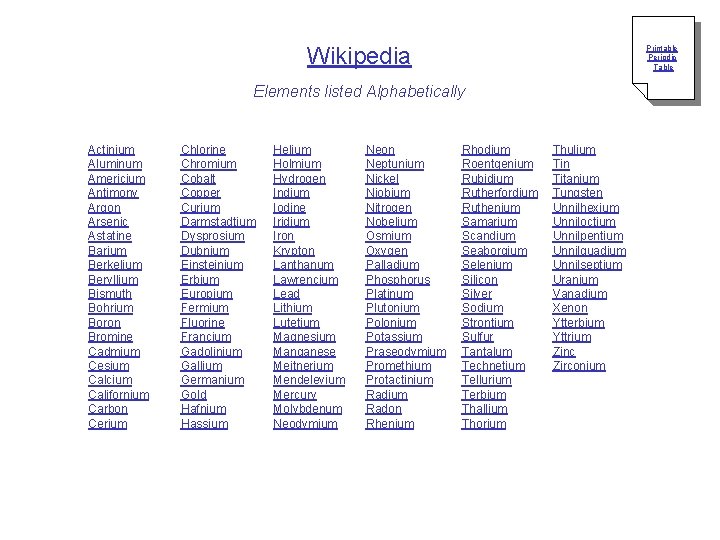
Wikipedia Printable Periodic Table Elements listed Alphabetically Actinium Aluminum Americium Antimony Argon Arsenic Astatine Barium Berkelium Beryllium Bismuth Bohrium Boron Bromine Cadmium Cesium Calcium Californium Carbon Cerium Chlorine Chromium Cobalt Copper Curium Darmstadtium Dysprosium Dubnium Einsteinium Erbium Europium Fermium Fluorine Francium Gadolinium Gallium Germanium Gold Hafnium Hassium Helium Holmium Hydrogen Indium Iodine Iridium Iron Krypton Lanthanum Lawrencium Lead Lithium Lutetium Magnesium Manganese Meitnerium Mendelevium Mercury Molybdenum Neodymium Neon Neptunium Nickel Niobium Nitrogen Nobelium Osmium Oxygen Palladium Phosphorus Platinum Plutonium Polonium Potassium Praseodymium Promethium Protactinium Radon Rhenium Rhodium Roentgenium Rubidium Rutherfordium Ruthenium Samarium Scandium Seaborgium Selenium Silicon Silver Sodium Strontium Sulfur Tantalum Technetium Tellurium Terbium Thallium Thorium Thulium Tin Titanium Tungsten Unnilhexium Unniloctium Unnilpentium Unnilquadium Unnilseptium Uranium Vanadium Xenon Ytterbium Yttrium Zinc Zirconium

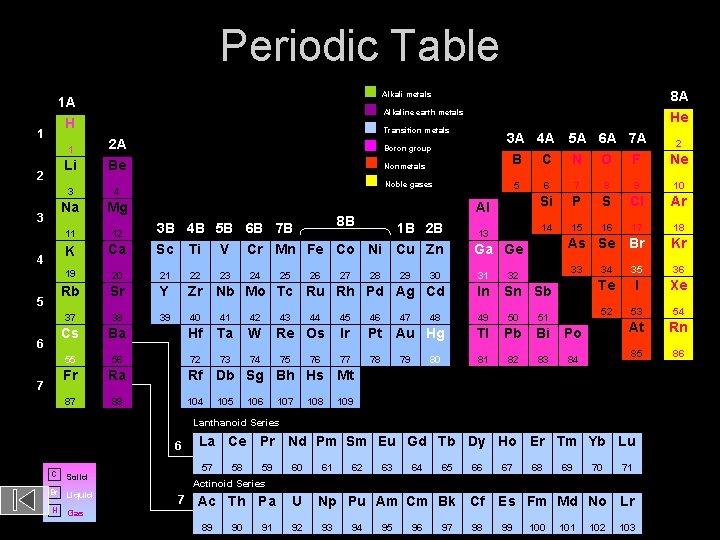
Periodic Table Alkaline earth metals H 1 2 3 4 5 6 7 8 A He Alkali metals 1 A Transition metals 3 A 4 A 5 A 6 A 7 A B C N O F 1 2 A Boron group Li Be Nonmetals 3 4 Na Mg 11 12 K Ca 19 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 37 38 39 40 41 42 49 50 51 Cs Ba Hf Ta W 55 56 72 73 74 Fr Ra 87 88 Noble gases 5 8 B 3 B 4 B 5 B 6 B 7 B 1 B 2 B Sc Ti V Cr Mn Fe Co Ni Cu Zn 23 24 25 26 43 27 44 Re Os 75 76 28 29 30 47 Al 13 48 7 8 9 10 Si P S Cl Ar 14 15 16 17 18 As Se Br Kr 33 32 Sn Sb 45 46 Ir Pt Au Hg Tl Pb Bi Po 77 78 81 82 83 84 79 80 34 35 36 Te I Xe 52 53 54 At Rn 85 86 Rf Db Sg Bh Hs Mt 104 105 106 107 108 109 Lanthanoid Series 6 C Br Liquid H Gas La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 Solid 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Actinoid Series 7 Ac Th Pa 89 90 91 U 92 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 Es Fm Md No Lr 99 Ne 6 Ga Ge 31 2 100 101 102 103

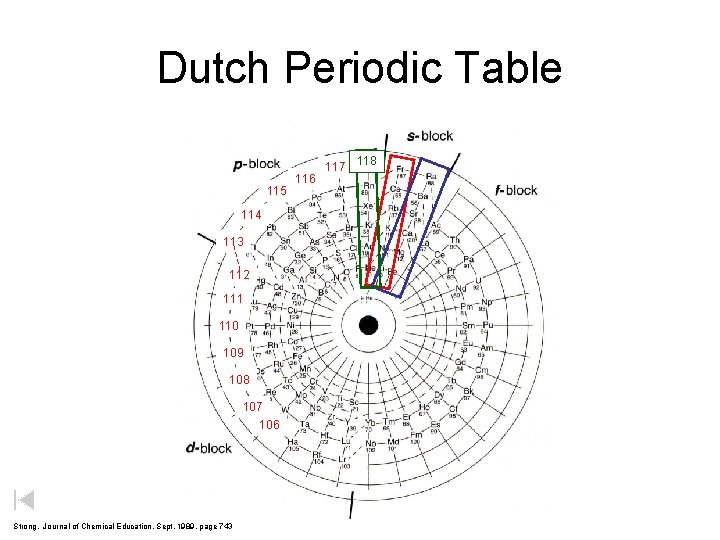
Dutch Periodic Table 115 114 113 112 111 110 109 108 107 106 Strong, Journal of Chemical Education, Sept. 1989, page 743 116 117 118

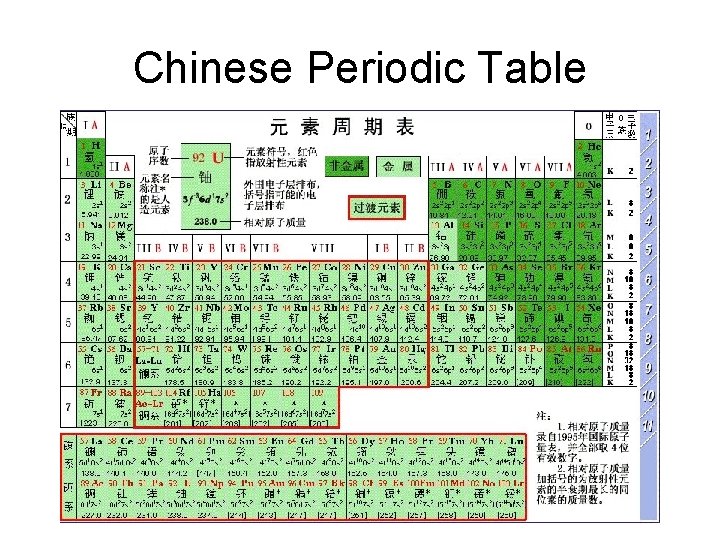
Chinese Periodic Table

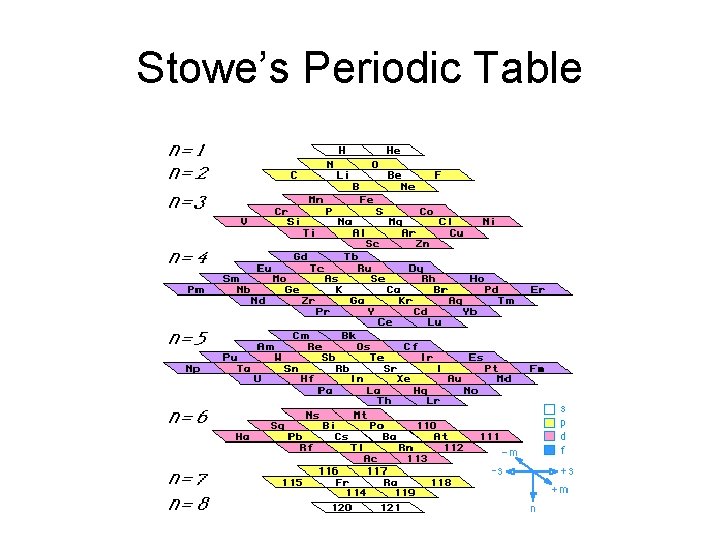
Stowe’s Periodic Table

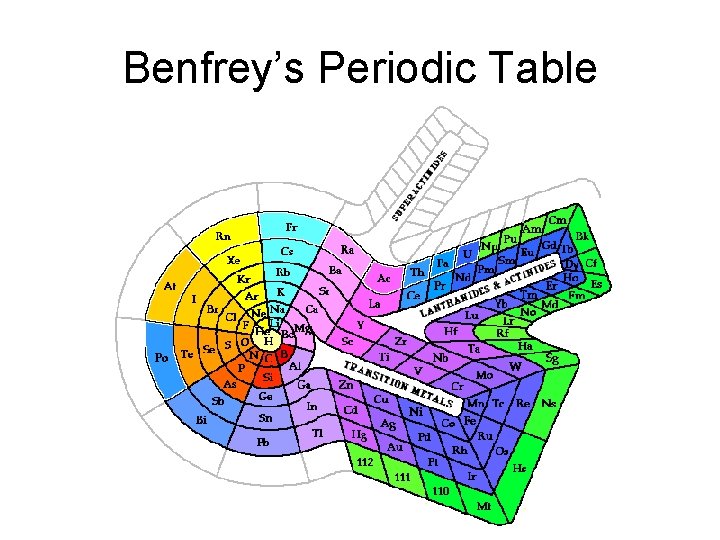
Benfrey’s Periodic Table

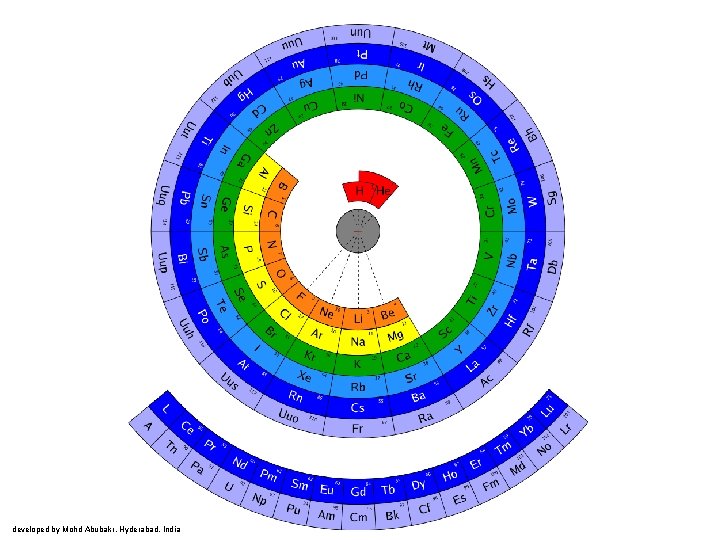
developed by Mohd Abubakr, Hyderabad, India

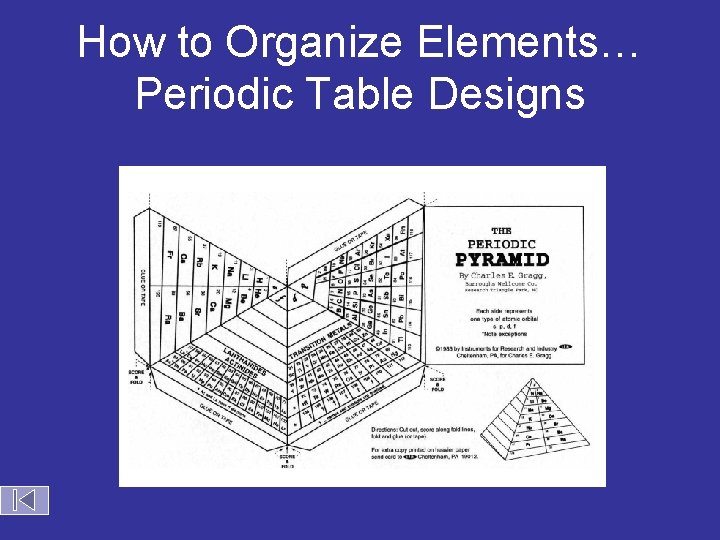
How to Organize Elements… Periodic Table Designs

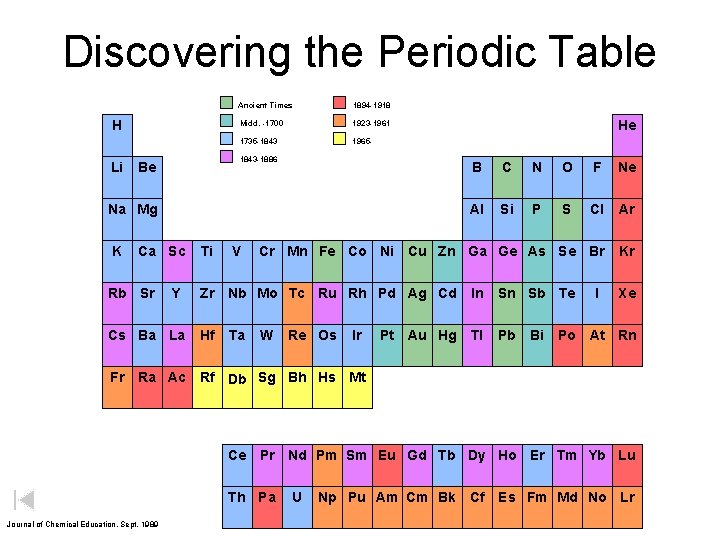
Discovering the Periodic Table H Li Ancient Times 1894 -1918 Midd. -1700 1923 -1961 1735 -1843 1965 - 1843 -1886 Be B C N O F Ne Al Si P S Cl Ar Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Na Mg K Ca Sc Rb Sr Y Cs Ba La Fr Ti V Zr Nb Mo Tc Ru Rh Pd Ag Cd Hf Ta He W Re Os Ir Pt Au Hg In Sn Sb Te Tl Pb Bi I Xe Po At Rn Ra Ac Rf Db Sg Bh Hs Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa Journal of Chemical Education, Sept. 1989 U Np Pu Am Cm Bk Cf Es Fm Md No Lr

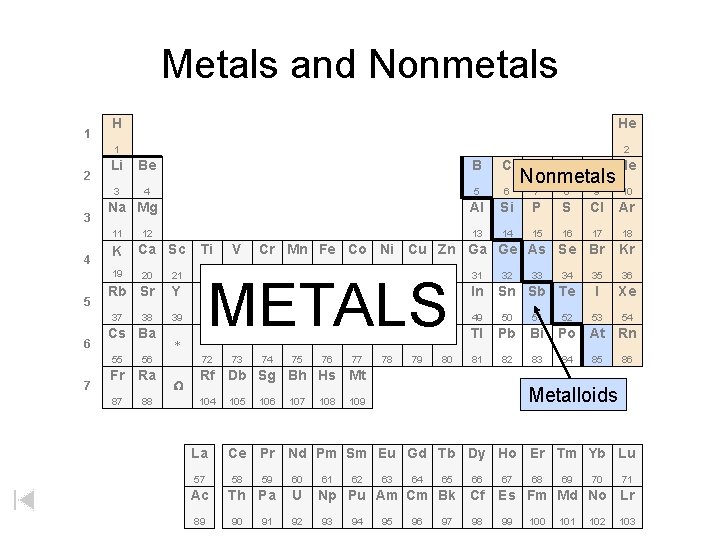
Metals and Nonmetals 1 2 3 H He 1 2 Li Be B C 3 4 5 6 Al 13 Na Mg 11 4 K 19 5 7 Ca Sc O F Ne 7 8 9 10 Si P S Cl Ar 14 15 16 17 18 Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 37 6 12 N Nonmetals 38 Cs Ba 55 56 Fr Ra 87 88 * W 25 26 27 28 29 30 METALS 43 44 Re Os 75 76 47 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 34 Sn Sb Te 45 79 48 31 52 Po At Rn 84 85 86 Rf Db Sg Bh Hs Mt 104 La 57 Ac 89 105 106 107 108 Metalloids 109 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 58 59 60 Th Pa U 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103

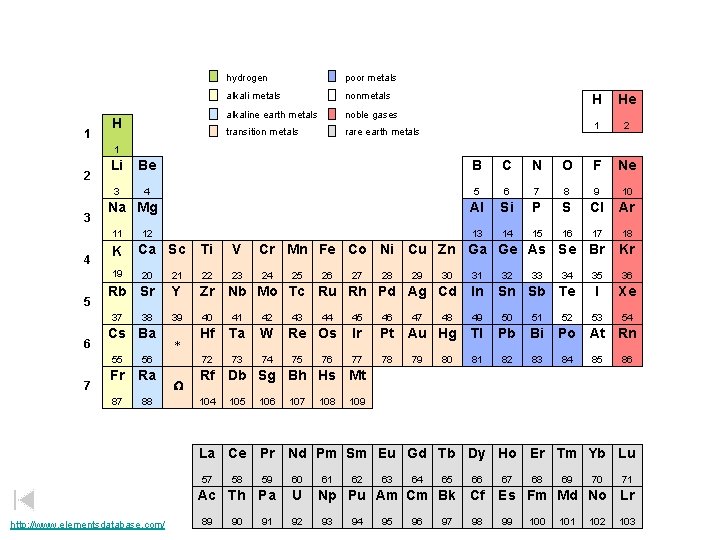
1 H hydrogen poor metals alkali metals nonmetals alkaline earth metals noble gases transition metals rare earth metals H He 1 2 3 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Na Mg 11 4 K 19 5 7 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 37 6 12 38 Cs Ba 55 56 Fr Ra 87 88 * W 25 43 26 44 Re Os 75 76 27 28 29 47 30 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 34 Sn Sb Te 45 79 48 31 52 Po At Rn 84 85 86 Rf Db Sg Bh Hs Mt 104 105 106 107 108 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 http: //www. elementsdatabase. com/ 59 60 Ac Th Pa U 89 58 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103

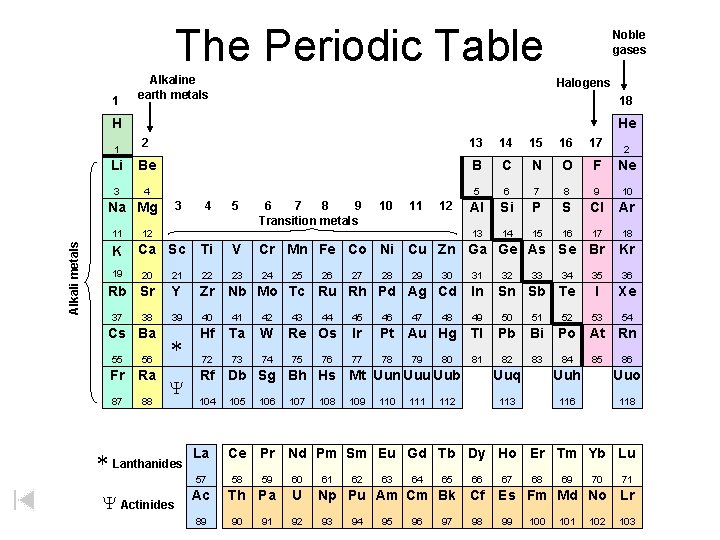
The Periodic Table 1 Alkaline earth metals Noble gases Halogens 18 H He 2 13 14 15 16 17 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 1 3 Na Mg Alkali metals 11 K 19 4 5 6 7 8 9 Transition metals 10 11 12 12 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 * Hf Ta W 72 73 74 37 38 Cs Ba 55 56 Fr Ra 87 88 Y * Lanthanides 25 43 26 44 Re Os 75 76 27 28 29 47 30 104 La Ac 89 105 106 107 108 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 109 110 111 112 34 Sn Sb Te 45 79 48 31 Rf Db Sg Bh Hs Mt Uun Uuu Uub 57 Y Actinides 2 52 Po At Rn 84 85 86 Uuq Uuh Uuo 113 116 118 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 58 59 60 Th Pa U 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103

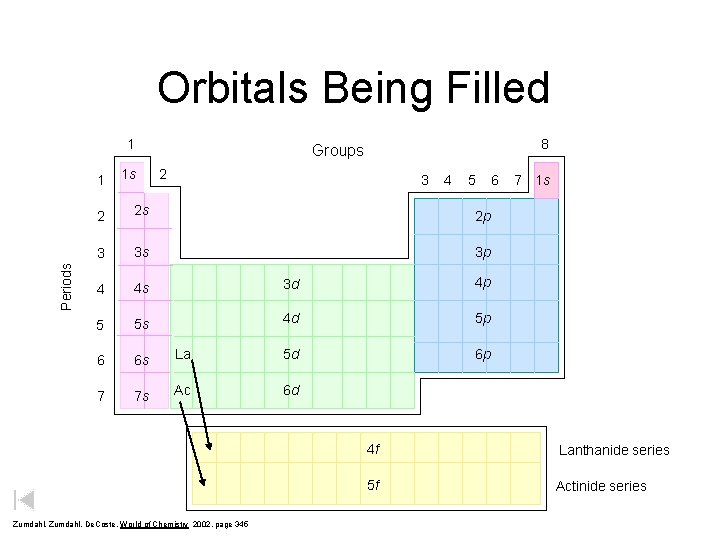
Orbitals Being Filled 1 Periods 1 1 s 8 Groups 2 3 4 5 2 2 s 2 p 3 3 s 3 p 4 4 s 3 d 4 p 5 5 s 4 d 5 p 6 6 s La 5 d 6 p 7 7 s Ac 6 d Zumdahl, De. Coste, World of Chemistry 2002, page 345 6 7 1 s 4 f Lanthanide series 5 f Actinide series

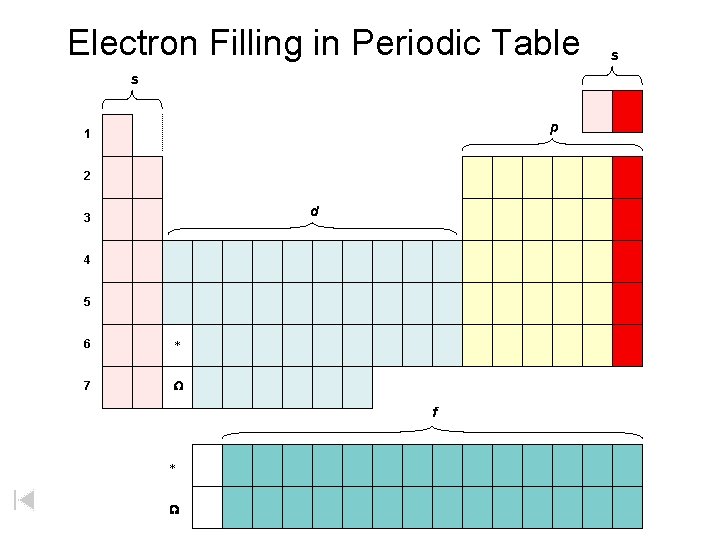
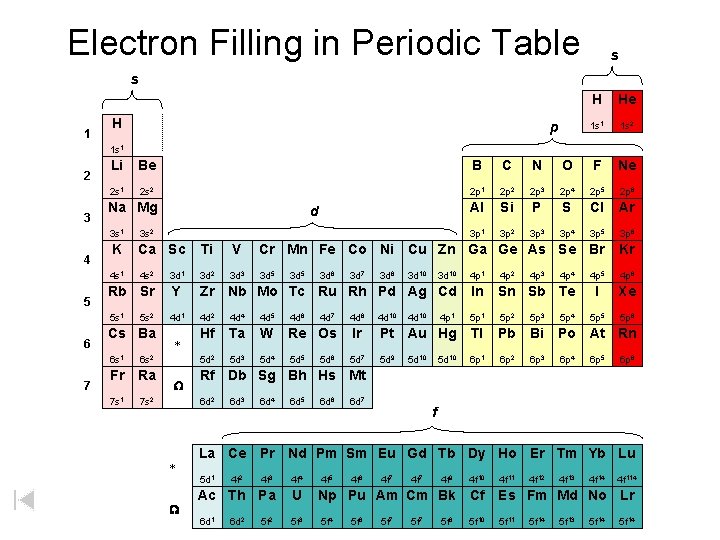
Electron Filling in Periodic Table s p 1 2 d 3 4 5 6 * 7 W f * W s

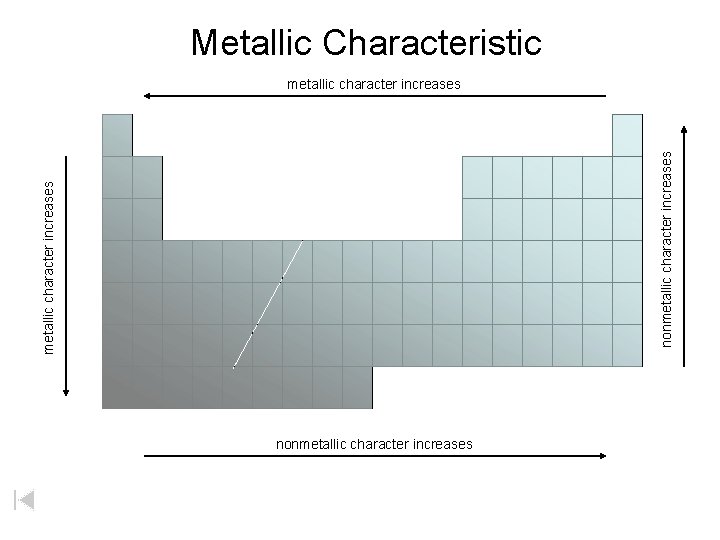
Metallic Characteristic metallic character increases nonmetallic character increases

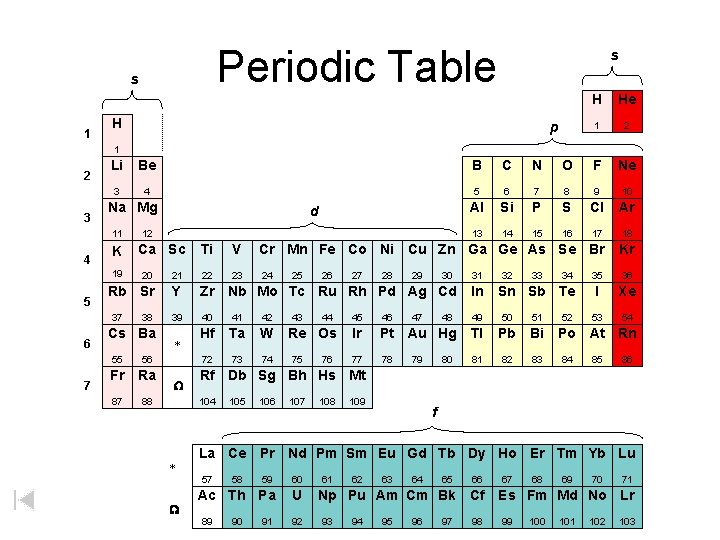
Periodic Table s 1 s H p H He 1 2 3 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Na Mg 11 4 K 19 5 7 12 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 37 6 d 38 Cs Ba 55 56 Fr Ra 87 88 * W 44 Re Os 75 76 27 28 29 30 47 48 31 32 33 Sn Sb Te 45 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 79 80 34 52 Po At Rn 84 85 86 105 106 107 108 109 f La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 W 43 26 Rf Db Sg Bh Hs Mt 104 * 25 59 60 Ac Th Pa U 89 58 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103

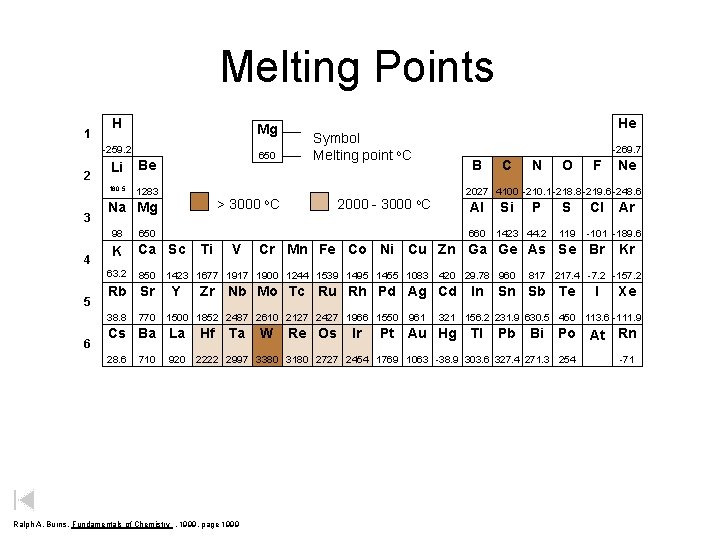
Melting Points 1 H Mg -259. 2 2 3 4 Li Be 180. 5 1283 98 650 K Ca Sc 850 Rb Sr 38. 8 6 > 3000 Na Mg 63. 2 5 650 770 710 o. C 2000 - 3000 -269. 7 B Ti V Al 1500 1852 2487 2610 2127 2427 1966 1550 920 Ta Si P 1423 44. 2 420 29. 78 960 Zr Nb Mo Tc Ru Rh Pd Ag Cd Hf N O S 119 W Re Os Ir 961 Ne Cl In Ar -101 -189. 6 Kr 817 217. 4 -7. 2 -157. 2 Sn Sb Te I Xe 321 156. 2 231. 9 630. 5 450 113. 6 -111. 9 Pt Au Hg Tl Pb Bi Po At Rn 2222 2997 3380 3180 2727 2454 1769 1063 -38. 9 303. 6 327. 4 271. 3 254 Ralph A. Burns, Fundamentals of Chemistry , 1999, page 1999 F Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 1423 1677 1917 1900 1244 1539 1495 1455 1083 Y C 2027 4100 -210. 1 -218. 8 -219. 6 -248. 6 o. C 660 Cs Ba La 28. 6 He Symbol Melting point o. C -71

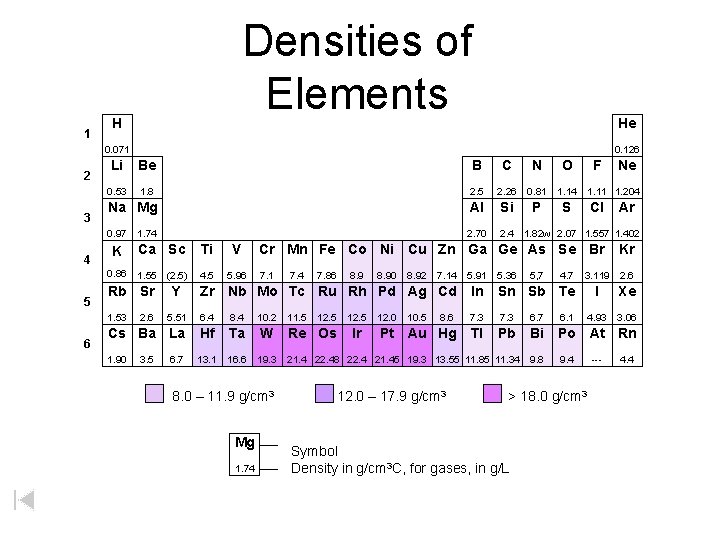
1 Densities of Elements H He 0. 071 2 3 4 5 Li Be B C N O 0. 53 1. 8 2. 5 2. 26 0. 81 1. 14 Na Mg Al Si P S 0. 97 2. 70 2. 4 1. 82 w 2. 07 1. 557 1. 402 1. 74 K Ca Sc Ti V 0. 86 1. 55 (2. 5) 4. 5 5. 96 Rb Sr Ne 1. 11 1. 204 Cl Ar Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 7. 1 3. 119 2. 6 I Xe 4. 93 3. 06 7. 4 7. 86 8. 90 8. 92 7. 14 5. 91 5. 36 5, 7 4. 7 In Sn Sb Te 5. 51 6. 4 8. 4 10. 2 8. 6 7. 3 6. 7 6. 1 Cs Ba La Hf Ta W Pt Au Hg Tl Pb Bi Po At Rn 1. 90 13. 1 16. 6 19. 3 9. 8 9. 4 2. 6 3. 5 Y F Zr Nb Mo Tc Ru Rh Pd Ag Cd 1. 53 6 0. 126 6. 7 8. 0 – 11. 9 g/cm 3 Mg 1. 74 W 11. 5 12. 5 Re Os 12. 5 Ir 12. 0 10. 5 21. 4 22. 48 22. 4 21. 45 19. 3 13. 55 11. 85 11. 34 12. 0 – 17. 9 g/cm 3 > 18. 0 g/cm 3 Symbol Density in g/cm 3 C, for gases, in g/L --- 4. 4

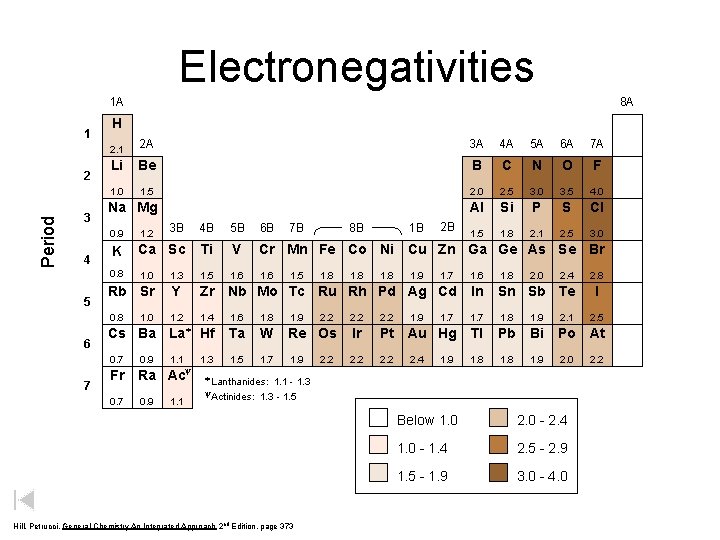
Electronegativities 1 A 1 Period 2 3 4 5 6 7 8 A H 2. 1 2 A 3 A 4 A 5 A 6 A 7 A Li Be B C N O F 1. 0 1. 5 2. 0 2. 5 3. 0 3. 5 4. 0 Al Si P S Cl 1. 5 1. 8 2. 1 2. 5 3. 0 Na Mg 1. 2 3 B 4 B 5 B 6 B K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 0. 8 1. 0 1. 3 1. 5 1. 6 1. 7 1. 6 1. 8 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 0. 8 1. 2 1. 4 1. 6 1. 8 1. 9 2. 2 1. 7 1. 8 1. 9 2. 1 Cs Ba La* Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At 0. 7 1. 1 1. 3 1. 5 1. 7 1. 9 2. 2 1. 8 1. 9 2. 0 Fr 0. 7 1. 0 0. 9 y Ra Ac 0. 9 1. 1 8 B 7 B 1. 5 1. 8 2. 2 1. 8 1 B 2 B 0. 9 1. 8 1. 9 2. 4 1. 9 2. 0 2. 4 * Lanthanides: 1. 1 - 1. 3 - 1. 5 y. Actinides: Hill, Petrucci, General Chemistry An Integrated Approach 2 nd Edition, page 373 Below 1. 0 2. 0 - 2. 4 1. 0 - 1. 4 2. 5 - 2. 9 1. 5 - 1. 9 3. 0 - 4. 0 2. 8 I 2. 5 2. 2

Electron Filling in Periodic Table s s 1 H p H He 1 s 1 1 s 2 1 s 1 2 3 4 5 6 7 Li Be B C N O F Ne 2 s 1 2 s 2 2 p 1 2 p 2 2 p 3 2 p 4 2 p 5 2 p 6 Al Si P S Cl Ar 3 p 1 3 p 2 3 p 3 3 p 4 3 p 5 3 p 6 Na Mg d 3 s 1 3 s 2 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 4 s 1 4 s 2 3 d 1 3 d 2 3 d 3 3 d 5 3 d 10 4 p 1 4 p 2 4 p 5 4 p 6 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 5 s 1 4 d 2 4 d 4 4 d 5 4 d 6 4 d 7 4 d 8 4 d 10 4 p 1 5 p 2 5 p 3 5 p 4 5 p 5 5 p 6 Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 5 d 2 5 d 3 5 d 4 5 d 5 5 d 7 5 d 9 6 p 1 6 p 2 6 p 3 6 p 4 5 s 2 Cs Ba 6 s 1 6 s 2 Fr Ra 7 s 1 7 s 2 * W 5 d 6 3 d 7 3 d 8 3 d 10 4 d 10 5 d 10 4 p 3 4 p 4 6 p 5 6 p 6 6 d 3 6 d 4 6 d 5 6 d 6 6 d 7 f La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 5 d 1 W 3 d 6 Rf Db Sg Bh Hs Mt 6 d 2 * 3 d 5 4 f 2 4 f 3 4 f 4 Ac Th Pa U 6 d 1 5 f 3 6 d 2 5 f 2 4 f 5 4 f 6 4 f 7 4 f 9 4 f 10 Np Pu Am Cm Bk Cf 5 f 4 5 f 6 5 f 7 5 f 8 5 f 10 4 f 11 4 f 12 4 f 13 4 f 14 4 f 114 Es Fm Md No Lr 5 f 11 5 f 14 5 f 13 5 f 14

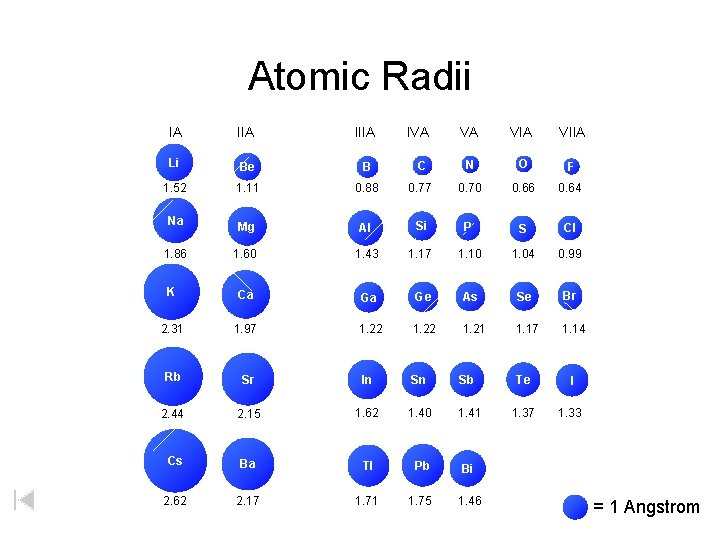
Atomic Radii IA IIIA Li Be B 1. 52 1. 11 Na 1. 86 IVA VA VIIA C N O F 0. 88 0. 77 0. 70 0. 66 0. 64 Mg Al Si P S Cl 1. 60 1. 43 1. 17 1. 10 1. 04 0. 99 K Ca Ga Ge As Se Br 2. 31 1. 97 1. 22 1. 21 1. 17 1. 14 Rb Sr In Sn Sb Te I 2. 44 2. 15 1. 62 1. 40 1. 41 1. 37 1. 33 Cs Ba Tl Pb Bi 2. 62 2. 17 1. 71 1. 75 1. 46 = 1 Angstrom

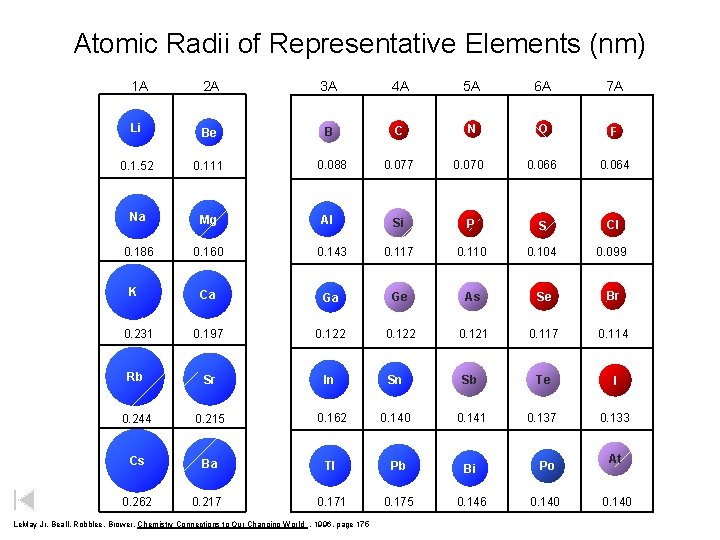
Atomic Radii of Representative Elements (nm) 1 A 2 A 3 A 4 A 5 A 6 A 7 A Li Be B C N O F 0. 1. 52 0. 111 0. 088 0. 077 0. 070 0. 066 0. 064 Na Mg Si P S Cl 0. 186 0. 160 0. 143 0. 117 0. 110 0. 104 0. 099 Ca Ga Ge As Se Br 0. 197 0. 122 0. 121 0. 117 0. 114 Rb Sr In Sn Sb Te I 0. 244 0. 215 0. 162 0. 140 0. 141 0. 137 0. 133 Cs Ba Tl Pb Bi Po 0. 262 0. 217 0. 171 0. 175 0. 146 0. 140 K 0. 231 Al Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 175 At 0. 140

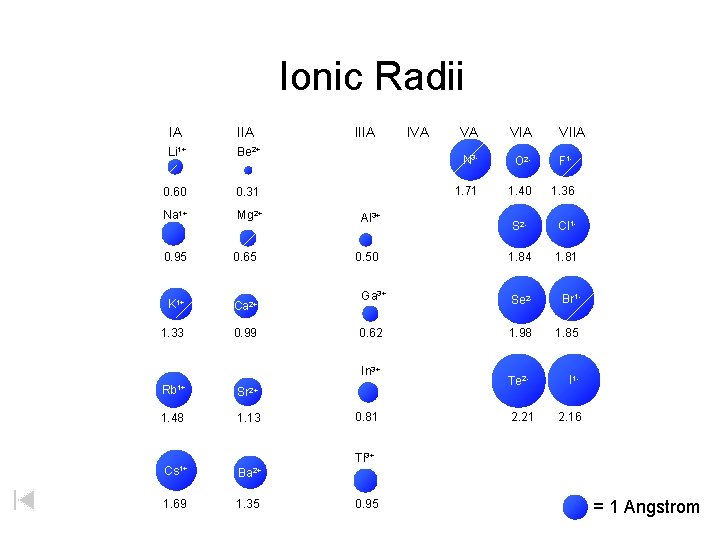
Atomic Ionic Radii IA IIIA IVA VA VIA Li 1+ Li Be 2+ Be 1. 52 0. 60 1+ Na Na VIIA B C NN 3 - OO 2 - F 1 F 1. 11 0. 31 0. 88 0. 77 0. 70 1. 71 0. 66 1. 40 0. 64 1. 36 Mg 2+ Mg Al 3+ Al Si P 2 SS 1 Cl. Cl 1. 43 0. 50 1. 17 1. 10 1. 04 1. 84 0. 99 1. 81 1. 86 0. 95 1. 60 0. 65 K K 1+ Ca Ca 2+ Ga 3+ Ge As Se 2 Se Br 1 Br 2. 31 1. 33 1. 97 0. 99 1. 22 0. 62 1. 21 1. 17 1. 98 1. 14 1. 85 Rb Rb 1+ Sr Sr 2+ In 3+ In Sn Sb 2. 44 1. 48 2. 15 1. 13 1. 62 0. 81 1. 40 1. 41 Tl 3+ Tl Pb Bi 1. 71 0. 95 1. 75 1. 46 Cs Cs 1+ Ba Ba 2+ 2. 62 1. 69 2. 17 1. 35 2 Te. Te 1. 37 2. 21 II 11. 33 2. 16 = 1 Angstrom

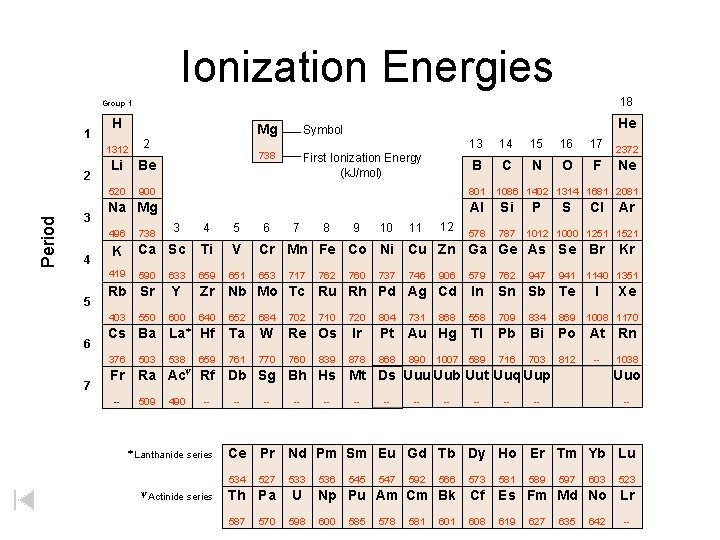
Ionization Energies 18 Group 1 1 Period 2 3 H 6 7 738 First Ionization Energy (k. J/mol) 13 14 15 16 17 B C N O F 2 Li Be 520 900 801 Na Mg Al Si 578 787 12 Cl Ar 590 633 659 651 906 579 762 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 403 600 640 652 684 702 868 558 709 834 869 Cs Ba La* Hf Ta W Re Os Pt Au Hg Tl Pb Bi Po At Rn 376 538 659 761 770 760 868 589 716 703 812 Fr -- 503 11 S V 550 10 P Ti 7 9 1086 1402 1314 1681 2081 Ca Sc K 3 8 Ne 5 738 6 2372 4 419 5 Symbol 1312 496 4 He Mg 1012 1000 1251 1521 Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 653 717 762 710 839 760 720 Ir 878 737 804 746 731 890 1007 941 1140 1351 I -- Ra Ac Rf Db Sg Bh Hs Mt Ds Uuu Uub Uut Uuq Uup 490 -- * Lanthanide series -- -- -- 1038 Uuo -- -- Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 534 y Actinide -- Xe 1008 1170 y 509 Kr 527 Th Pa 587 570 533 U 598 536 545 547 592 566 573 581 589 597 603 523 Np Pu Am Cm Bk Cf Es Fm Md No Lr 600 619 585 578 581 608 627 635 642 --

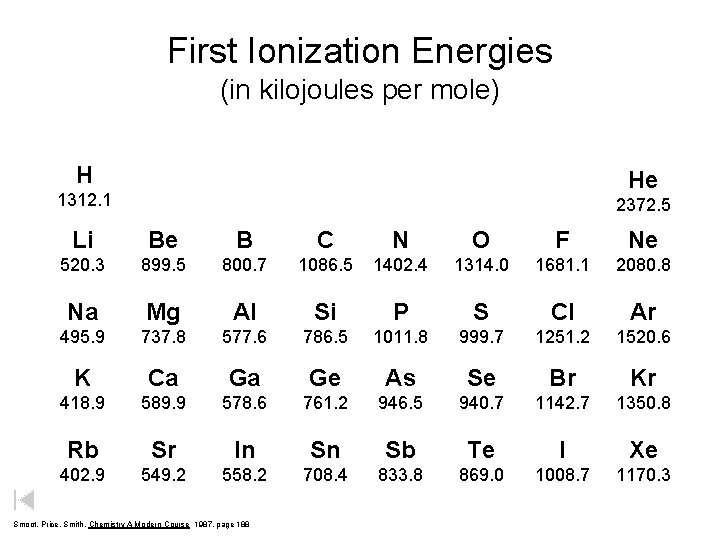
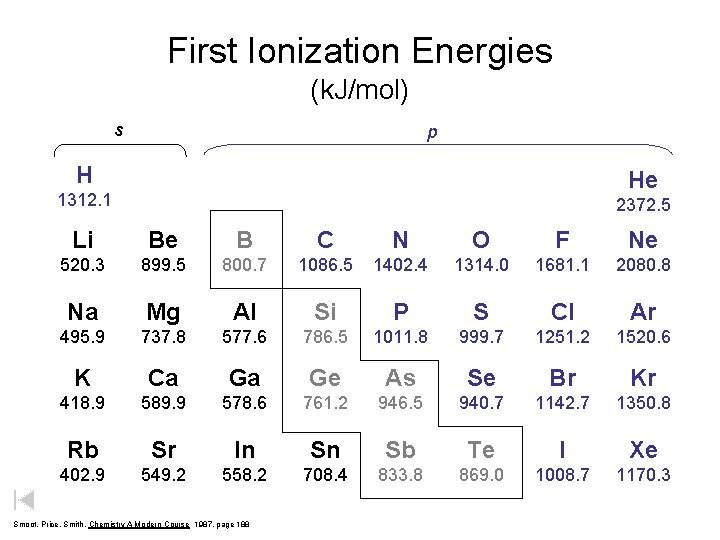
First Ionization Energies (in kilojoules per mole) H He 1312. 1 2372. 5 Li Be B C N O F Ne 520. 3 899. 5 800. 7 1086. 5 1402. 4 1314. 0 1681. 1 2080. 8 Na Mg Al Si P S Cl Ar 495. 9 737. 8 577. 6 786. 5 1011. 8 999. 7 1251. 2 1520. 6 K Ca Ga Ge As Se Br Kr 418. 9 589. 9 578. 6 761. 2 946. 5 940. 7 1142. 7 1350. 8 Rb Sr In Sn Sb Te I Xe 402. 9 549. 2 558. 2 708. 4 833. 8 869. 0 1008. 7 1170. 3 Smoot, Price, Smith, Chemistry A Modern Course 1987, page 188

First Ionization Energies (k. J/mol) s p H He 1312. 1 2372. 5 Li Be B C N O F Ne 520. 3 899. 5 800. 7 1086. 5 1402. 4 1314. 0 1681. 1 2080. 8 Na Mg Al Si P S Cl Ar 495. 9 737. 8 577. 6 786. 5 1011. 8 999. 7 1251. 2 1520. 6 K Ca Ga Ge As Se Br Kr 418. 9 589. 9 578. 6 761. 2 946. 5 940. 7 1142. 7 1350. 8 Rb Sr In Sn Sb Te I Xe 402. 9 549. 2 558. 2 708. 4 833. 8 869. 0 1008. 7 1170. 3 Smoot, Price, Smith, Chemistry A Modern Course 1987, page 188

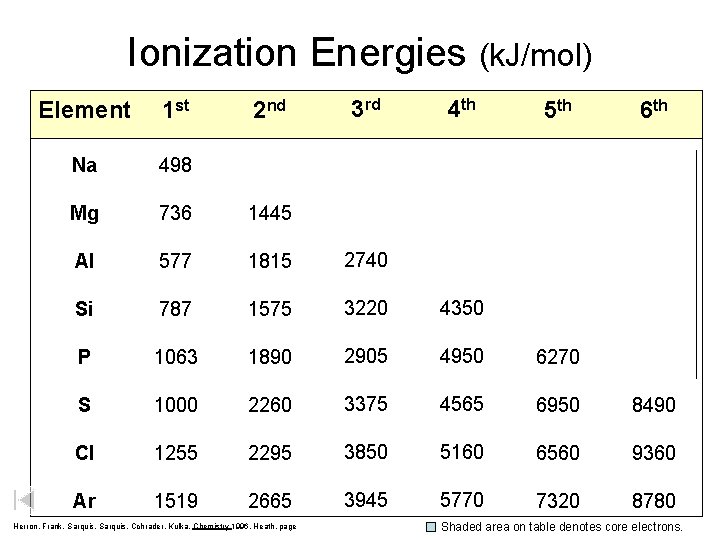
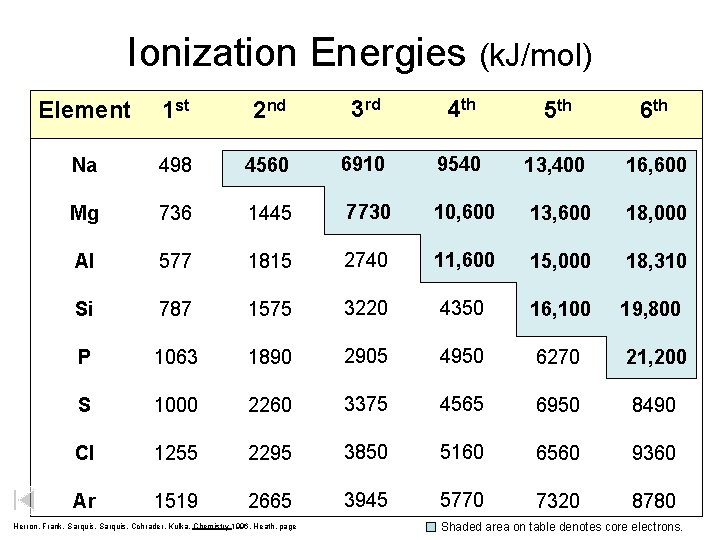
Ionization Energies (k. J/mol) Element 1 st 2 nd 3 rd 4 th 5 th 6 th Na 498 4560 6910 9540 13, 400 16, 600 Mg 736 1445 7730 10, 600 13, 600 18, 000 Al 577 1815 2740 11, 600 15, 000 18, 310 Si 787 1575 3220 4350 16, 100 19, 800 P 1063 1890 2905 4950 6270 21, 200 S 1000 2260 3375 4565 6950 8490 Cl 1255 2295 3850 5160 6560 9360 Ar 1519 2665 3945 5770 7320 8780 Herron, Frank, Sarquis, Cchrader, Kulka, Chemistry 1996, Heath, page Shaded area on table denotes core electrons.

Ionization Energies (k. J/mol) Element 1 st 2 nd 3 rd 4 th 5 th 6 th Na 498 4560 6910 9540 13, 400 16, 600 Mg 736 1445 7730 10, 600 13, 600 18, 000 Al 577 1815 2740 11, 600 15, 000 18, 310 Si 787 1575 3220 4350 16, 100 19, 800 P 1063 1890 2905 4950 6270 21, 200 S 1000 2260 3375 4565 6950 8490 Cl 1255 2295 3850 5160 6560 9360 Ar 1519 2665 3945 5770 7320 8780 Herron, Frank, Sarquis, Cchrader, Kulka, Chemistry 1996, Heath, page Shaded area on table denotes core electrons.

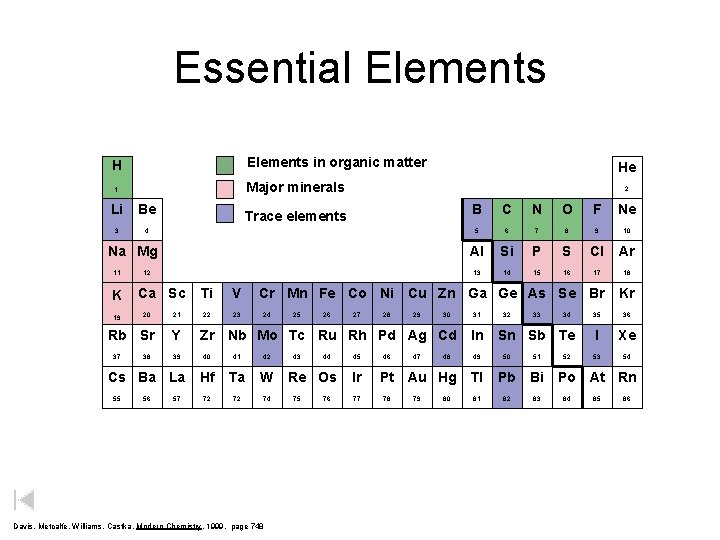
Essential Elements H Elements in organic matter 1 Major minerals Li Be 3 4 He 2 Trace elements Na Mg 11 K 19 12 Ca Sc 20 Rb Sr 37 38 21 Y 39 Cs Ba La 55 56 57 Ti V 22 23 B C N O F Ne 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 24 25 26 27 28 29 30 Zr Nb Mo Tc Ru Rh Pd Ag Cd 40 41 42 Hf Ta W 72 72 74 Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 748 43 44 Re Os 75 76 45 Ir 77 46 47 48 Pt Au Hg 78 79 80 31 In 32 33 34 Sn Sb Te 49 50 51 Tl Pb Bi 81 82 83 52 Kr 35 36 I Xe 53 54 Po At Rn 84 85 86

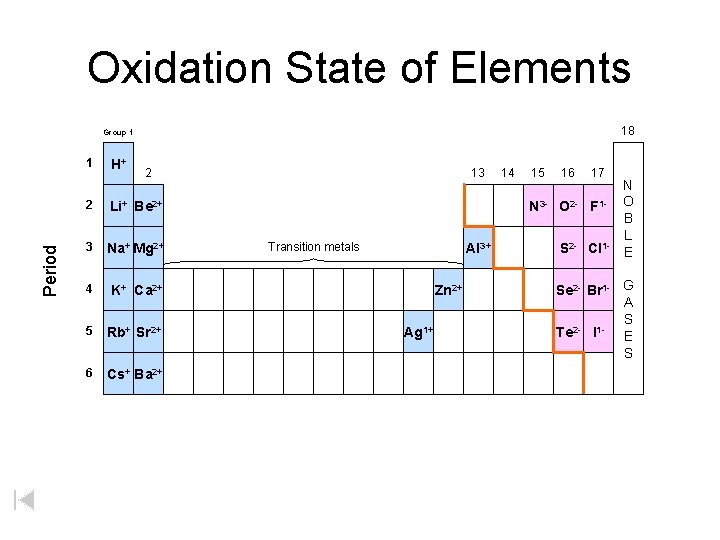
Oxidation State of Elements 18 Group 1 Period 1 H+ 13 2 2 Li+ Be 2+ 3 Na+ Mg 2+ 4 K+ Ca 2+ 5 Rb+ Sr 2+ 6 Cs+ Ba 2+ 14 15 16 17 N 3 - O 2 - F 1 Transition metals Al 3+ Zn 2+ Ag 1+ S 2 - Cl 1 - N O B L E Se 2 - Br 1 - G A S Te 2 - I 1 - E S

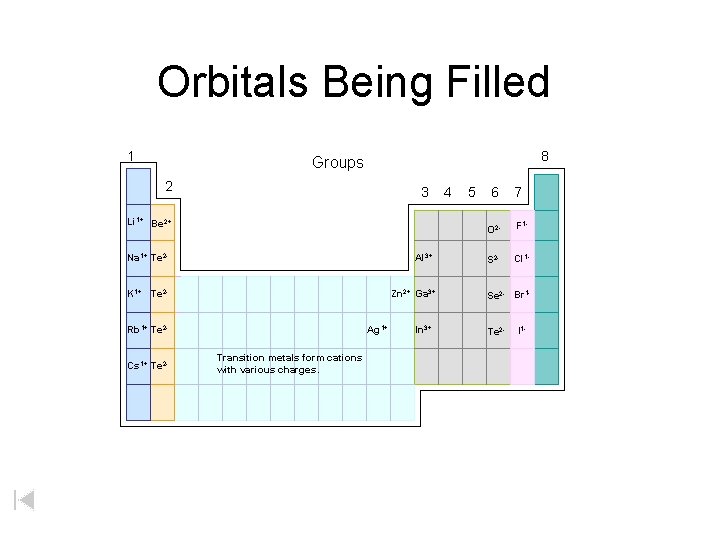
Orbitals Being Filled 1 8 Groups 2 3 Li 1+ Be 2+ Na 1+ Te 2 - Al 3+ K 1+ Te 2 - Zn 2+ Ga 3+ Rb 1+ Te 2 Cs 1+ Te 2 - Ag 1+ Transition metals form cations with various charges. In 3+ 4 5 6 7 O 2 - F 1 - S 2 - Cl 1 - Se 2 - Br 1 Te 2 - I 1 -

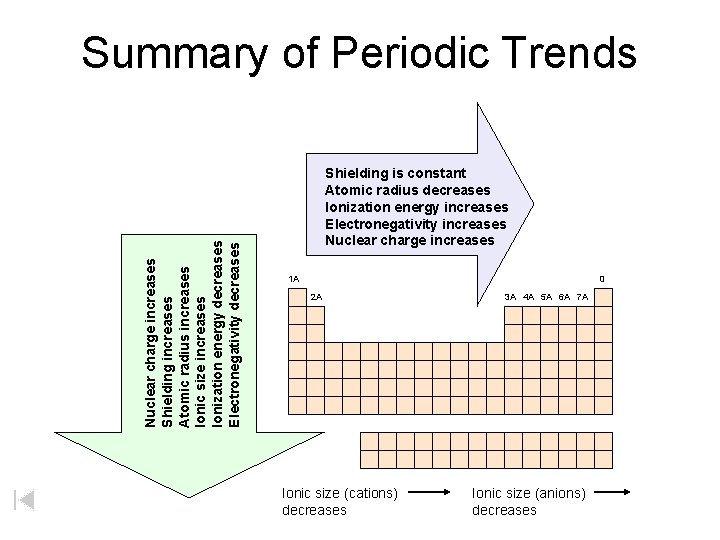
Nuclear charge increases Shielding increases Atomic radius increases Ionic size increases Ionization energy decreases Electronegativity decreases Summary of Periodic Trends Shielding is constant Atomic radius decreases Ionization energy increases Electronegativity increases Nuclear charge increases 1 A 0 2 A Ionic size (cations) decreases 3 A 4 A 5 A 6 A 7 A Ionic size (anions) decreases

1 H H He 1 2 3 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Na Mg 11 4 K 19 5 7 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 37 6 12 38 Cs Ba 55 56 Fr Ra 87 88 * W 25 43 26 44 Re Os 75 76 27 28 29 47 30 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 34 Sn Sb Te 45 79 48 31 52 Po At Rn 84 85 86 Rf Db Sg Bh Hs Mt 104 105 106 107 108 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 59 60 Ac Th Pa U 89 58 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103

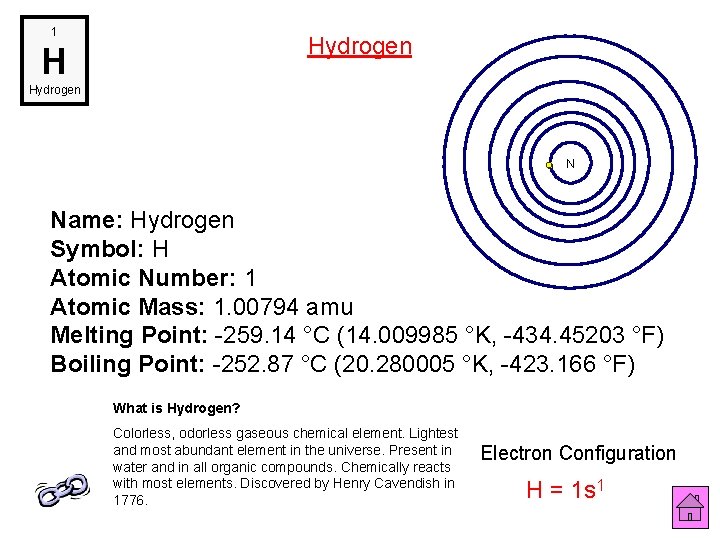
1 Hydrogen H Hydrogen N Name: Hydrogen Symbol: H Atomic Number: 1 Atomic Mass: 1. 00794 amu Melting Point: -259. 14 °C (14. 009985 °K, -434. 45203 °F) Boiling Point: -252. 87 °C (20. 280005 °K, -423. 166 °F) What is Hydrogen? Colorless, odorless gaseous chemical element. Lightest and most abundant element in the universe. Present in water and in all organic compounds. Chemically reacts with most elements. Discovered by Henry Cavendish in 1776. Electron Configuration H = 1 s 1

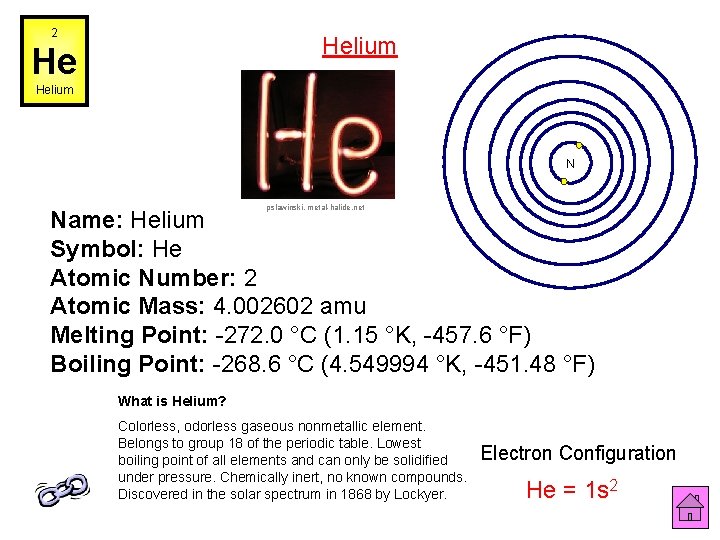
2 Helium He Helium N pslawinski, metal-halide. net Name: Helium Symbol: He Atomic Number: 2 Atomic Mass: 4. 002602 amu Melting Point: -272. 0 °C (1. 15 °K, -457. 6 °F) Boiling Point: -268. 6 °C (4. 549994 °K, -451. 48 °F) What is Helium? Colorless, odorless gaseous nonmetallic element. Belongs to group 18 of the periodic table. Lowest boiling point of all elements and can only be solidified under pressure. Chemically inert, no known compounds. Discovered in the solar spectrum in 1868 by Lockyer. Electron Configuration He = 1 s 2

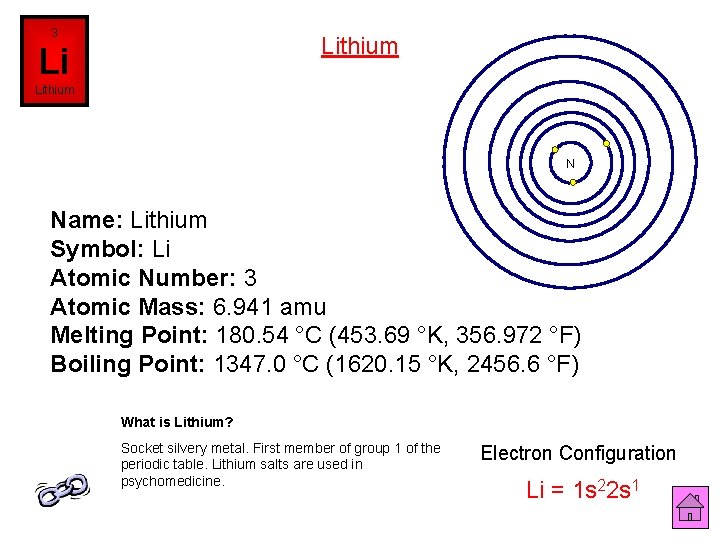
3 Lithium Li Lithium N Name: Lithium Symbol: Li Atomic Number: 3 Atomic Mass: 6. 941 amu Melting Point: 180. 54 °C (453. 69 °K, 356. 972 °F) Boiling Point: 1347. 0 °C (1620. 15 °K, 2456. 6 °F) What is Lithium? Socket silvery metal. First member of group 1 of the periodic table. Lithium salts are used in psychomedicine. Electron Configuration Li = 1 s 22 s 1

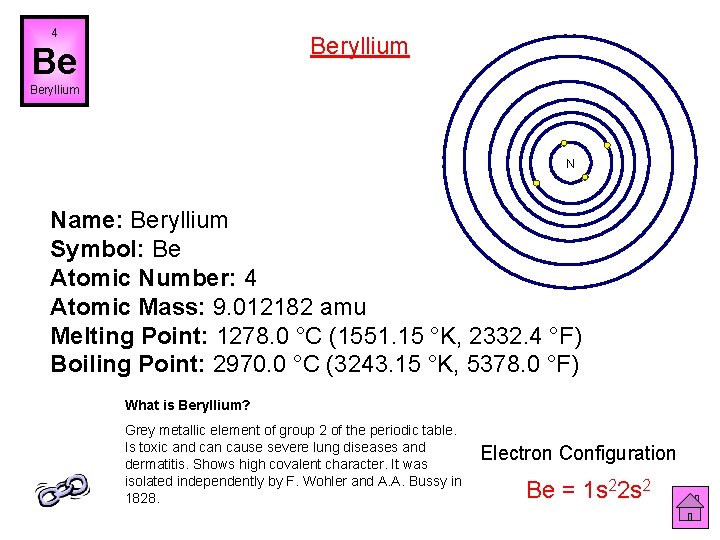
4 Beryllium Be Beryllium N Name: Beryllium Symbol: Be Atomic Number: 4 Atomic Mass: 9. 012182 amu Melting Point: 1278. 0 °C (1551. 15 °K, 2332. 4 °F) Boiling Point: 2970. 0 °C (3243. 15 °K, 5378. 0 °F) What is Beryllium? Grey metallic element of group 2 of the periodic table. Is toxic and can cause severe lung diseases and dermatitis. Shows high covalent character. It was isolated independently by F. Wohler and A. A. Bussy in 1828. Electron Configuration Be = 1 s 22 s 2

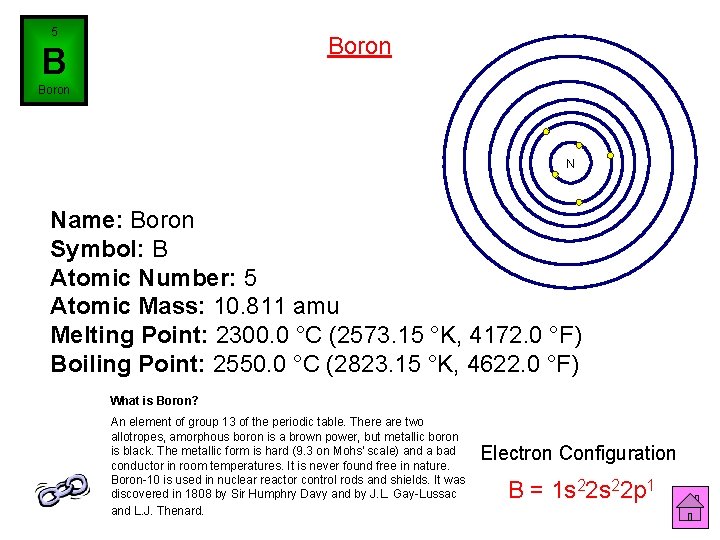
5 Boron B Boron N Name: Boron Symbol: B Atomic Number: 5 Atomic Mass: 10. 811 amu Melting Point: 2300. 0 °C (2573. 15 °K, 4172. 0 °F) Boiling Point: 2550. 0 °C (2823. 15 °K, 4622. 0 °F) What is Boron? An element of group 13 of the periodic table. There are two allotropes, amorphous boron is a brown power, but metallic boron is black. The metallic form is hard (9. 3 on Mohs' scale) and a bad conductor in room temperatures. It is never found free in nature. Boron-10 is used in nuclear reactor control rods and shields. It was discovered in 1808 by Sir Humphry Davy and by J. L. Gay-Lussac and L. J. Thenard. Electron Configuration B = 1 s 22 p 1

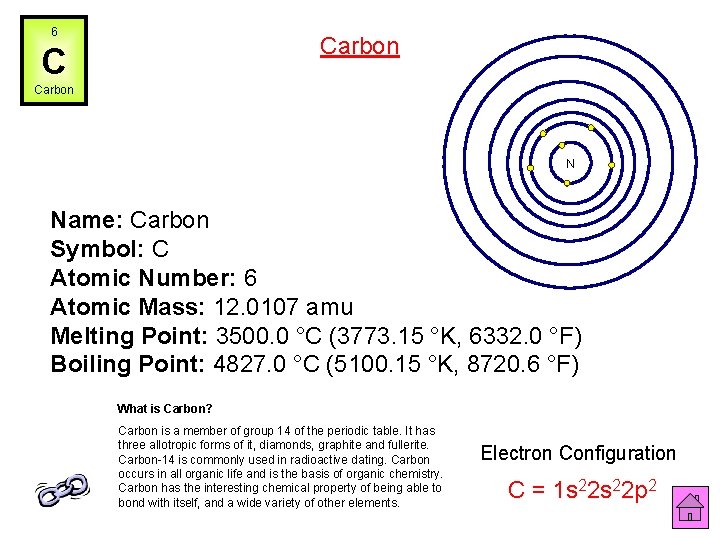
6 Carbon C Carbon N Name: Carbon Symbol: C Atomic Number: 6 Atomic Mass: 12. 0107 amu Melting Point: 3500. 0 °C (3773. 15 °K, 6332. 0 °F) Boiling Point: 4827. 0 °C (5100. 15 °K, 8720. 6 °F) What is Carbon? Carbon is a member of group 14 of the periodic table. It has three allotropic forms of it, diamonds, graphite and fullerite. Carbon-14 is commonly used in radioactive dating. Carbon occurs in all organic life and is the basis of organic chemistry. Carbon has the interesting chemical property of being able to bond with itself, and a wide variety of other elements. Electron Configuration C = 1 s 22 p 2

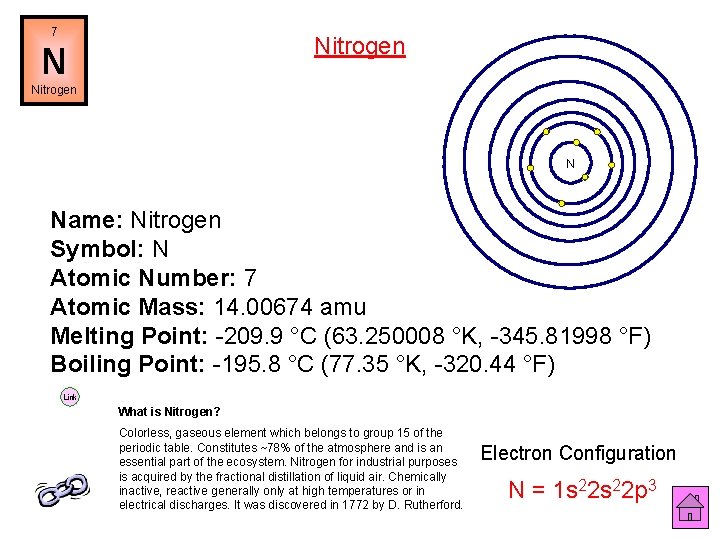
7 Nitrogen N Name: Nitrogen Symbol: N Atomic Number: 7 Atomic Mass: 14. 00674 amu Melting Point: -209. 9 °C (63. 250008 °K, -345. 81998 °F) Boiling Point: -195. 8 °C (77. 35 °K, -320. 44 °F) Link What is Nitrogen? Colorless, gaseous element which belongs to group 15 of the periodic table. Constitutes ~78% of the atmosphere and is an essential part of the ecosystem. Nitrogen for industrial purposes is acquired by the fractional distillation of liquid air. Chemically inactive, reactive generally only at high temperatures or in electrical discharges. It was discovered in 1772 by D. Rutherford. Electron Configuration N = 1 s 22 p 3

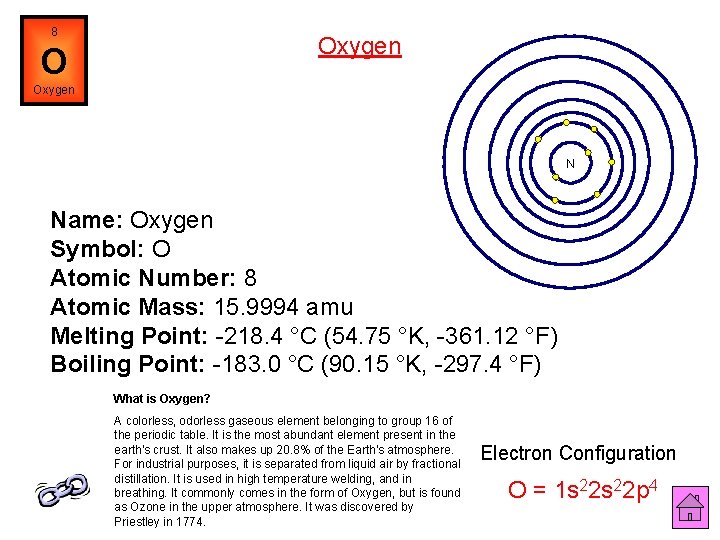
8 Oxygen O Oxygen N Name: Oxygen Symbol: O Atomic Number: 8 Atomic Mass: 15. 9994 amu Melting Point: -218. 4 °C (54. 75 °K, -361. 12 °F) Boiling Point: -183. 0 °C (90. 15 °K, -297. 4 °F) What is Oxygen? A colorless, odorless gaseous element belonging to group 16 of the periodic table. It is the most abundant element present in the earth's crust. It also makes up 20. 8% of the Earth's atmosphere. For industrial purposes, it is separated from liquid air by fractional distillation. It is used in high temperature welding, and in breathing. It commonly comes in the form of Oxygen, but is found as Ozone in the upper atmosphere. It was discovered by Priestley in 1774. Electron Configuration O = 1 s 22 p 4

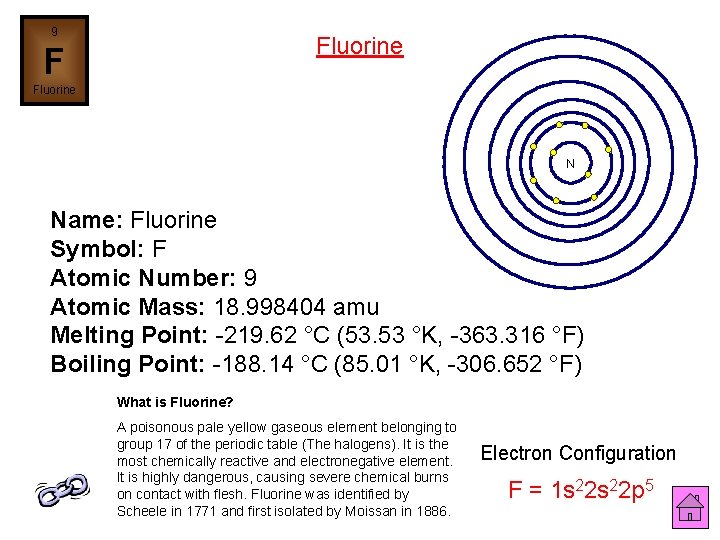
9 Fluorine F Fluorine N Name: Fluorine Symbol: F Atomic Number: 9 Atomic Mass: 18. 998404 amu Melting Point: -219. 62 °C (53. 53 °K, -363. 316 °F) Boiling Point: -188. 14 °C (85. 01 °K, -306. 652 °F) What is Fluorine? A poisonous pale yellow gaseous element belonging to group 17 of the periodic table (The halogens). It is the most chemically reactive and electronegative element. It is highly dangerous, causing severe chemical burns on contact with flesh. Fluorine was identified by Scheele in 1771 and first isolated by Moissan in 1886. Electron Configuration F = 1 s 22 p 5

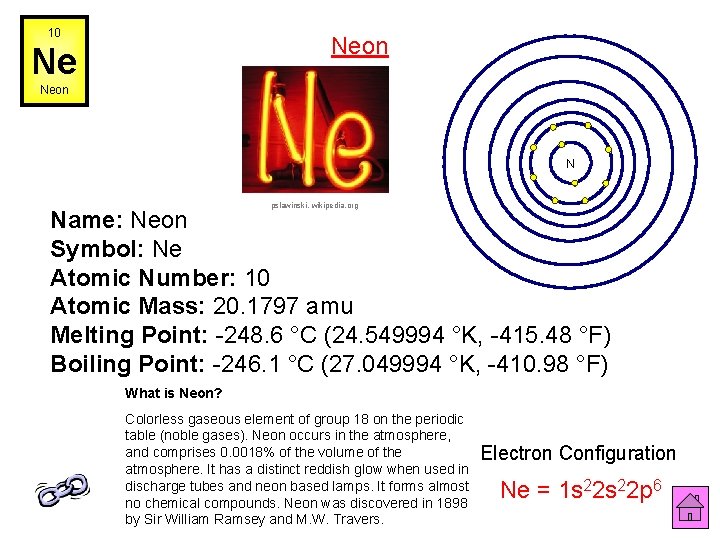
10 Neon Ne Neon N pslawinski, wikipedia. org Name: Neon Symbol: Ne Atomic Number: 10 Atomic Mass: 20. 1797 amu Melting Point: -248. 6 °C (24. 549994 °K, -415. 48 °F) Boiling Point: -246. 1 °C (27. 049994 °K, -410. 98 °F) What is Neon? Colorless gaseous element of group 18 on the periodic table (noble gases). Neon occurs in the atmosphere, and comprises 0. 0018% of the volume of the atmosphere. It has a distinct reddish glow when used in discharge tubes and neon based lamps. It forms almost no chemical compounds. Neon was discovered in 1898 by Sir William Ramsey and M. W. Travers. Electron Configuration Ne = 1 s 22 p 6

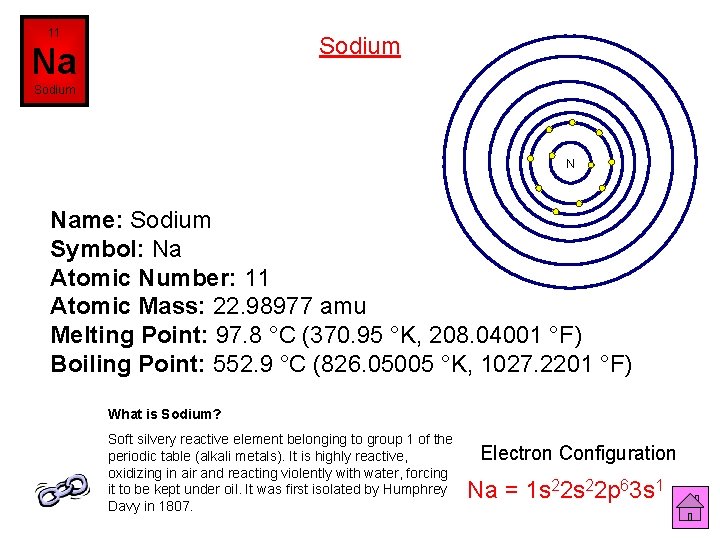
11 Sodium Na Sodium N Name: Sodium Symbol: Na Atomic Number: 11 Atomic Mass: 22. 98977 amu Melting Point: 97. 8 °C (370. 95 °K, 208. 04001 °F) Boiling Point: 552. 9 °C (826. 05005 °K, 1027. 2201 °F) What is Sodium? Soft silvery reactive element belonging to group 1 of the periodic table (alkali metals). It is highly reactive, oxidizing in air and reacting violently with water, forcing it to be kept under oil. It was first isolated by Humphrey Davy in 1807. Electron Configuration Na = 1 s 22 p 63 s 1

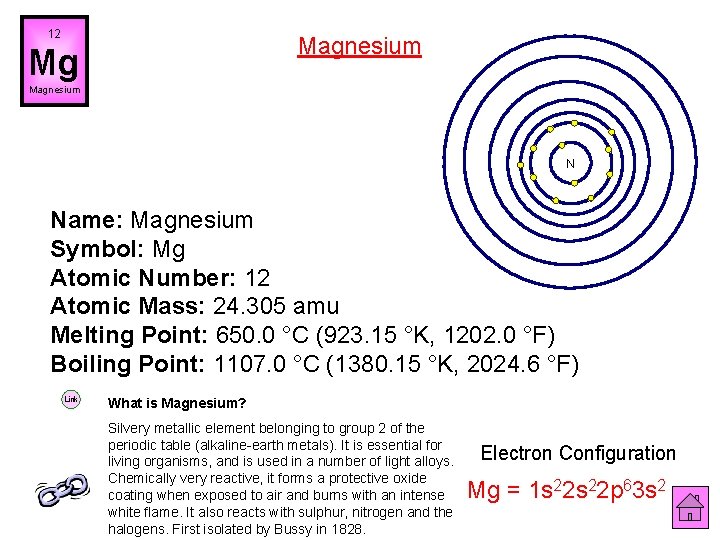
12 Magnesium Mg Magnesium N Name: Magnesium Symbol: Mg Atomic Number: 12 Atomic Mass: 24. 305 amu Melting Point: 650. 0 °C (923. 15 °K, 1202. 0 °F) Boiling Point: 1107. 0 °C (1380. 15 °K, 2024. 6 °F) Link What is Magnesium? Silvery metallic element belonging to group 2 of the periodic table (alkaline-earth metals). It is essential for living organisms, and is used in a number of light alloys. Chemically very reactive, it forms a protective oxide coating when exposed to air and burns with an intense white flame. It also reacts with sulphur, nitrogen and the halogens. First isolated by Bussy in 1828. Electron Configuration Mg = 1 s 22 p 63 s 2

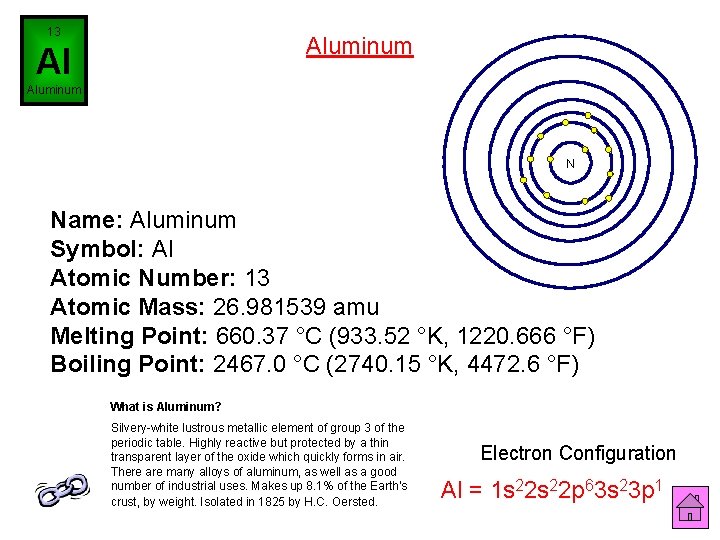
13 Aluminum Al Aluminum N Name: Aluminum Symbol: Al Atomic Number: 13 Atomic Mass: 26. 981539 amu Melting Point: 660. 37 °C (933. 52 °K, 1220. 666 °F) Boiling Point: 2467. 0 °C (2740. 15 °K, 4472. 6 °F) What is Aluminum? Silvery-white lustrous metallic element of group 3 of the periodic table. Highly reactive but protected by a thin transparent layer of the oxide which quickly forms in air. There are many alloys of aluminum, as well as a good number of industrial uses. Makes up 8. 1% of the Earth's crust, by weight. Isolated in 1825 by H. C. Oersted. Electron Configuration Al = 1 s 22 p 63 s 23 p 1

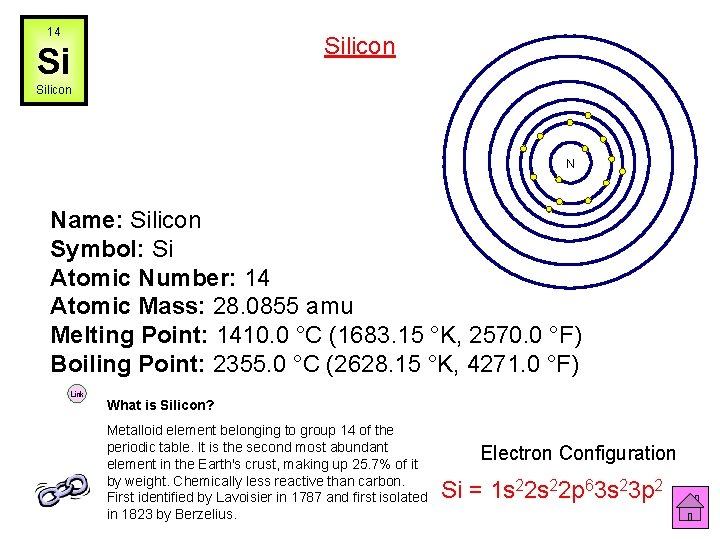
14 Silicon Si Silicon N Name: Silicon Symbol: Si Atomic Number: 14 Atomic Mass: 28. 0855 amu Melting Point: 1410. 0 °C (1683. 15 °K, 2570. 0 °F) Boiling Point: 2355. 0 °C (2628. 15 °K, 4271. 0 °F) Link What is Silicon? Metalloid element belonging to group 14 of the periodic table. It is the second most abundant element in the Earth's crust, making up 25. 7% of it by weight. Chemically less reactive than carbon. First identified by Lavoisier in 1787 and first isolated in 1823 by Berzelius. Electron Configuration Si = 1 s 22 p 63 s 23 p 2

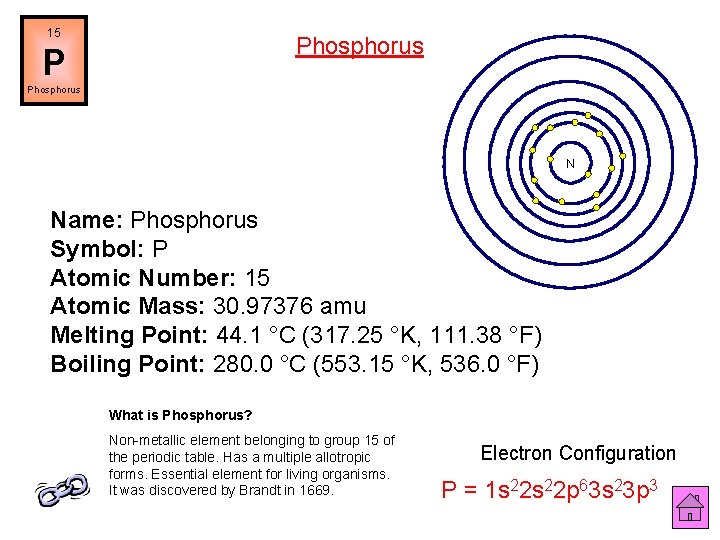
15 Phosphorus P Phosphorus N Name: Phosphorus Symbol: P Atomic Number: 15 Atomic Mass: 30. 97376 amu Melting Point: 44. 1 °C (317. 25 °K, 111. 38 °F) Boiling Point: 280. 0 °C (553. 15 °K, 536. 0 °F) What is Phosphorus? Non-metallic element belonging to group 15 of the periodic table. Has a multiple allotropic forms. Essential element for living organisms. It was discovered by Brandt in 1669. Electron Configuration P = 1 s 22 p 63 s 23 p 3

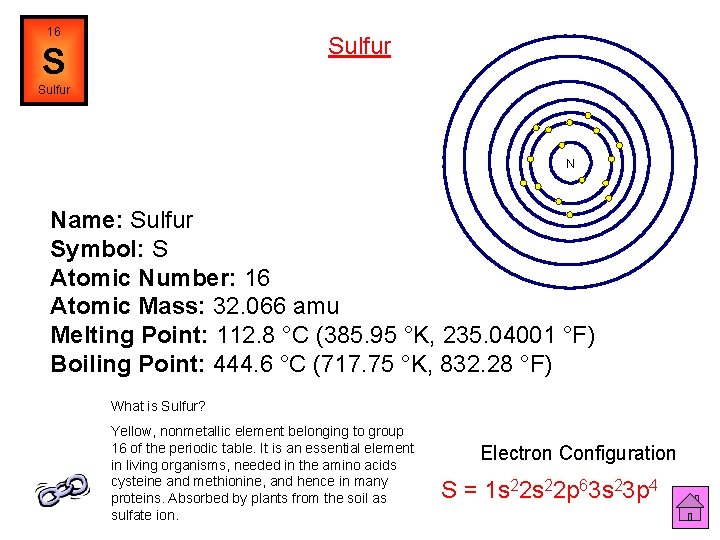
16 Sulfur S Sulfur N Name: Sulfur Symbol: S Atomic Number: 16 Atomic Mass: 32. 066 amu Melting Point: 112. 8 °C (385. 95 °K, 235. 04001 °F) Boiling Point: 444. 6 °C (717. 75 °K, 832. 28 °F) What is Sulfur? Yellow, nonmetallic element belonging to group 16 of the periodic table. It is an essential element in living organisms, needed in the amino acids cysteine and methionine, and hence in many proteins. Absorbed by plants from the soil as sulfate ion. Electron Configuration S = 1 s 22 p 63 s 23 p 4

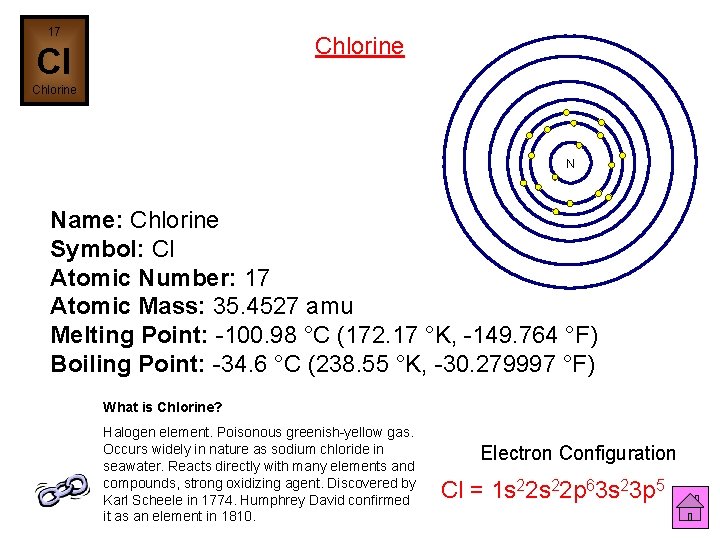
17 Chlorine Cl Chlorine N Name: Chlorine Symbol: Cl Atomic Number: 17 Atomic Mass: 35. 4527 amu Melting Point: -100. 98 °C (172. 17 °K, -149. 764 °F) Boiling Point: -34. 6 °C (238. 55 °K, -30. 279997 °F) What is Chlorine? Halogen element. Poisonous greenish-yellow gas. Occurs widely in nature as sodium chloride in seawater. Reacts directly with many elements and compounds, strong oxidizing agent. Discovered by Karl Scheele in 1774. Humphrey David confirmed it as an element in 1810. Electron Configuration Cl = 1 s 22 p 63 s 23 p 5

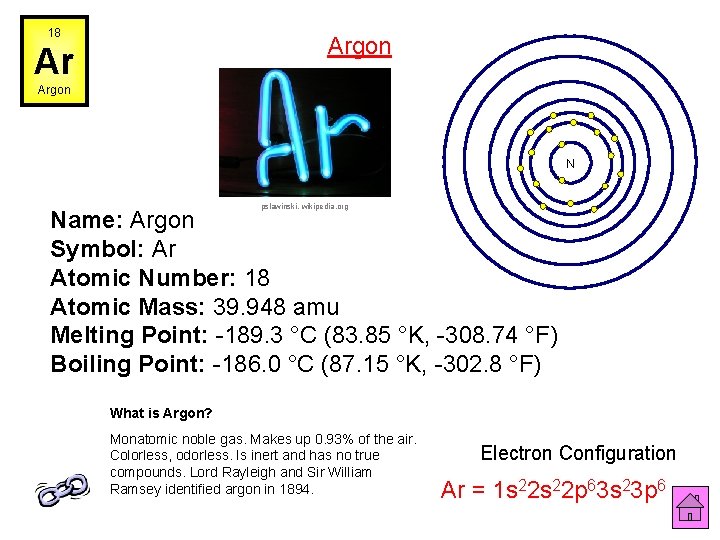
18 Argon Ar Argon N pslawinski, wikipedia. org Name: Argon Symbol: Ar Atomic Number: 18 Atomic Mass: 39. 948 amu Melting Point: -189. 3 °C (83. 85 °K, -308. 74 °F) Boiling Point: -186. 0 °C (87. 15 °K, -302. 8 °F) What is Argon? Monatomic noble gas. Makes up 0. 93% of the air. Colorless, odorless. Is inert and has no true compounds. Lord Rayleigh and Sir William Ramsey identified argon in 1894. Electron Configuration Ar = 1 s 22 p 63 s 23 p 6

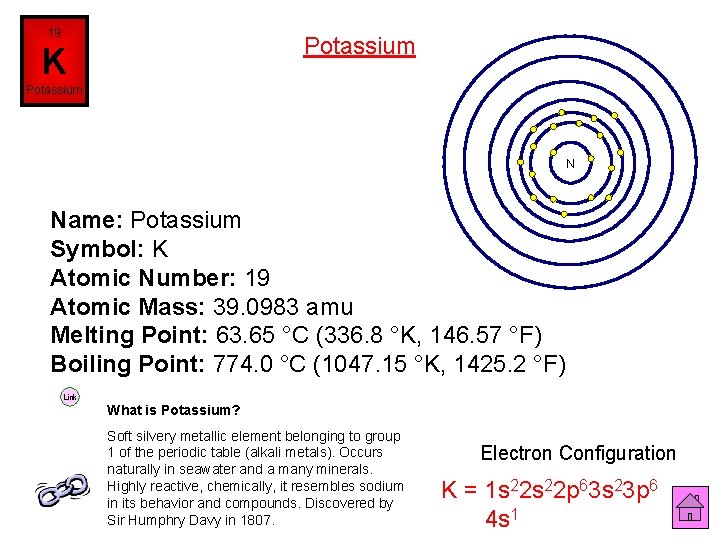
19 Potassium K Potassium N Name: Potassium Symbol: K Atomic Number: 19 Atomic Mass: 39. 0983 amu Melting Point: 63. 65 °C (336. 8 °K, 146. 57 °F) Boiling Point: 774. 0 °C (1047. 15 °K, 1425. 2 °F) Link What is Potassium? Soft silvery metallic element belonging to group 1 of the periodic table (alkali metals). Occurs naturally in seawater and a many minerals. Highly reactive, chemically, it resembles sodium in its behavior and compounds. Discovered by Sir Humphry Davy in 1807. Electron Configuration K = 1 s 22 p 63 s 23 p 6 4 s 1

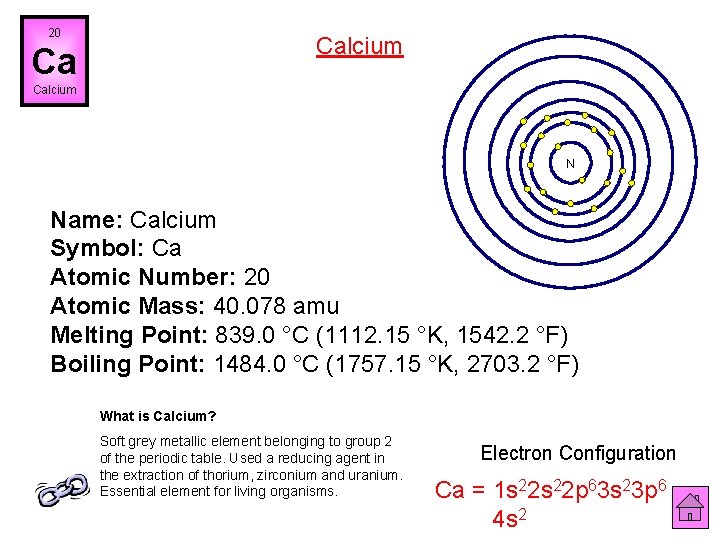
20 Calcium Ca Calcium N Name: Calcium Symbol: Ca Atomic Number: 20 Atomic Mass: 40. 078 amu Melting Point: 839. 0 °C (1112. 15 °K, 1542. 2 °F) Boiling Point: 1484. 0 °C (1757. 15 °K, 2703. 2 °F) What is Calcium? Soft grey metallic element belonging to group 2 of the periodic table. Used a reducing agent in the extraction of thorium, zirconium and uranium. Essential element for living organisms. Electron Configuration Ca = 1 s 22 p 63 s 23 p 6 4 s 2

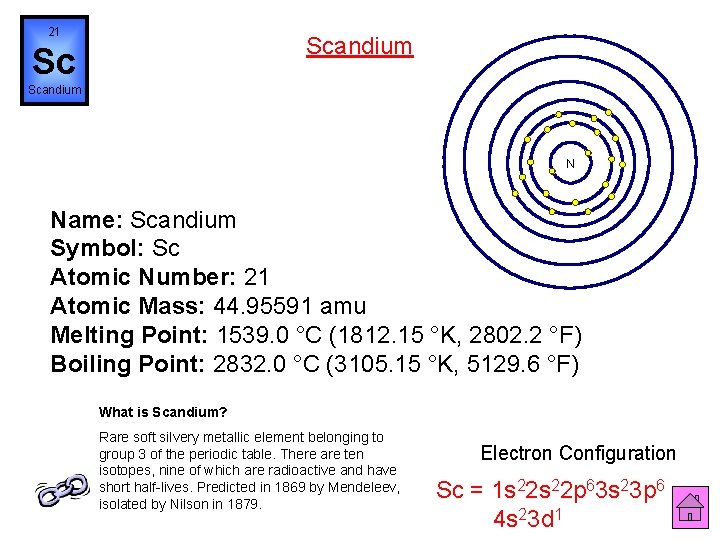
21 Scandium Sc Scandium N Name: Scandium Symbol: Sc Atomic Number: 21 Atomic Mass: 44. 95591 amu Melting Point: 1539. 0 °C (1812. 15 °K, 2802. 2 °F) Boiling Point: 2832. 0 °C (3105. 15 °K, 5129. 6 °F) What is Scandium? Rare soft silvery metallic element belonging to group 3 of the periodic table. There are ten isotopes, nine of which are radioactive and have short half-lives. Predicted in 1869 by Mendeleev, isolated by Nilson in 1879. Electron Configuration Sc = 1 s 22 p 63 s 23 p 6 4 s 23 d 1

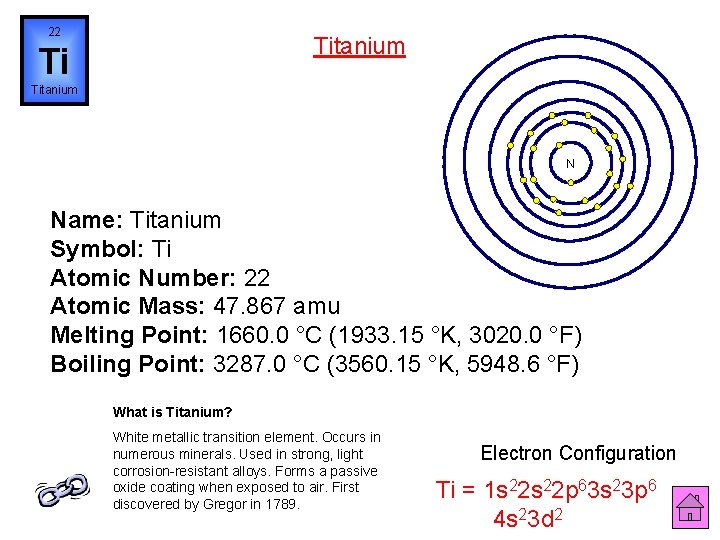
22 Titanium Ti Titanium N Name: Titanium Symbol: Ti Atomic Number: 22 Atomic Mass: 47. 867 amu Melting Point: 1660. 0 °C (1933. 15 °K, 3020. 0 °F) Boiling Point: 3287. 0 °C (3560. 15 °K, 5948. 6 °F) What is Titanium? White metallic transition element. Occurs in numerous minerals. Used in strong, light corrosion-resistant alloys. Forms a passive oxide coating when exposed to air. First discovered by Gregor in 1789. Electron Configuration Ti = 1 s 22 p 63 s 23 p 6 4 s 23 d 2

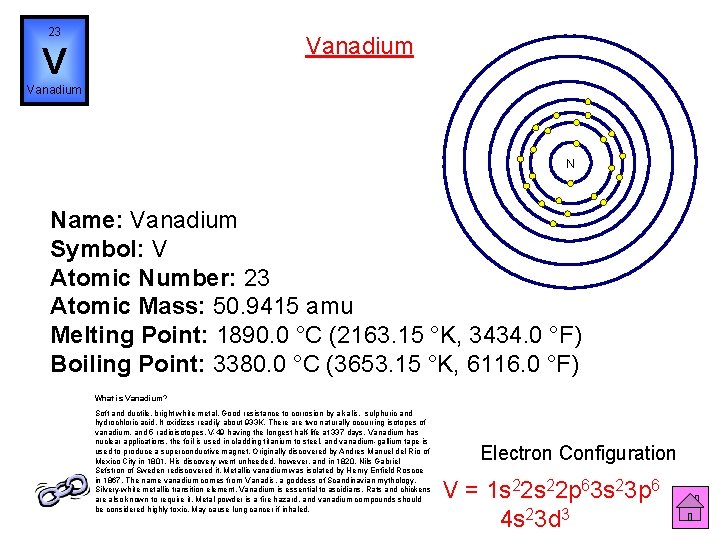
23 Vanadium V Vanadium N Name: Vanadium Symbol: V Atomic Number: 23 Atomic Mass: 50. 9415 amu Melting Point: 1890. 0 °C (2163. 15 °K, 3434. 0 °F) Boiling Point: 3380. 0 °C (3653. 15 °K, 6116. 0 °F) What is Vanadium? Soft and ductile, bright white metal. Good resistance to corrosion by alkalis, sulphuric and hydrochloric acid. It oxidizes readily about 933 K. There are two naturally occurring isotopes of vanadium, and 5 radioisotopes, V-49 having the longest half-life at 337 days. Vanadium has nuclear applications, the foil is used in cladding titanium to steel, and vanadium-gallium tape is used to produce a superconductive magnet. Originally discovered by Andres Manuel del Rio of Mexico City in 1801. His discovery went unheeded, however, and in 1820, Nils Gabriel Sefstron of Sweden rediscovered it. Metallic vanadium was isolated by Henry Enfield Roscoe in 1867. The name vanadium comes from Vanadis, a goddess of Scandinavian mythology. Silvery-white metallic transition element. Vanadium is essential to ascidians. Rats and chickens are also known to require it. Metal powder is a fire hazard, and vanadium compounds should be considered highly toxic. May cause lung cancer if inhaled. Electron Configuration V = 1 s 22 p 63 s 23 p 6 4 s 23 d 3

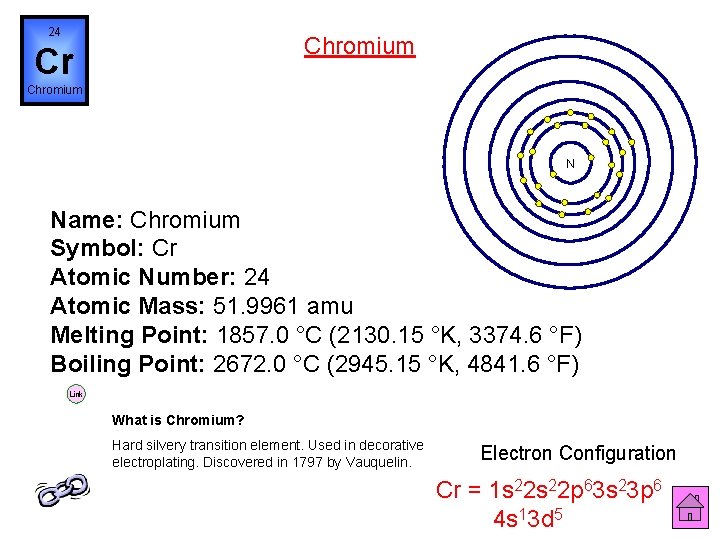
24 Chromium Cr Chromium N Name: Chromium Symbol: Cr Atomic Number: 24 Atomic Mass: 51. 9961 amu Melting Point: 1857. 0 °C (2130. 15 °K, 3374. 6 °F) Boiling Point: 2672. 0 °C (2945. 15 °K, 4841. 6 °F) Link What is Chromium? Hard silvery transition element. Used in decorative electroplating. Discovered in 1797 by Vauquelin. Electron Configuration Cr = 1 s 22 p 63 s 23 p 6 4 s 13 d 5

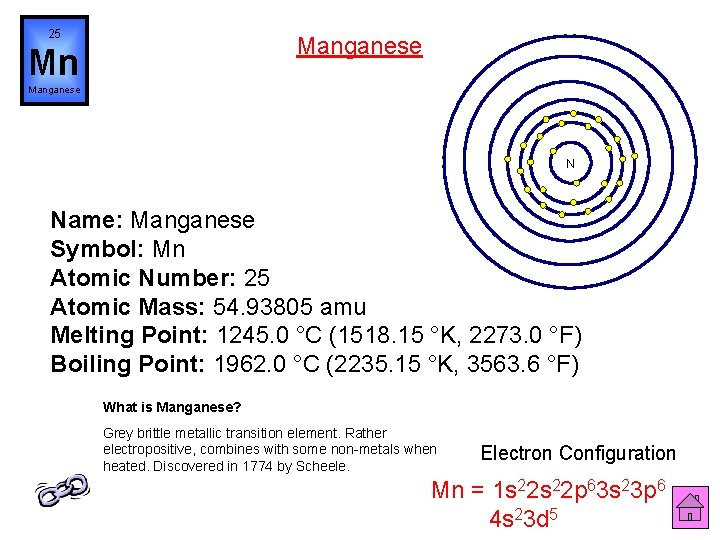
25 Manganese Mn Manganese N Name: Manganese Symbol: Mn Atomic Number: 25 Atomic Mass: 54. 93805 amu Melting Point: 1245. 0 °C (1518. 15 °K, 2273. 0 °F) Boiling Point: 1962. 0 °C (2235. 15 °K, 3563. 6 °F) What is Manganese? Grey brittle metallic transition element. Rather electropositive, combines with some non-metals when heated. Discovered in 1774 by Scheele. Electron Configuration Mn = 1 s 22 p 63 s 23 p 6 4 s 23 d 5

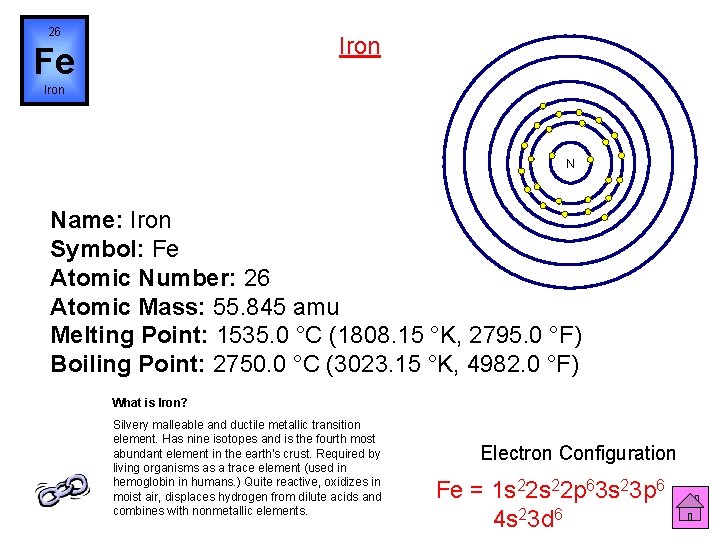
26 Iron Fe Iron N Name: Iron Symbol: Fe Atomic Number: 26 Atomic Mass: 55. 845 amu Melting Point: 1535. 0 °C (1808. 15 °K, 2795. 0 °F) Boiling Point: 2750. 0 °C (3023. 15 °K, 4982. 0 °F) What is Iron? Silvery malleable and ductile metallic transition element. Has nine isotopes and is the fourth most abundant element in the earth's crust. Required by living organisms as a trace element (used in hemoglobin in humans. ) Quite reactive, oxidizes in moist air, displaces hydrogen from dilute acids and combines with nonmetallic elements. Electron Configuration Fe = 1 s 22 p 63 s 23 p 6 4 s 23 d 6

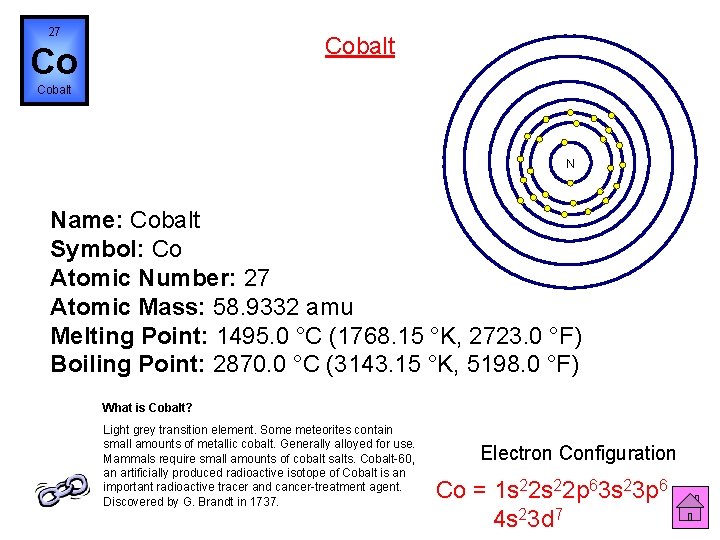
27 Cobalt Co Cobalt N Name: Cobalt Symbol: Co Atomic Number: 27 Atomic Mass: 58. 9332 amu Melting Point: 1495. 0 °C (1768. 15 °K, 2723. 0 °F) Boiling Point: 2870. 0 °C (3143. 15 °K, 5198. 0 °F) What is Cobalt? Light grey transition element. Some meteorites contain small amounts of metallic cobalt. Generally alloyed for use. Mammals require small amounts of cobalt salts. Cobalt-60, an artificially produced radioactive isotope of Cobalt is an important radioactive tracer and cancer-treatment agent. Discovered by G. Brandt in 1737. Electron Configuration Co = 1 s 22 p 63 s 23 p 6 4 s 23 d 7

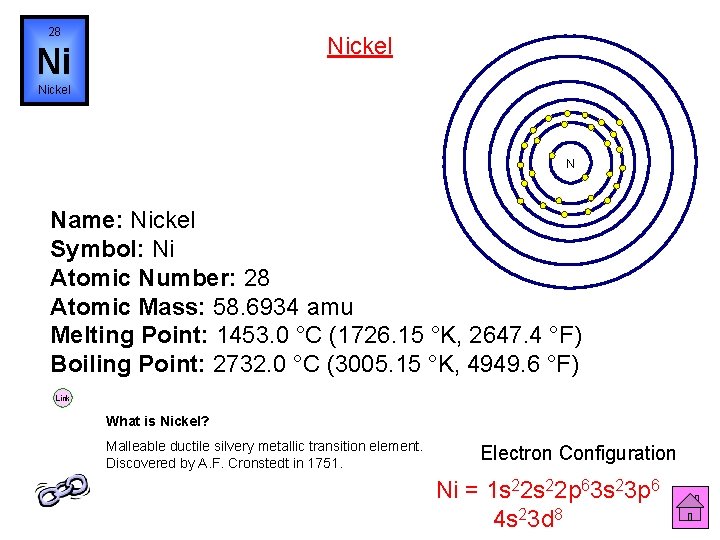
28 Nickel Ni Nickel N Name: Nickel Symbol: Ni Atomic Number: 28 Atomic Mass: 58. 6934 amu Melting Point: 1453. 0 °C (1726. 15 °K, 2647. 4 °F) Boiling Point: 2732. 0 °C (3005. 15 °K, 4949. 6 °F) Link What is Nickel? Malleable ductile silvery metallic transition element. Discovered by A. F. Cronstedt in 1751. Electron Configuration Ni = 1 s 22 p 63 s 23 p 6 4 s 23 d 8

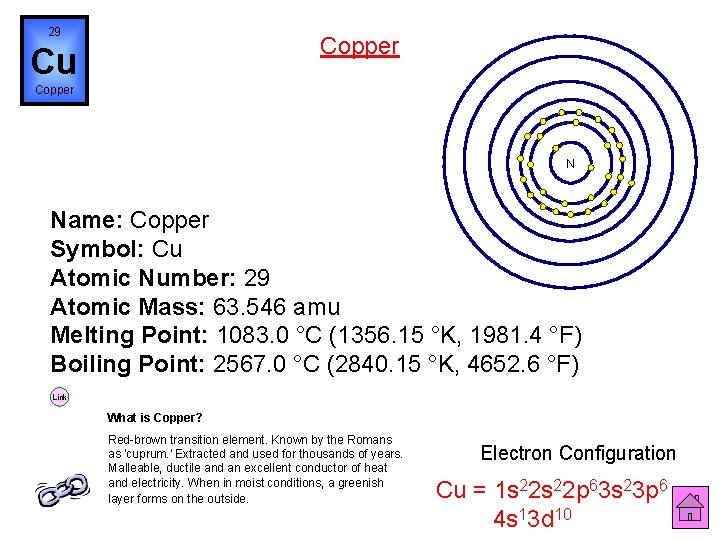
29 Copper Cu Copper N Name: Copper Symbol: Cu Atomic Number: 29 Atomic Mass: 63. 546 amu Melting Point: 1083. 0 °C (1356. 15 °K, 1981. 4 °F) Boiling Point: 2567. 0 °C (2840. 15 °K, 4652. 6 °F) Link What is Copper? Red-brown transition element. Known by the Romans as 'cuprum. ' Extracted and used for thousands of years. Malleable, ductile and an excellent conductor of heat and electricity. When in moist conditions, a greenish layer forms on the outside. Electron Configuration Cu = 1 s 22 p 63 s 23 p 6 4 s 13 d 10

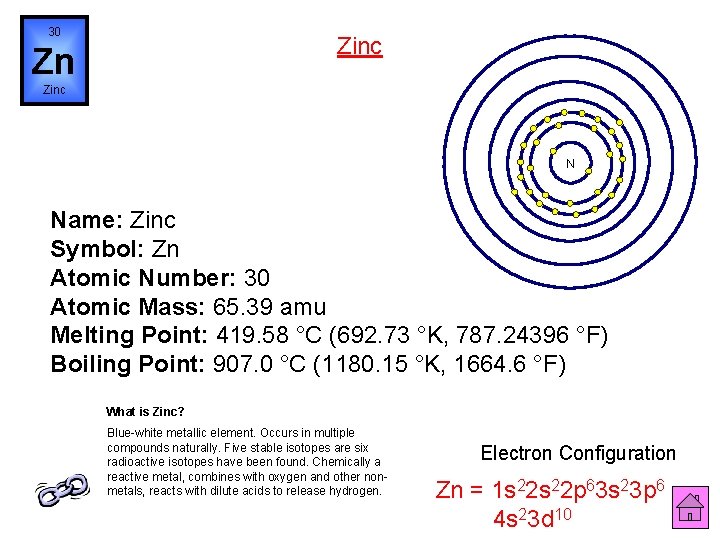
30 Zinc Zn Zinc N Name: Zinc Symbol: Zn Atomic Number: 30 Atomic Mass: 65. 39 amu Melting Point: 419. 58 °C (692. 73 °K, 787. 24396 °F) Boiling Point: 907. 0 °C (1180. 15 °K, 1664. 6 °F) What is Zinc? Blue-white metallic element. Occurs in multiple compounds naturally. Five stable isotopes are six radioactive isotopes have been found. Chemically a reactive metal, combines with oxygen and other nonmetals, reacts with dilute acids to release hydrogen. Electron Configuration Zn = 1 s 22 p 63 s 23 p 6 4 s 23 d 10

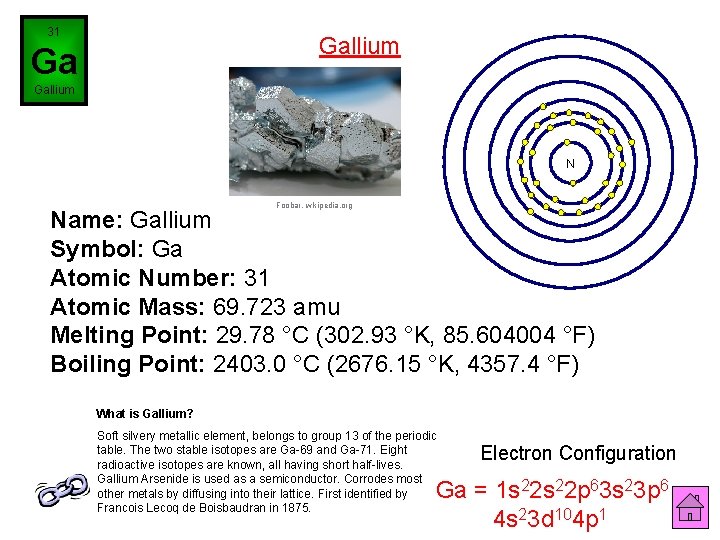
31 Gallium Ga Gallium N Foobar, wikipedia. org Name: Gallium Symbol: Ga Atomic Number: 31 Atomic Mass: 69. 723 amu Melting Point: 29. 78 °C (302. 93 °K, 85. 604004 °F) Boiling Point: 2403. 0 °C (2676. 15 °K, 4357. 4 °F) What is Gallium? Soft silvery metallic element, belongs to group 13 of the periodic table. The two stable isotopes are Ga-69 and Ga-71. Eight radioactive isotopes are known, all having short half-lives. Gallium Arsenide is used as a semiconductor. Corrodes most other metals by diffusing into their lattice. First identified by Francois Lecoq de Boisbaudran in 1875. Electron Configuration Ga = 1 s 22 p 63 s 23 p 6 4 s 23 d 104 p 1

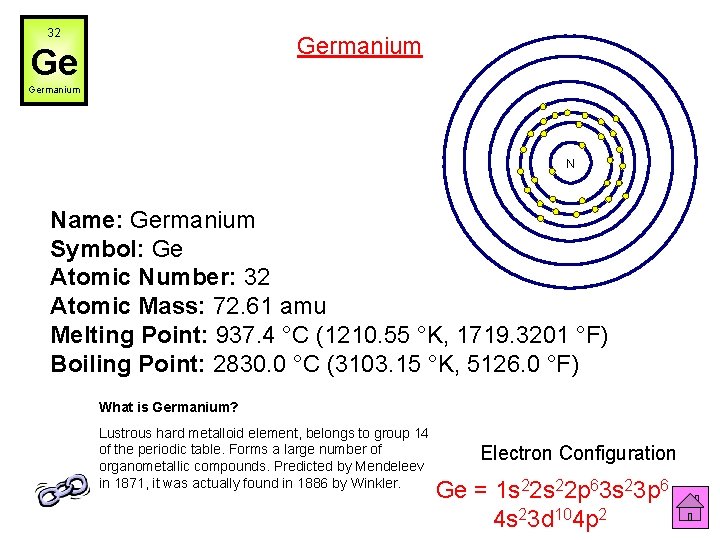
32 Germanium Ge Germanium N Name: Germanium Symbol: Ge Atomic Number: 32 Atomic Mass: 72. 61 amu Melting Point: 937. 4 °C (1210. 55 °K, 1719. 3201 °F) Boiling Point: 2830. 0 °C (3103. 15 °K, 5126. 0 °F) What is Germanium? Lustrous hard metalloid element, belongs to group 14 of the periodic table. Forms a large number of organometallic compounds. Predicted by Mendeleev in 1871, it was actually found in 1886 by Winkler. Electron Configuration Ge = 1 s 22 p 63 s 23 p 6 4 s 23 d 104 p 2

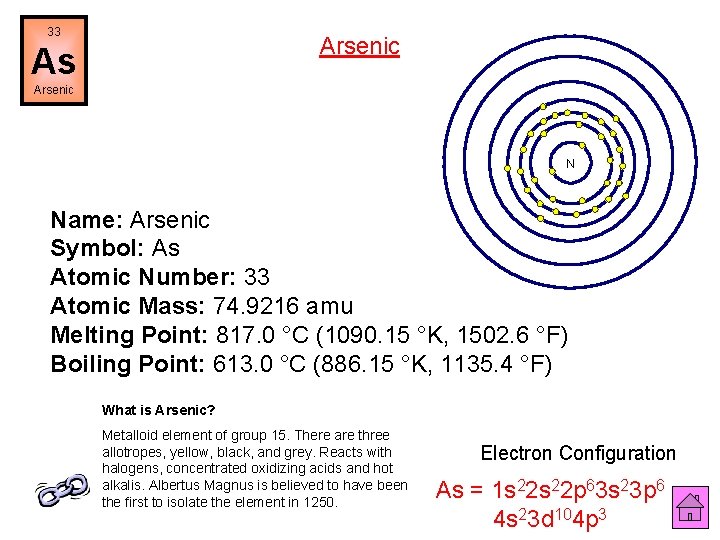
33 Arsenic As Arsenic N Name: Arsenic Symbol: As Atomic Number: 33 Atomic Mass: 74. 9216 amu Melting Point: 817. 0 °C (1090. 15 °K, 1502. 6 °F) Boiling Point: 613. 0 °C (886. 15 °K, 1135. 4 °F) What is Arsenic? Metalloid element of group 15. There are three allotropes, yellow, black, and grey. Reacts with halogens, concentrated oxidizing acids and hot alkalis. Albertus Magnus is believed to have been the first to isolate the element in 1250. Electron Configuration As = 1 s 22 p 63 s 23 p 6 4 s 23 d 104 p 3

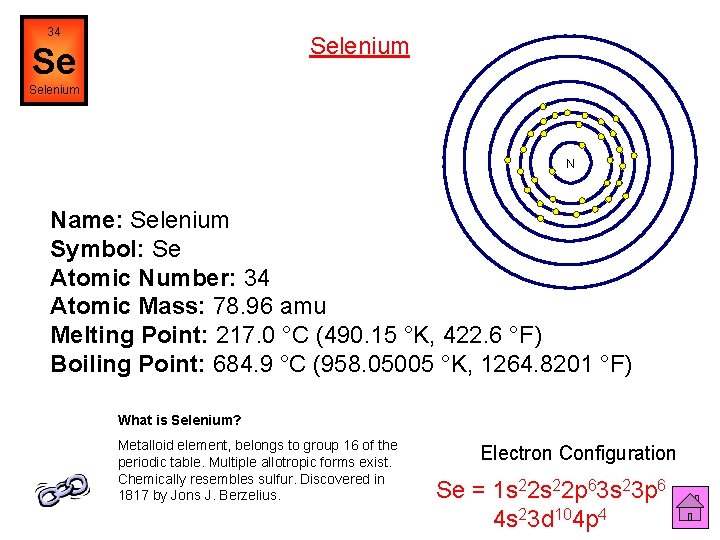
34 Selenium Se Selenium N Name: Selenium Symbol: Se Atomic Number: 34 Atomic Mass: 78. 96 amu Melting Point: 217. 0 °C (490. 15 °K, 422. 6 °F) Boiling Point: 684. 9 °C (958. 05005 °K, 1264. 8201 °F) What is Selenium? Metalloid element, belongs to group 16 of the periodic table. Multiple allotropic forms exist. Chemically resembles sulfur. Discovered in 1817 by Jons J. Berzelius. Electron Configuration Se = 1 s 22 p 63 s 23 p 6 4 s 23 d 104 p 4

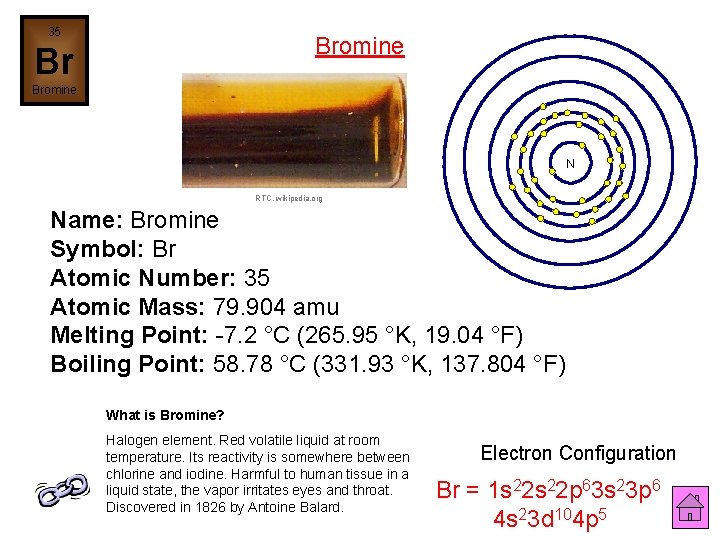
35 Bromine Br Bromine N RTC, wikipedia. org Name: Bromine Symbol: Br Atomic Number: 35 Atomic Mass: 79. 904 amu Melting Point: -7. 2 °C (265. 95 °K, 19. 04 °F) Boiling Point: 58. 78 °C (331. 93 °K, 137. 804 °F) What is Bromine? Halogen element. Red volatile liquid at room temperature. Its reactivity is somewhere between chlorine and iodine. Harmful to human tissue in a liquid state, the vapor irritates eyes and throat. Discovered in 1826 by Antoine Balard. Electron Configuration Br = 1 s 22 p 63 s 23 p 6 4 s 23 d 104 p 5

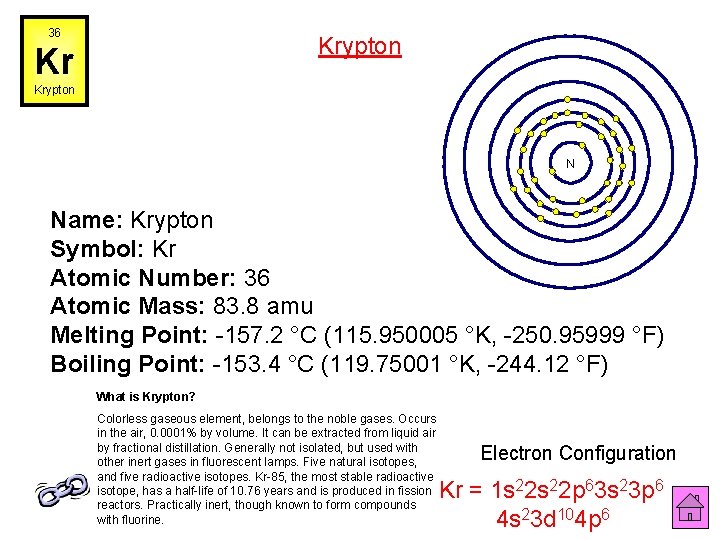
36 Krypton Kr Krypton N Name: Krypton Symbol: Kr Atomic Number: 36 Atomic Mass: 83. 8 amu Melting Point: -157. 2 °C (115. 950005 °K, -250. 95999 °F) Boiling Point: -153. 4 °C (119. 75001 °K, -244. 12 °F) What is Krypton? Colorless gaseous element, belongs to the noble gases. Occurs in the air, 0. 0001% by volume. It can be extracted from liquid air by fractional distillation. Generally not isolated, but used with other inert gases in fluorescent lamps. Five natural isotopes, and five radioactive isotopes. Kr-85, the most stable radioactive isotope, has a half-life of 10. 76 years and is produced in fission reactors. Practically inert, though known to form compounds with fluorine. Electron Configuration Kr = 1 s 22 p 63 s 23 p 6 4 s 23 d 104 p 6

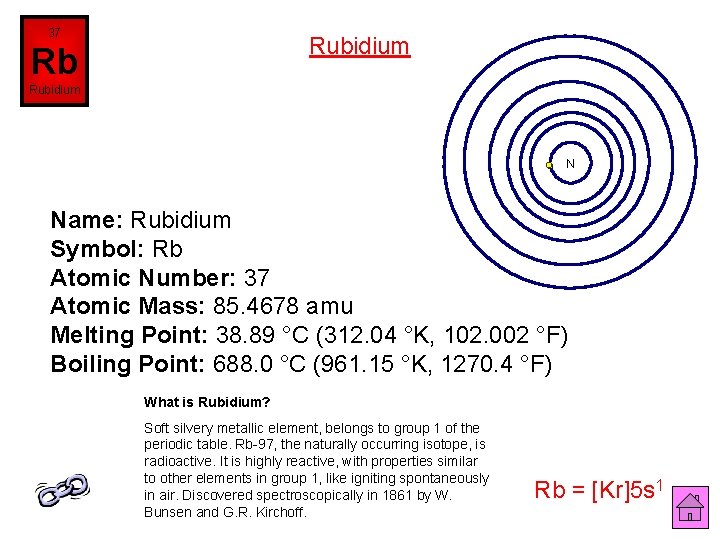
37 Rubidium Rb Rubidium N Name: Rubidium Symbol: Rb Atomic Number: 37 Atomic Mass: 85. 4678 amu Melting Point: 38. 89 °C (312. 04 °K, 102. 002 °F) Boiling Point: 688. 0 °C (961. 15 °K, 1270. 4 °F) What is Rubidium? Soft silvery metallic element, belongs to group 1 of the periodic table. Rb-97, the naturally occurring isotope, is radioactive. It is highly reactive, with properties similar to other elements in group 1, like igniting spontaneously in air. Discovered spectroscopically in 1861 by W. Bunsen and G. R. Kirchoff. Rb = [Kr]5 s 1

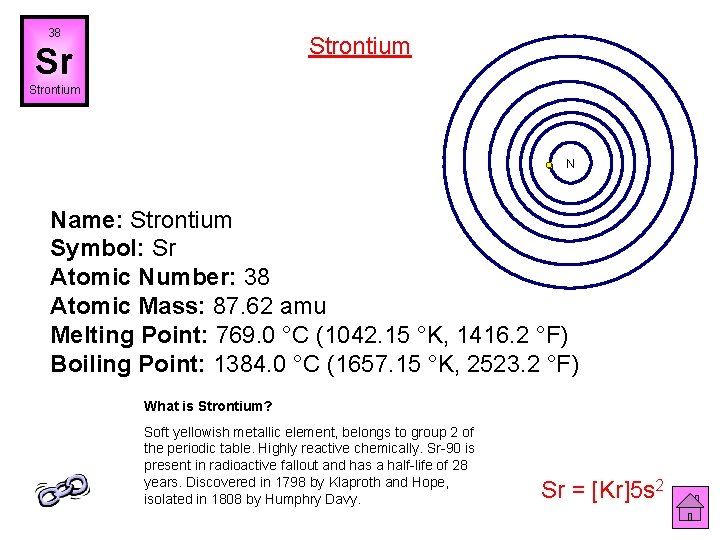
38 Strontium Sr Strontium N Name: Strontium Symbol: Sr Atomic Number: 38 Atomic Mass: 87. 62 amu Melting Point: 769. 0 °C (1042. 15 °K, 1416. 2 °F) Boiling Point: 1384. 0 °C (1657. 15 °K, 2523. 2 °F) What is Strontium? Soft yellowish metallic element, belongs to group 2 of the periodic table. Highly reactive chemically. Sr-90 is present in radioactive fallout and has a half-life of 28 years. Discovered in 1798 by Klaproth and Hope, isolated in 1808 by Humphry Davy. Sr = [Kr]5 s 2

39 Yttrium Y Yttrium N Name: Yttrium Symbol: Y Atomic Number: 39 Atomic Mass: 88. 90585 amu Melting Point: 1523. 0 °C (1796. 15 °K, 2773. 4 °F) Boiling Point: 3337. 0 °C (3610. 15 °K, 6038. 6 °F) What is Yttrium? Silvery-grey metallic element of group 3 on the periodic table. Found in uranium ores. The only natural isotope is Y-89, there are 14 other artificial isotopes. Chemically resembles the lanthanoids. Stable in the air below 400 degrees, Celsius. Discovered in 1828 by Friedrich Wohler. Y = [Kr]5 s 24 d 1

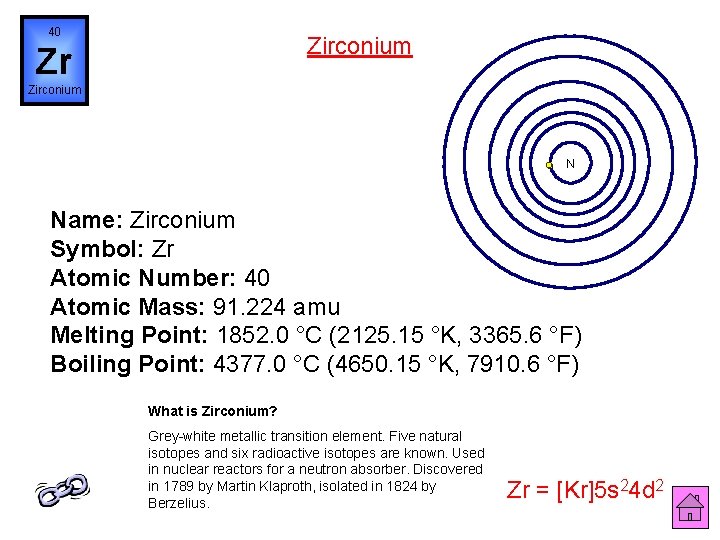
40 Zirconium Zr Zirconium N Name: Zirconium Symbol: Zr Atomic Number: 40 Atomic Mass: 91. 224 amu Melting Point: 1852. 0 °C (2125. 15 °K, 3365. 6 °F) Boiling Point: 4377. 0 °C (4650. 15 °K, 7910. 6 °F) What is Zirconium? Grey-white metallic transition element. Five natural isotopes and six radioactive isotopes are known. Used in nuclear reactors for a neutron absorber. Discovered in 1789 by Martin Klaproth, isolated in 1824 by Berzelius. Zr = [Kr]5 s 24 d 2

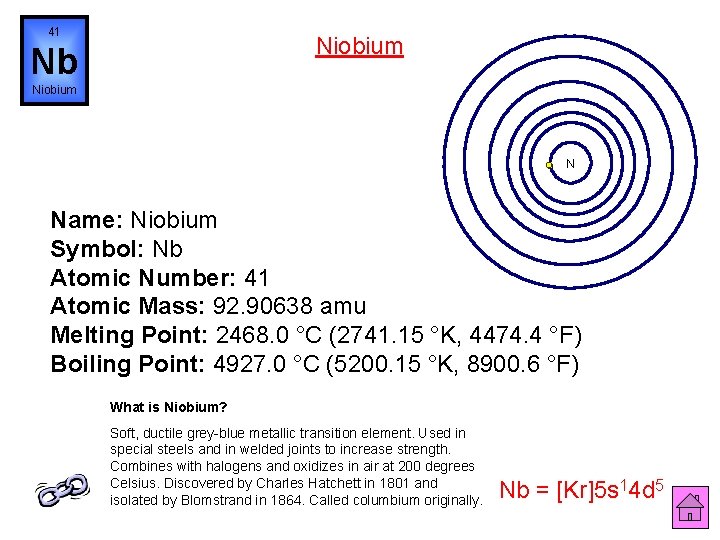
41 Niobium Nb Niobium N Name: Niobium Symbol: Nb Atomic Number: 41 Atomic Mass: 92. 90638 amu Melting Point: 2468. 0 °C (2741. 15 °K, 4474. 4 °F) Boiling Point: 4927. 0 °C (5200. 15 °K, 8900. 6 °F) What is Niobium? Soft, ductile grey-blue metallic transition element. Used in special steels and in welded joints to increase strength. Combines with halogens and oxidizes in air at 200 degrees Celsius. Discovered by Charles Hatchett in 1801 and isolated by Blomstrand in 1864. Called columbium originally. Nb = [Kr]5 s 14 d 5

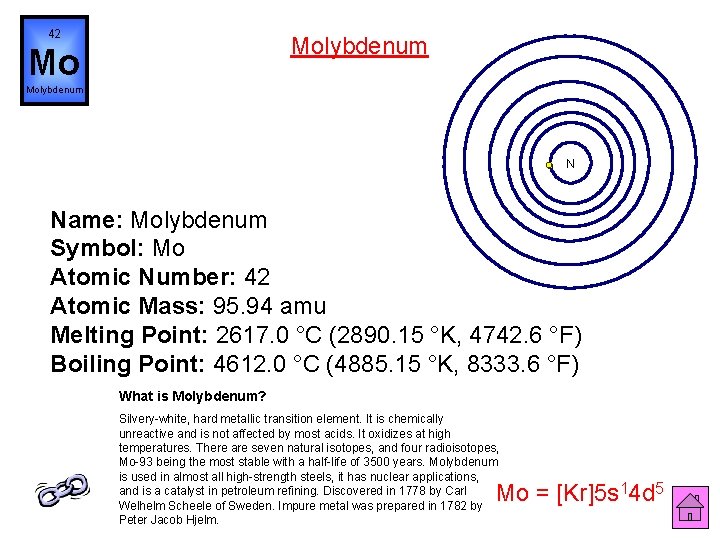
42 Molybdenum Mo Molybdenum N Name: Molybdenum Symbol: Mo Atomic Number: 42 Atomic Mass: 95. 94 amu Melting Point: 2617. 0 °C (2890. 15 °K, 4742. 6 °F) Boiling Point: 4612. 0 °C (4885. 15 °K, 8333. 6 °F) What is Molybdenum? Silvery-white, hard metallic transition element. It is chemically unreactive and is not affected by most acids. It oxidizes at high temperatures. There are seven natural isotopes, and four radioisotopes, Mo-93 being the most stable with a half-life of 3500 years. Molybdenum is used in almost all high-strength steels, it has nuclear applications, and is a catalyst in petroleum refining. Discovered in 1778 by Carl Welhelm Scheele of Sweden. Impure metal was prepared in 1782 by Peter Jacob Hjelm. Mo = [Kr]5 s 14 d 5

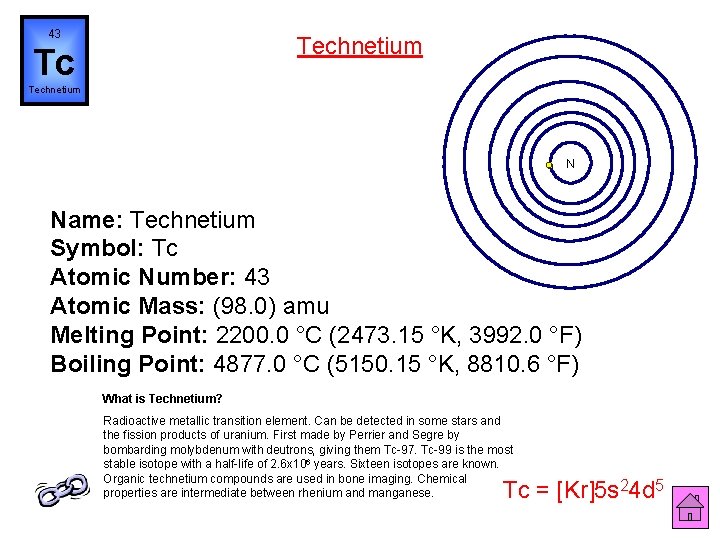
43 Technetium Tc Technetium N Name: Technetium Symbol: Tc Atomic Number: 43 Atomic Mass: (98. 0) amu Melting Point: 2200. 0 °C (2473. 15 °K, 3992. 0 °F) Boiling Point: 4877. 0 °C (5150. 15 °K, 8810. 6 °F) What is Technetium? Radioactive metallic transition element. Can be detected in some stars and the fission products of uranium. First made by Perrier and Segre by bombarding molybdenum with deutrons, giving them Tc-97. Tc-99 is the most stable isotope with a half-life of 2. 6 x 106 years. Sixteen isotopes are known. Organic technetium compounds are used in bone imaging. Chemical properties are intermediate between rhenium and manganese. Tc = [Kr]5 s 24 d 5

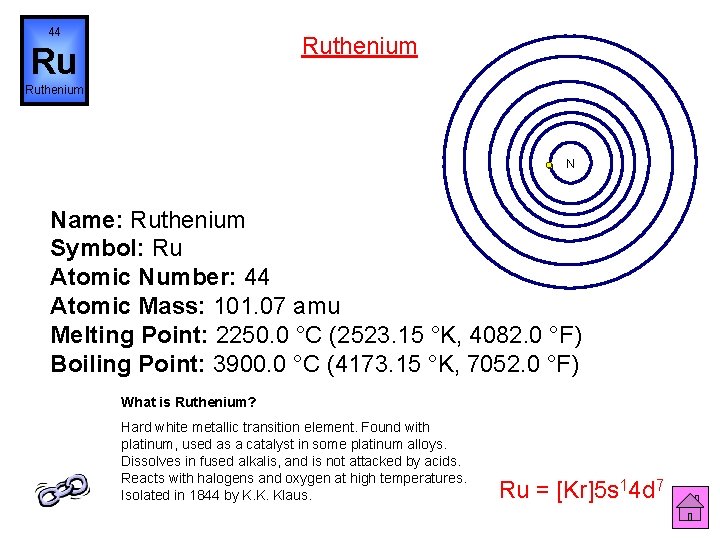
44 Ruthenium Ru Ruthenium N Name: Ruthenium Symbol: Ru Atomic Number: 44 Atomic Mass: 101. 07 amu Melting Point: 2250. 0 °C (2523. 15 °K, 4082. 0 °F) Boiling Point: 3900. 0 °C (4173. 15 °K, 7052. 0 °F) What is Ruthenium? Hard white metallic transition element. Found with platinum, used as a catalyst in some platinum alloys. Dissolves in fused alkalis, and is not attacked by acids. Reacts with halogens and oxygen at high temperatures. Isolated in 1844 by K. K. Klaus. Ru = [Kr]5 s 14 d 7

45 Rhodium Rh Rhodium N Name: Rhodium Symbol: Rh Atomic Number: 45 Atomic Mass: 102. 9055 amu Melting Point: 1966. 0 °C (2239. 15 °K, 3570. 8 °F) Boiling Point: 3727. 0 °C (4000. 15 °K, 6740. 6 °F) What is Rhodium? Silvery white metallic transition element. Found with platinum and used in some platinum alloys. Not attacked by acids, dissolves only in aqua regia. Discovered in 1803 by W. H. Wollaston. Rh = [Kr]5 s 14 d 8

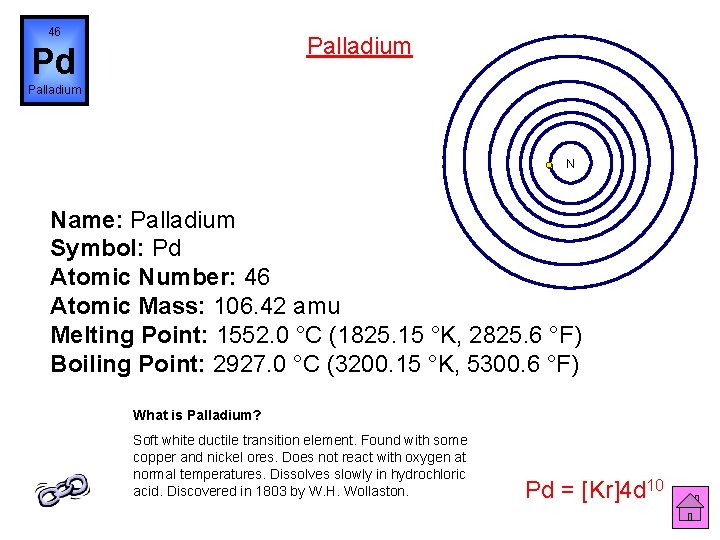
46 Palladium Pd Palladium N Name: Palladium Symbol: Pd Atomic Number: 46 Atomic Mass: 106. 42 amu Melting Point: 1552. 0 °C (1825. 15 °K, 2825. 6 °F) Boiling Point: 2927. 0 °C (3200. 15 °K, 5300. 6 °F) What is Palladium? Soft white ductile transition element. Found with some copper and nickel ores. Does not react with oxygen at normal temperatures. Dissolves slowly in hydrochloric acid. Discovered in 1803 by W. H. Wollaston. Pd = [Kr]4 d 10

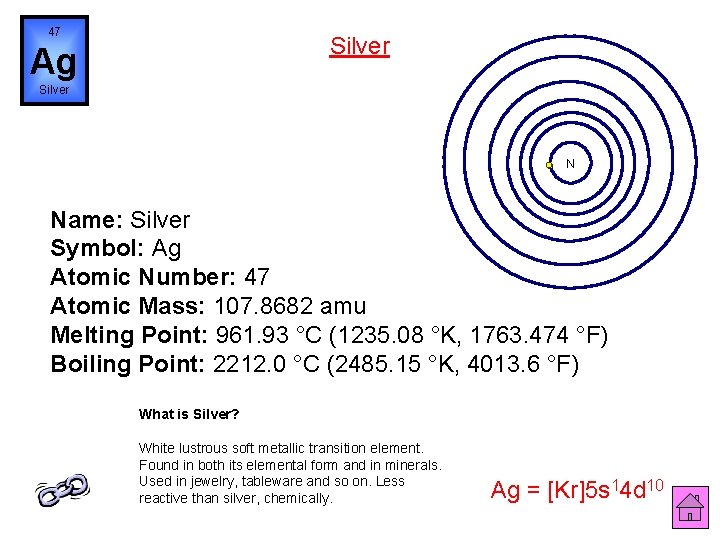
47 Silver Ag Silver N Name: Silver Symbol: Ag Atomic Number: 47 Atomic Mass: 107. 8682 amu Melting Point: 961. 93 °C (1235. 08 °K, 1763. 474 °F) Boiling Point: 2212. 0 °C (2485. 15 °K, 4013. 6 °F) What is Silver? White lustrous soft metallic transition element. Found in both its elemental form and in minerals. Used in jewelry, tableware and so on. Less reactive than silver, chemically. Ag = [Kr]5 s 14 d 10

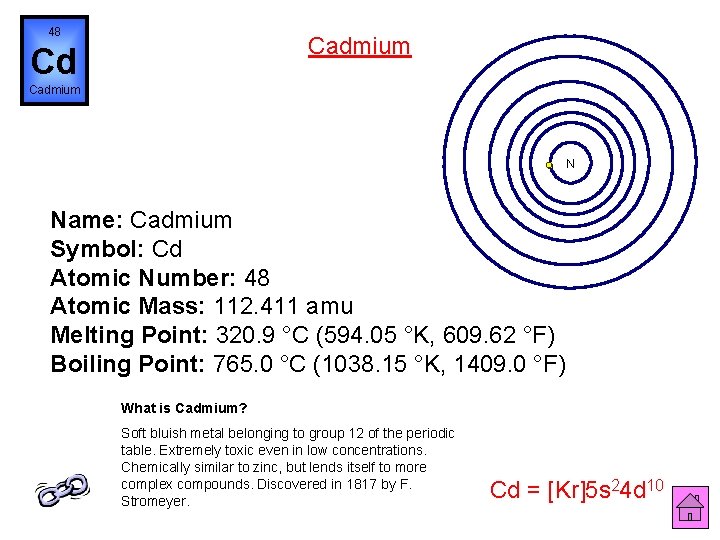
48 Cadmium Cd Cadmium N Name: Cadmium Symbol: Cd Atomic Number: 48 Atomic Mass: 112. 411 amu Melting Point: 320. 9 °C (594. 05 °K, 609. 62 °F) Boiling Point: 765. 0 °C (1038. 15 °K, 1409. 0 °F) What is Cadmium? Soft bluish metal belonging to group 12 of the periodic table. Extremely toxic even in low concentrations. Chemically similar to zinc, but lends itself to more complex compounds. Discovered in 1817 by F. Stromeyer. Cd = [Kr]5 s 24 d 10

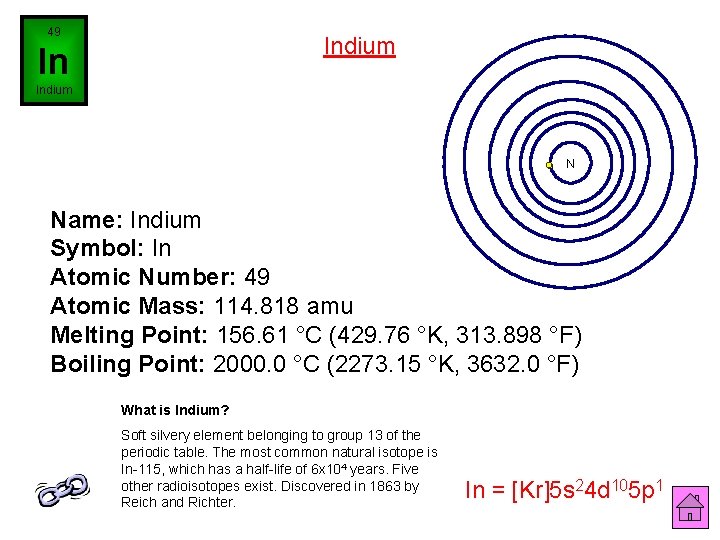
49 Indium In Indium N Name: Indium Symbol: In Atomic Number: 49 Atomic Mass: 114. 818 amu Melting Point: 156. 61 °C (429. 76 °K, 313. 898 °F) Boiling Point: 2000. 0 °C (2273. 15 °K, 3632. 0 °F) What is Indium? Soft silvery element belonging to group 13 of the periodic table. The most common natural isotope is In-115, which has a half-life of 6 x 104 years. Five other radioisotopes exist. Discovered in 1863 by Reich and Richter. In = [Kr]5 s 24 d 105 p 1

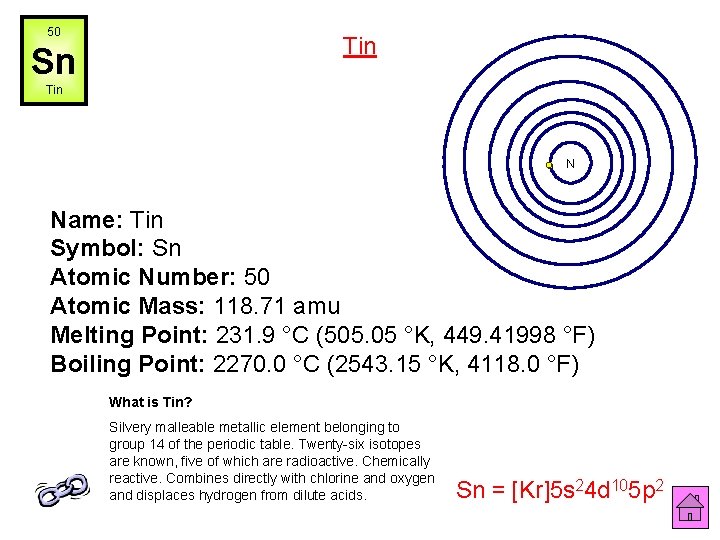
50 Tin Sn Tin N Name: Tin Symbol: Sn Atomic Number: 50 Atomic Mass: 118. 71 amu Melting Point: 231. 9 °C (505. 05 °K, 449. 41998 °F) Boiling Point: 2270. 0 °C (2543. 15 °K, 4118. 0 °F) What is Tin? Silvery malleable metallic element belonging to group 14 of the periodic table. Twenty-six isotopes are known, five of which are radioactive. Chemically reactive. Combines directly with chlorine and oxygen and displaces hydrogen from dilute acids. Sn = [Kr]5 s 24 d 105 p 2

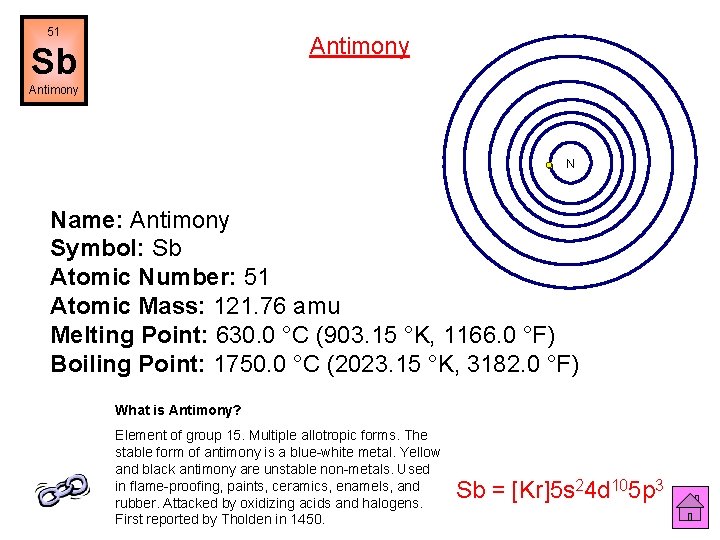
51 Antimony Sb Antimony N Name: Antimony Symbol: Sb Atomic Number: 51 Atomic Mass: 121. 76 amu Melting Point: 630. 0 °C (903. 15 °K, 1166. 0 °F) Boiling Point: 1750. 0 °C (2023. 15 °K, 3182. 0 °F) What is Antimony? Element of group 15. Multiple allotropic forms. The stable form of antimony is a blue-white metal. Yellow and black antimony are unstable non-metals. Used in flame-proofing, paints, ceramics, enamels, and rubber. Attacked by oxidizing acids and halogens. First reported by Tholden in 1450. Sb = [Kr]5 s 24 d 105 p 3

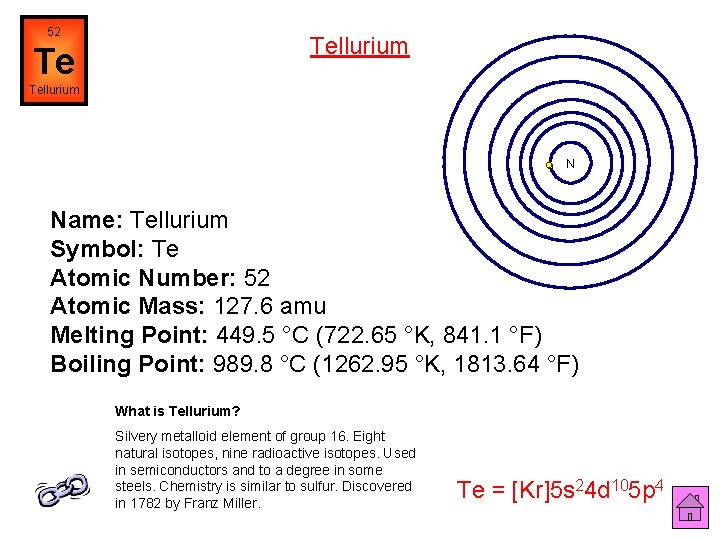
52 Tellurium Te Tellurium N Name: Tellurium Symbol: Te Atomic Number: 52 Atomic Mass: 127. 6 amu Melting Point: 449. 5 °C (722. 65 °K, 841. 1 °F) Boiling Point: 989. 8 °C (1262. 95 °K, 1813. 64 °F) What is Tellurium? Silvery metalloid element of group 16. Eight natural isotopes, nine radioactive isotopes. Used in semiconductors and to a degree in some steels. Chemistry is similar to sulfur. Discovered in 1782 by Franz Miller. Te = [Kr]5 s 24 d 105 p 4

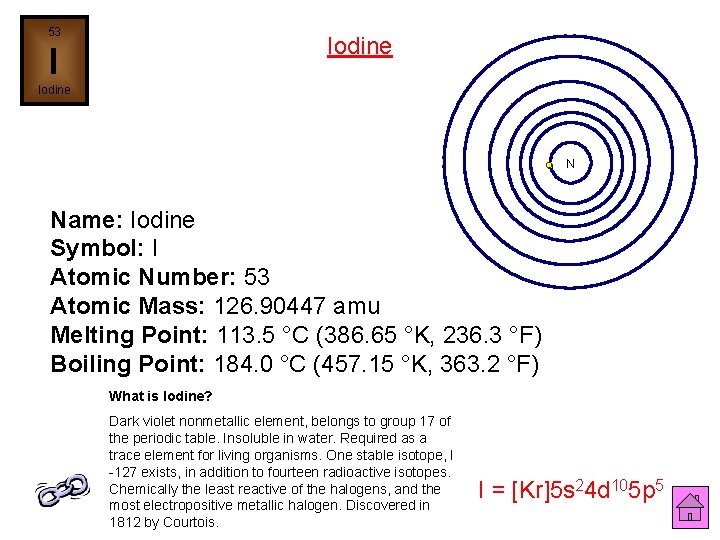
53 Iodine I Iodine N Name: Iodine Symbol: I Atomic Number: 53 Atomic Mass: 126. 90447 amu Melting Point: 113. 5 °C (386. 65 °K, 236. 3 °F) Boiling Point: 184. 0 °C (457. 15 °K, 363. 2 °F) What is Iodine? Dark violet nonmetallic element, belongs to group 17 of the periodic table. Insoluble in water. Required as a trace element for living organisms. One stable isotope, I -127 exists, in addition to fourteen radioactive isotopes. Chemically the least reactive of the halogens, and the most electropositive metallic halogen. Discovered in 1812 by Courtois. I = [Kr]5 s 24 d 105 p 5

54 Xenon Xe Xenon Name: Xenon Symbol: Xe Atomic Number: 54 Atomic Mass: 131. 29 amu Melting Point: -111. 9 °C (161. 25 °K, -169. 42 °F) Boiling Point: -108. 1 °C (165. 05 °K, -162. 58 °F) pslawinski, wikipedia. org What is Xenon? Colorless, odorless gas belonging to group 18 on the periodic table (the noble gases. ) Nine natural isotopes and seven radioactive isotopes are known. Xenon was part of the first noble-gas compound synthesized. Several others involving Xenon have been found since then. Xenon was discovered by Ramsey and Travers in 1898. Xe = [Kr]5 s 24 d 105 p 6

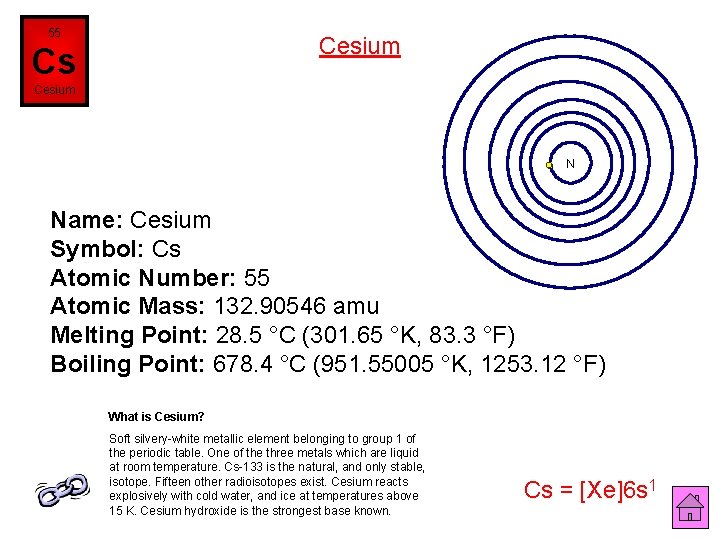
55 Cesium Cs Cesium N Name: Cesium Symbol: Cs Atomic Number: 55 Atomic Mass: 132. 90546 amu Melting Point: 28. 5 °C (301. 65 °K, 83. 3 °F) Boiling Point: 678. 4 °C (951. 55005 °K, 1253. 12 °F) What is Cesium? Soft silvery-white metallic element belonging to group 1 of the periodic table. One of the three metals which are liquid at room temperature. Cs-133 is the natural, and only stable, isotope. Fifteen other radioisotopes exist. Cesium reacts explosively with cold water, and ice at temperatures above 15 K. Cesium hydroxide is the strongest base known. Cs = [Xe]6 s 1

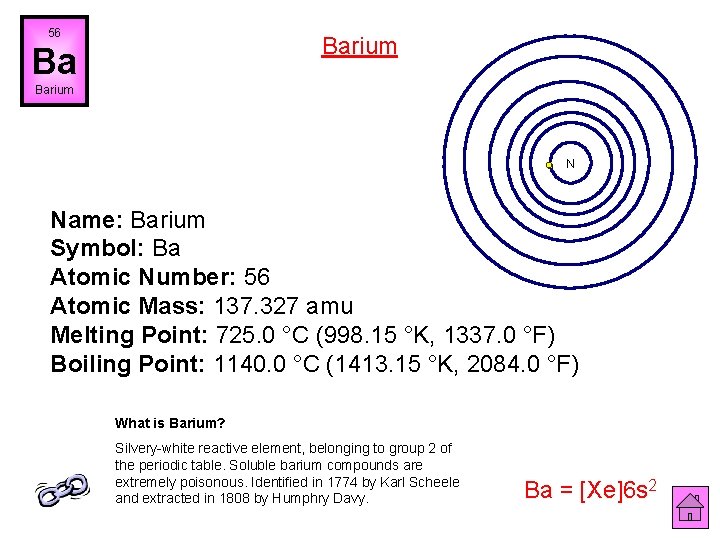
56 Barium Ba Barium N Name: Barium Symbol: Ba Atomic Number: 56 Atomic Mass: 137. 327 amu Melting Point: 725. 0 °C (998. 15 °K, 1337. 0 °F) Boiling Point: 1140. 0 °C (1413. 15 °K, 2084. 0 °F) What is Barium? Silvery-white reactive element, belonging to group 2 of the periodic table. Soluble barium compounds are extremely poisonous. Identified in 1774 by Karl Scheele and extracted in 1808 by Humphry Davy. Ba = [Xe]6 s 2

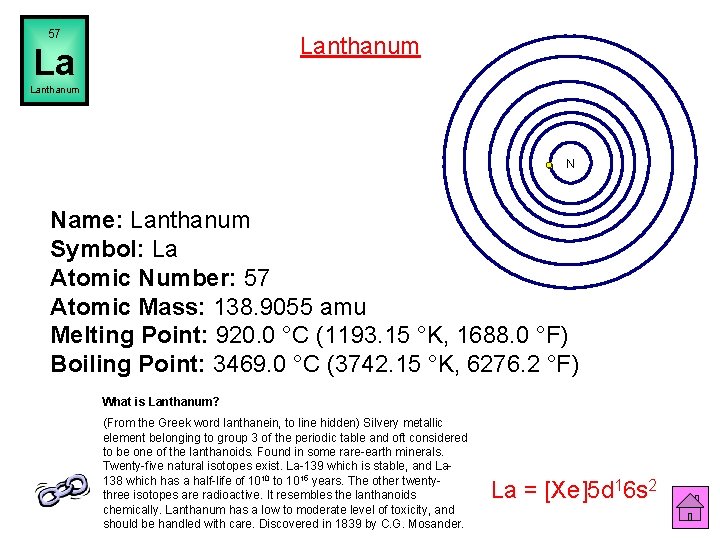
57 Lanthanum La Lanthanum N Name: Lanthanum Symbol: La Atomic Number: 57 Atomic Mass: 138. 9055 amu Melting Point: 920. 0 °C (1193. 15 °K, 1688. 0 °F) Boiling Point: 3469. 0 °C (3742. 15 °K, 6276. 2 °F) What is Lanthanum? (From the Greek word lanthanein, to line hidden) Silvery metallic element belonging to group 3 of the periodic table and oft considered to be one of the lanthanoids. Found in some rare-earth minerals. Twenty-five natural isotopes exist. La-139 which is stable, and La 138 which has a half-life of 1010 to 1015 years. The other twentythree isotopes are radioactive. It resembles the lanthanoids chemically. Lanthanum has a low to moderate level of toxicity, and should be handled with care. Discovered in 1839 by C. G. Mosander. La = [Xe]5 d 16 s 2

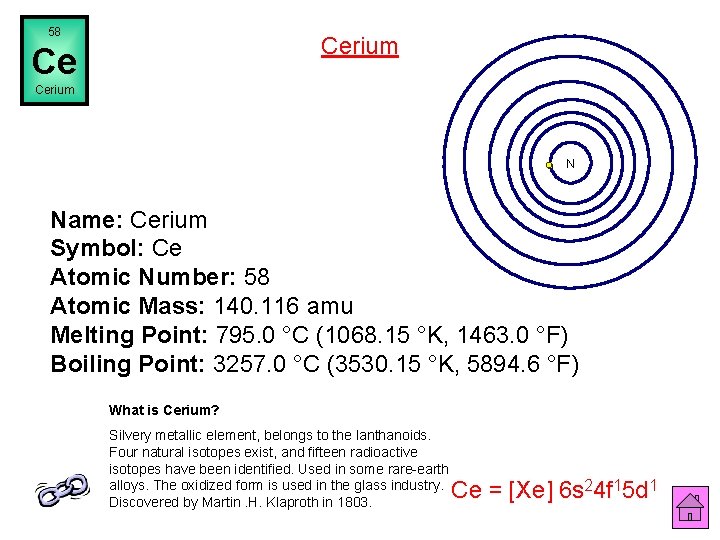
58 Cerium Ce Cerium N Name: Cerium Symbol: Ce Atomic Number: 58 Atomic Mass: 140. 116 amu Melting Point: 795. 0 °C (1068. 15 °K, 1463. 0 °F) Boiling Point: 3257. 0 °C (3530. 15 °K, 5894. 6 °F) What is Cerium? Silvery metallic element, belongs to the lanthanoids. Four natural isotopes exist, and fifteen radioactive isotopes have been identified. Used in some rare-earth alloys. The oxidized form is used in the glass industry. Discovered by Martin. H. Klaproth in 1803. Ce = [Xe] 6 s 24 f 15 d 1

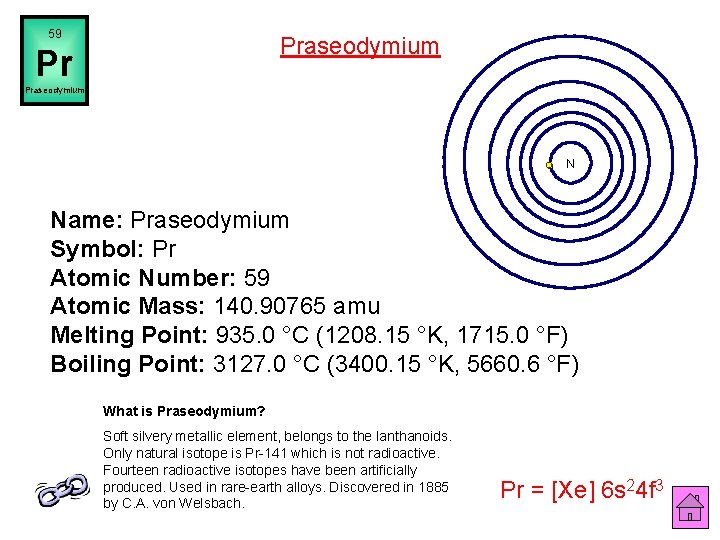
59 Praseodymium Pr Praseodymium N Name: Praseodymium Symbol: Pr Atomic Number: 59 Atomic Mass: 140. 90765 amu Melting Point: 935. 0 °C (1208. 15 °K, 1715. 0 °F) Boiling Point: 3127. 0 °C (3400. 15 °K, 5660. 6 °F) What is Praseodymium? Soft silvery metallic element, belongs to the lanthanoids. Only natural isotope is Pr-141 which is not radioactive. Fourteen radioactive isotopes have been artificially produced. Used in rare-earth alloys. Discovered in 1885 by C. A. von Welsbach. Pr = [Xe] 6 s 24 f 3

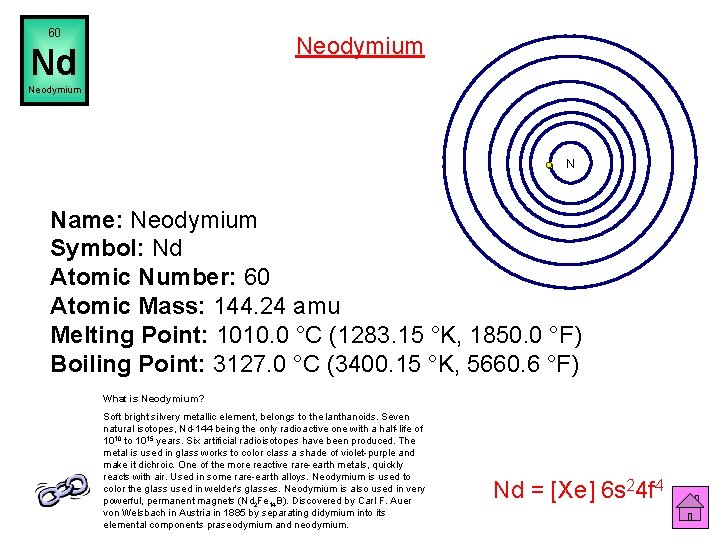
60 Neodymium Nd Neodymium N Name: Neodymium Symbol: Nd Atomic Number: 60 Atomic Mass: 144. 24 amu Melting Point: 1010. 0 °C (1283. 15 °K, 1850. 0 °F) Boiling Point: 3127. 0 °C (3400. 15 °K, 5660. 6 °F) What is Neodymium? Soft bright silvery metallic element, belongs to the lanthanoids. Seven natural isotopes, Nd-144 being the only radioactive one with a half-life of 1010 to 1015 years. Six artificial radioisotopes have been produced. The metal is used in glass works to color class a shade of violet-purple and make it dichroic. One of the more reactive rare-earth metals, quickly reacts with air. Used in some rare-earth alloys. Neodymium is used to color the glass used in welder's glasses. Neodymium is also used in very powerful, permanent magnets (Nd 2 Fe 14 B). Discovered by Carl F. Auer von Welsbach in Austria in 1885 by separating didymium into its elemental components praseodymium and neodymium. Nd = [Xe] 6 s 24 f 4

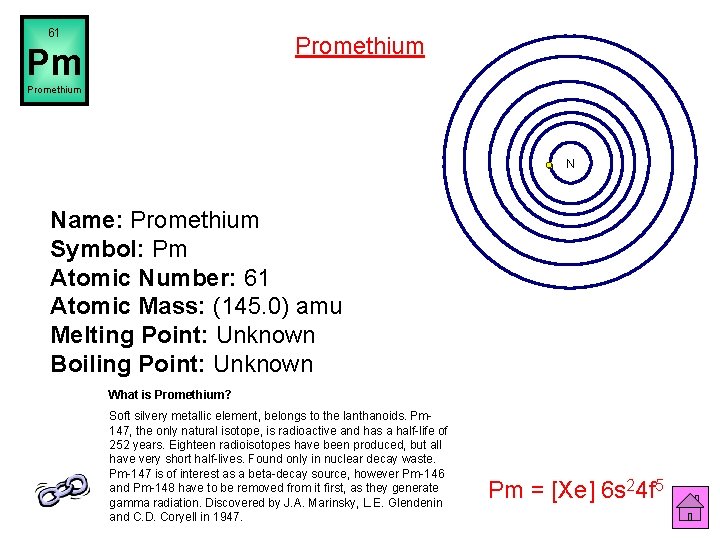
61 Promethium Pm Promethium N Name: Promethium Symbol: Pm Atomic Number: 61 Atomic Mass: (145. 0) amu Melting Point: Unknown Boiling Point: Unknown What is Promethium? Soft silvery metallic element, belongs to the lanthanoids. Pm 147, the only natural isotope, is radioactive and has a half-life of 252 years. Eighteen radioisotopes have been produced, but all have very short half-lives. Found only in nuclear decay waste. Pm-147 is of interest as a beta-decay source, however Pm-146 and Pm-148 have to be removed from it first, as they generate gamma radiation. Discovered by J. A. Marinsky, L. E. Glendenin and C. D. Coryell in 1947. Pm = [Xe] 6 s 24 f 5

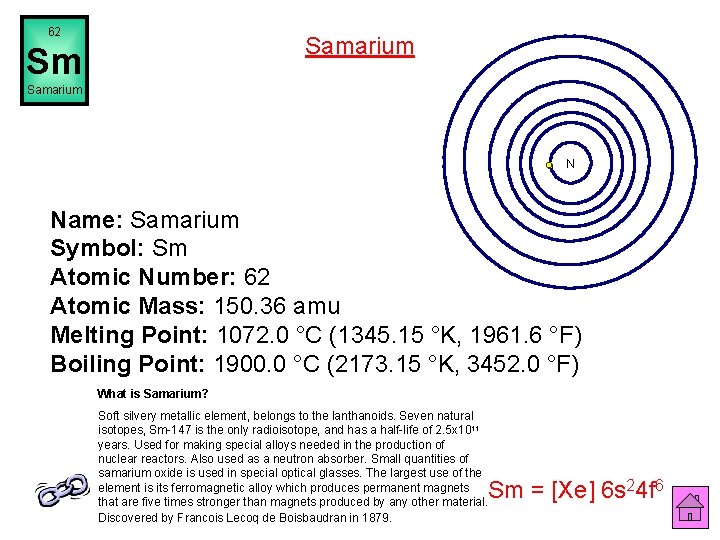
62 Samarium Sm Samarium N Name: Samarium Symbol: Sm Atomic Number: 62 Atomic Mass: 150. 36 amu Melting Point: 1072. 0 °C (1345. 15 °K, 1961. 6 °F) Boiling Point: 1900. 0 °C (2173. 15 °K, 3452. 0 °F) What is Samarium? Soft silvery metallic element, belongs to the lanthanoids. Seven natural isotopes, Sm-147 is the only radioisotope, and has a half-life of 2. 5 x 1011 years. Used for making special alloys needed in the production of nuclear reactors. Also used as a neutron absorber. Small quantities of samarium oxide is used in special optical glasses. The largest use of the element is its ferromagnetic alloy which produces permanent magnets that are five times stronger than magnets produced by any other material. Discovered by Francois Lecoq de Boisbaudran in 1879. Sm = [Xe] 6 s 24 f 6

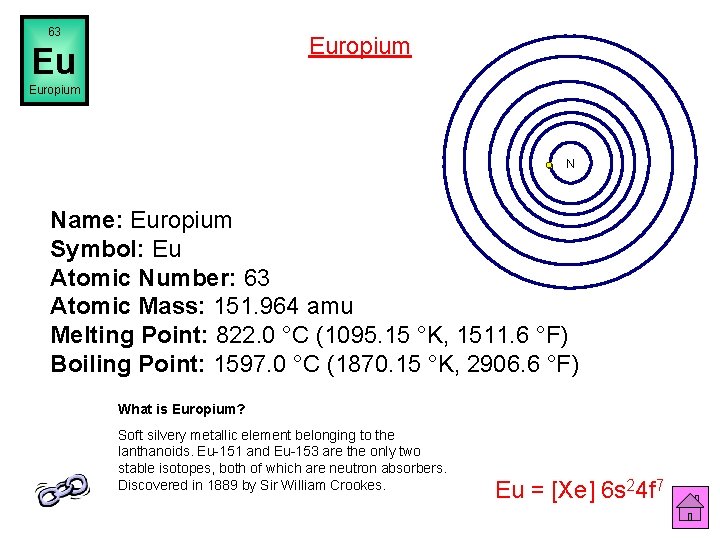
63 Europium Eu Europium N Name: Europium Symbol: Eu Atomic Number: 63 Atomic Mass: 151. 964 amu Melting Point: 822. 0 °C (1095. 15 °K, 1511. 6 °F) Boiling Point: 1597. 0 °C (1870. 15 °K, 2906. 6 °F) What is Europium? Soft silvery metallic element belonging to the lanthanoids. Eu-151 and Eu-153 are the only two stable isotopes, both of which are neutron absorbers. Discovered in 1889 by Sir William Crookes. Eu = [Xe] 6 s 24 f 7

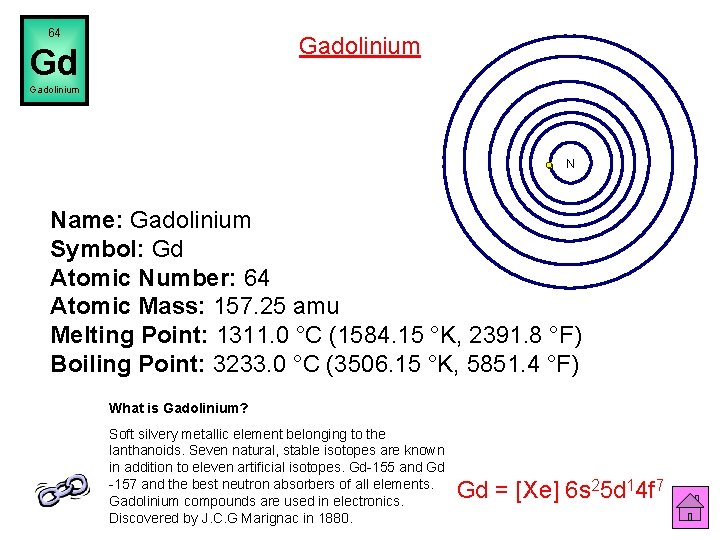
64 Gadolinium Gd Gadolinium N Name: Gadolinium Symbol: Gd Atomic Number: 64 Atomic Mass: 157. 25 amu Melting Point: 1311. 0 °C (1584. 15 °K, 2391. 8 °F) Boiling Point: 3233. 0 °C (3506. 15 °K, 5851. 4 °F) What is Gadolinium? Soft silvery metallic element belonging to the lanthanoids. Seven natural, stable isotopes are known in addition to eleven artificial isotopes. Gd-155 and Gd -157 and the best neutron absorbers of all elements. Gadolinium compounds are used in electronics. Discovered by J. C. G Marignac in 1880. Gd = [Xe] 6 s 25 d 14 f 7

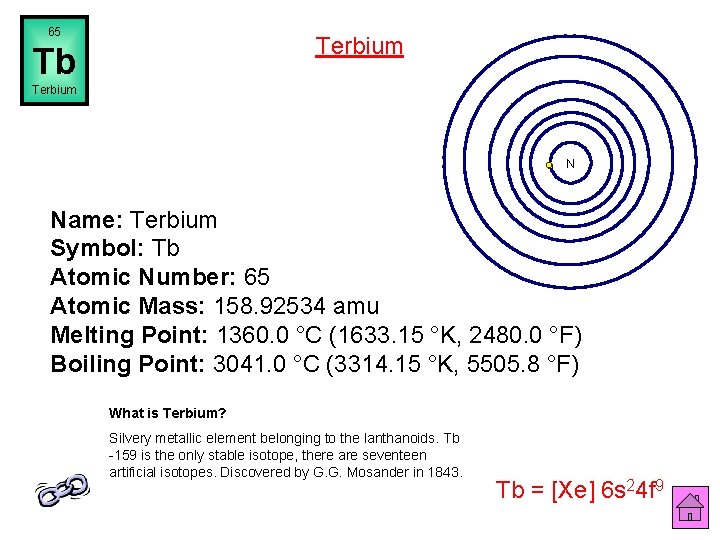
65 Terbium Tb Terbium N Name: Terbium Symbol: Tb Atomic Number: 65 Atomic Mass: 158. 92534 amu Melting Point: 1360. 0 °C (1633. 15 °K, 2480. 0 °F) Boiling Point: 3041. 0 °C (3314. 15 °K, 5505. 8 °F) What is Terbium? Silvery metallic element belonging to the lanthanoids. Tb -159 is the only stable isotope, there are seventeen artificial isotopes. Discovered by G. G. Mosander in 1843. Tb = [Xe] 6 s 24 f 9

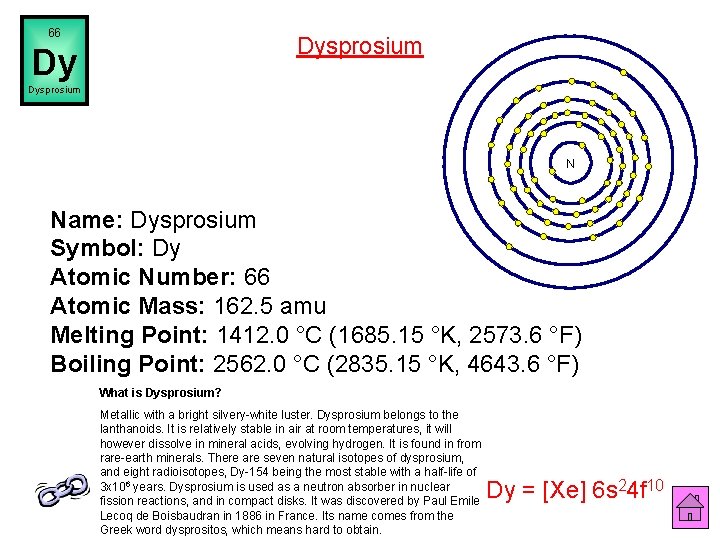
66 Dysprosium Dy Dysprosium N Name: Dysprosium Symbol: Dy Atomic Number: 66 Atomic Mass: 162. 5 amu Melting Point: 1412. 0 °C (1685. 15 °K, 2573. 6 °F) Boiling Point: 2562. 0 °C (2835. 15 °K, 4643. 6 °F) What is Dysprosium? Metallic with a bright silvery-white luster. Dysprosium belongs to the lanthanoids. It is relatively stable in air at room temperatures, it will however dissolve in mineral acids, evolving hydrogen. It is found in from rare-earth minerals. There are seven natural isotopes of dysprosium, and eight radioisotopes, Dy-154 being the most stable with a half-life of 3 x 106 years. Dysprosium is used as a neutron absorber in nuclear fission reactions, and in compact disks. It was discovered by Paul Emile Lecoq de Boisbaudran in 1886 in France. Its name comes from the Greek word dysprositos, which means hard to obtain. Dy = [Xe] 6 s 24 f 10

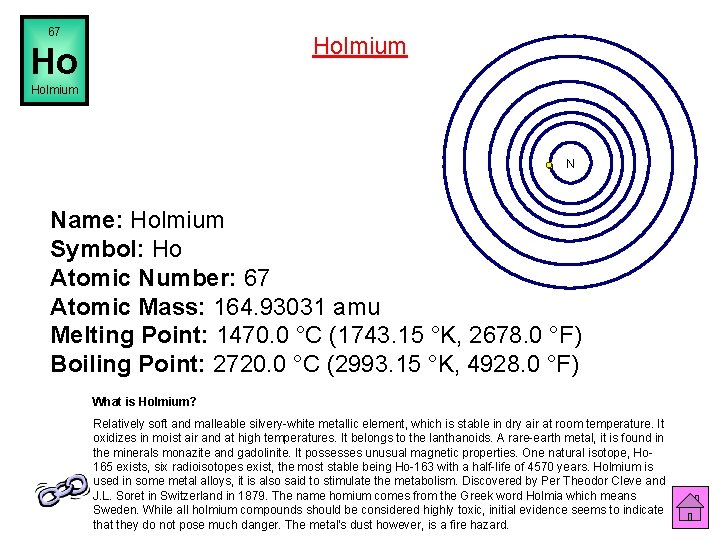
67 Holmium Ho Holmium N Name: Holmium Symbol: Ho Atomic Number: 67 Atomic Mass: 164. 93031 amu Melting Point: 1470. 0 °C (1743. 15 °K, 2678. 0 °F) Boiling Point: 2720. 0 °C (2993. 15 °K, 4928. 0 °F) What is Holmium? Relatively soft and malleable silvery-white metallic element, which is stable in dry air at room temperature. It oxidizes in moist air and at high temperatures. It belongs to the lanthanoids. A rare-earth metal, it is found in the minerals monazite and gadolinite. It possesses unusual magnetic properties. One natural isotope, Ho 165 exists, six radioisotopes exist, the most stable being Ho-163 with a half-life of 4570 years. Holmium is used in some metal alloys, it is also said to stimulate the metabolism. Discovered by Per Theodor Cleve and J. L. Soret in Switzerland in 1879. The name homium comes from the Greek word Holmia which means Sweden. While all holmium compounds should be considered highly toxic, initial evidence seems to indicate that they do not pose much danger. The metal's dust however, is a fire hazard.

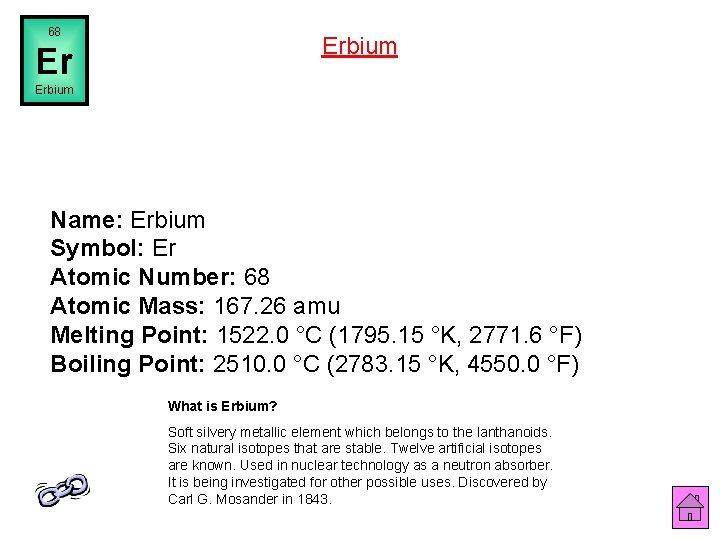
68 Erbium Er Erbium Name: Erbium Symbol: Er Atomic Number: 68 Atomic Mass: 167. 26 amu Melting Point: 1522. 0 °C (1795. 15 °K, 2771. 6 °F) Boiling Point: 2510. 0 °C (2783. 15 °K, 4550. 0 °F) What is Erbium? Soft silvery metallic element which belongs to the lanthanoids. Six natural isotopes that are stable. Twelve artificial isotopes are known. Used in nuclear technology as a neutron absorber. It is being investigated for other possible uses. Discovered by Carl G. Mosander in 1843.

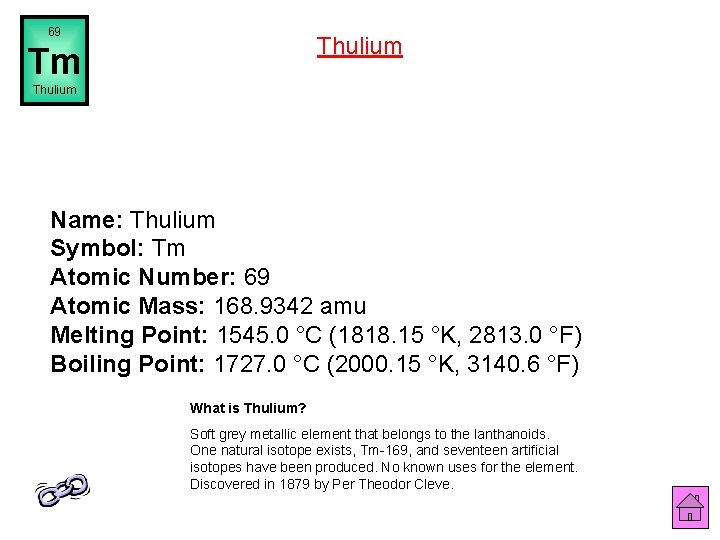
69 Thulium Tm Thulium Name: Thulium Symbol: Tm Atomic Number: 69 Atomic Mass: 168. 9342 amu Melting Point: 1545. 0 °C (1818. 15 °K, 2813. 0 °F) Boiling Point: 1727. 0 °C (2000. 15 °K, 3140. 6 °F) What is Thulium? Soft grey metallic element that belongs to the lanthanoids. One natural isotope exists, Tm-169, and seventeen artificial isotopes have been produced. No known uses for the element. Discovered in 1879 by Per Theodor Cleve.

70 Yb Ytterbium Name: Ytterbium Symbol: Yb Atomic Number: 70 Atomic Mass: 173. 04 amu Melting Point: 824. 0 °C (1097. 15 °K, 1515. 2 °F) Boiling Point: 1466. 0 °C (1739. 15 °K, 2670. 8 °F) What is Ytterbium? Silvery metallic element of the lanthanoids. Seven natural isotopes and ten artificial isotopes are known. Used in certain steels. Discovered by J. D. G. Marignac in 1878.

71 Lutetium Lu Lutetium Name: Lutetium Symbol: Lu Atomic Number: 71 Atomic Mass: 174. 967 amu Melting Point: 1656. 0 °C (1929. 15 °K, 3012. 8 °F) Boiling Point: 3315. 0 °C (3588. 15 °K, 5999. 0 °F) What is Lutetium? Silvery-white rare-earth metal which is relatively stable in air. It happens to be the most expensive rare-earth metal. Its found with almost all rare-earth metals, but is very difficult to separate from other elements. Least abundant of all natural elements. Used in metal alloys, and as a catalyst in various processes. There are two natural, stable isotopes, and seven radioisotopes, the most stable being Lu -174 with a half-life of 3. 3 years. The separation of lutetium from ytterbium was described by Georges Urbain in 1907. It was discovered at approximately the same time by Carl Auer von Welsbach. The name comes from the Greek word lutetia which means Paris.

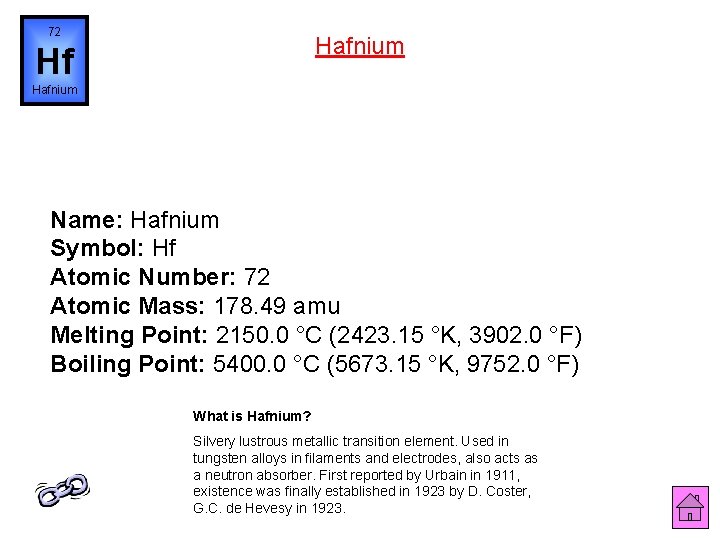
72 Hafnium Hf Hafnium Name: Hafnium Symbol: Hf Atomic Number: 72 Atomic Mass: 178. 49 amu Melting Point: 2150. 0 °C (2423. 15 °K, 3902. 0 °F) Boiling Point: 5400. 0 °C (5673. 15 °K, 9752. 0 °F) What is Hafnium? Silvery lustrous metallic transition element. Used in tungsten alloys in filaments and electrodes, also acts as a neutron absorber. First reported by Urbain in 1911, existence was finally established in 1923 by D. Coster, G. C. de Hevesy in 1923.

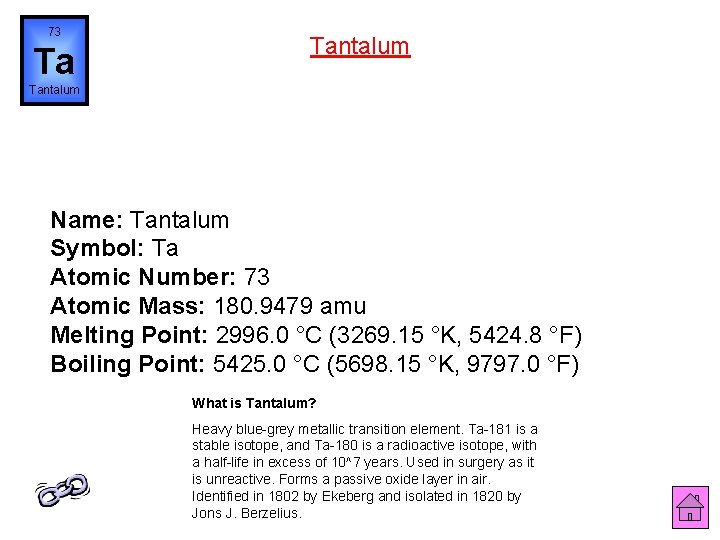
73 Ta Tantalum Name: Tantalum Symbol: Ta Atomic Number: 73 Atomic Mass: 180. 9479 amu Melting Point: 2996. 0 °C (3269. 15 °K, 5424. 8 °F) Boiling Point: 5425. 0 °C (5698. 15 °K, 9797. 0 °F) What is Tantalum? Heavy blue-grey metallic transition element. Ta-181 is a stable isotope, and Ta-180 is a radioactive isotope, with a half-life in excess of 10^7 years. Used in surgery as it is unreactive. Forms a passive oxide layer in air. Identified in 1802 by Ekeberg and isolated in 1820 by Jons J. Berzelius.

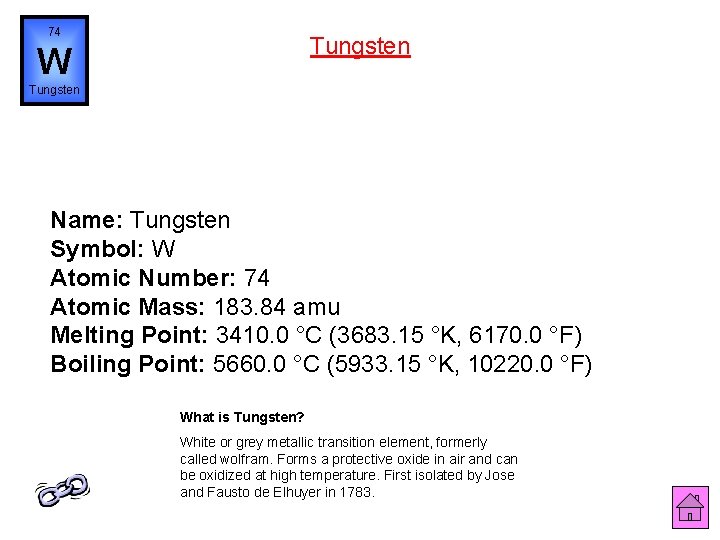
74 Tungsten W Tungsten Name: Tungsten Symbol: W Atomic Number: 74 Atomic Mass: 183. 84 amu Melting Point: 3410. 0 °C (3683. 15 °K, 6170. 0 °F) Boiling Point: 5660. 0 °C (5933. 15 °K, 10220. 0 °F) What is Tungsten? White or grey metallic transition element, formerly called wolfram. Forms a protective oxide in air and can be oxidized at high temperature. First isolated by Jose and Fausto de Elhuyer in 1783.

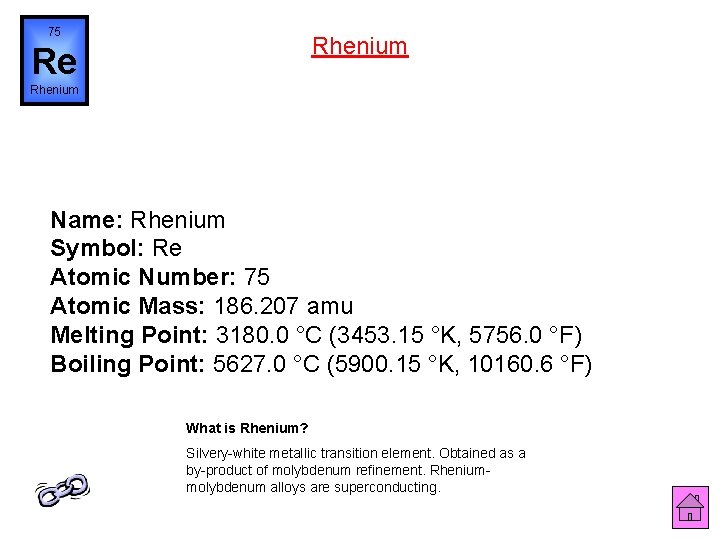
75 Rhenium Re Rhenium Name: Rhenium Symbol: Re Atomic Number: 75 Atomic Mass: 186. 207 amu Melting Point: 3180. 0 °C (3453. 15 °K, 5756. 0 °F) Boiling Point: 5627. 0 °C (5900. 15 °K, 10160. 6 °F) What is Rhenium? Silvery-white metallic transition element. Obtained as a by-product of molybdenum refinement. Rheniummolybdenum alloys are superconducting.

76 Osmium Os Osmium Name: Osmium Symbol: Os Atomic Number: 76 Atomic Mass: 190. 23 amu Melting Point: 3045. 0 °C (3318. 15 °K, 5513. 0 °F) Boiling Point: 5027. 0 °C (5300. 15 °K, 9080. 6 °F) What is Osmium? Hard blue-white metallic transition element. Found with platinum and used in some alloys with platinum and iridium.

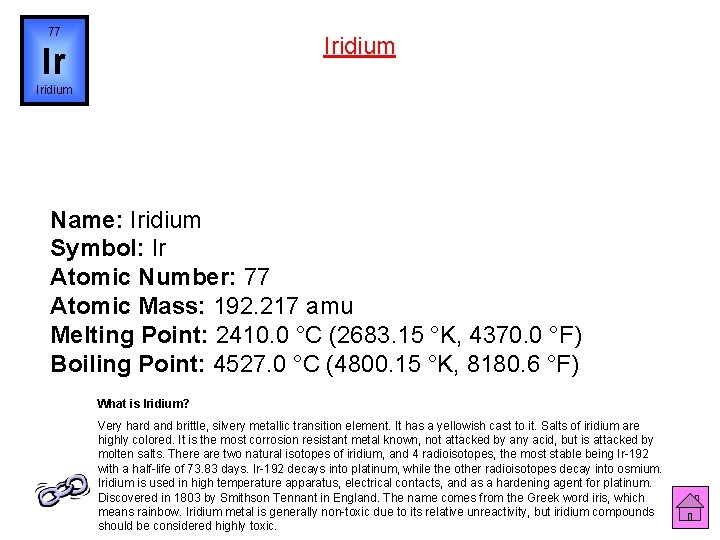
77 Iridium Ir Iridium Name: Iridium Symbol: Ir Atomic Number: 77 Atomic Mass: 192. 217 amu Melting Point: 2410. 0 °C (2683. 15 °K, 4370. 0 °F) Boiling Point: 4527. 0 °C (4800. 15 °K, 8180. 6 °F) What is Iridium? Very hard and brittle, silvery metallic transition element. It has a yellowish cast to it. Salts of iridium are highly colored. It is the most corrosion resistant metal known, not attacked by any acid, but is attacked by molten salts. There are two natural isotopes of iridium, and 4 radioisotopes, the most stable being Ir-192 with a half-life of 73. 83 days. Ir-192 decays into platinum, while the other radioisotopes decay into osmium. Iridium is used in high temperature apparatus, electrical contacts, and as a hardening agent for platinum. Discovered in 1803 by Smithson Tennant in England. The name comes from the Greek word iris, which means rainbow. Iridium metal is generally non-toxic due to its relative unreactivity, but iridium compounds should be considered highly toxic.

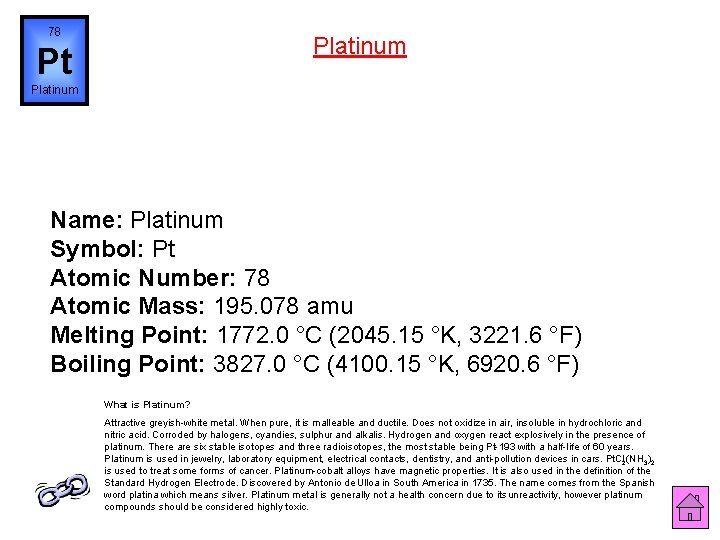
78 Platinum Pt Platinum Name: Platinum Symbol: Pt Atomic Number: 78 Atomic Mass: 195. 078 amu Melting Point: 1772. 0 °C (2045. 15 °K, 3221. 6 °F) Boiling Point: 3827. 0 °C (4100. 15 °K, 6920. 6 °F) What is Platinum? Attractive greyish-white metal. When pure, it is malleable and ductile. Does not oxidize in air, insoluble in hydrochloric and nitric acid. Corroded by halogens, cyandies, sulphur and alkalis. Hydrogen and oxygen react explosively in the presence of platinum. There are six stable isotopes and three radioisotopes, the most stable being Pt-193 with a half-life of 60 years. Platinum is used in jewelry, laboratory equipment, electrical contacts, dentistry, and anti-pollution devices in cars. Pt. Cl 2(NH 3)2 is used to treat some forms of cancer. Platinum-cobalt alloys have magnetic properties. It is also used in the definition of the Standard Hydrogen Electrode. Discovered by Antonio de Ulloa in South America in 1735. The name comes from the Spanish word platina which means silver. Platinum metal is generally not a health concern due to its unreactivity, however platinum compounds should be considered highly toxic.

79 Gold Au Gold Name: Gold Symbol: Au Atomic Number: 79 Atomic Mass: 196. 96655 amu Melting Point: 1064. 43 °C (1337. 5801 °K, 1947. 9741 °F) Boiling Point: 2807. 0 °C (3080. 15 °K, 5084. 6 °F) What is Gold? Gold is gold colored. It is the most malleable and ductile metal known. There is only one stable isotope of gold, and five radioisotopes of gold, Au-195 being the most stable with a half-life of 186 days. Gold is used as a monetary standard, in jewelry, dentistry, electronics. Au-198 is used in treating cancer and some other medical conditions. Gold has been known to exist as far back as 2600 BC. Gold comes from the Anglo-Saxon word gold. Its symbol, Au, comes from the Latin word aurum, which means gold. Gold is not particularly toxic, however it is known to cause damage to the liver and kidneys in some.

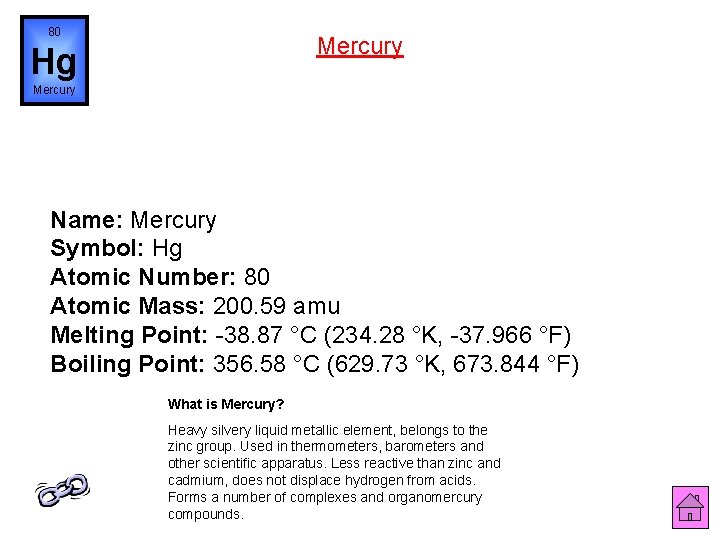
80 Mercury Hg Mercury Name: Mercury Symbol: Hg Atomic Number: 80 Atomic Mass: 200. 59 amu Melting Point: -38. 87 °C (234. 28 °K, -37. 966 °F) Boiling Point: 356. 58 °C (629. 73 °K, 673. 844 °F) What is Mercury? Heavy silvery liquid metallic element, belongs to the zinc group. Used in thermometers, barometers and other scientific apparatus. Less reactive than zinc and cadmium, does not displace hydrogen from acids. Forms a number of complexes and organomercury compounds.

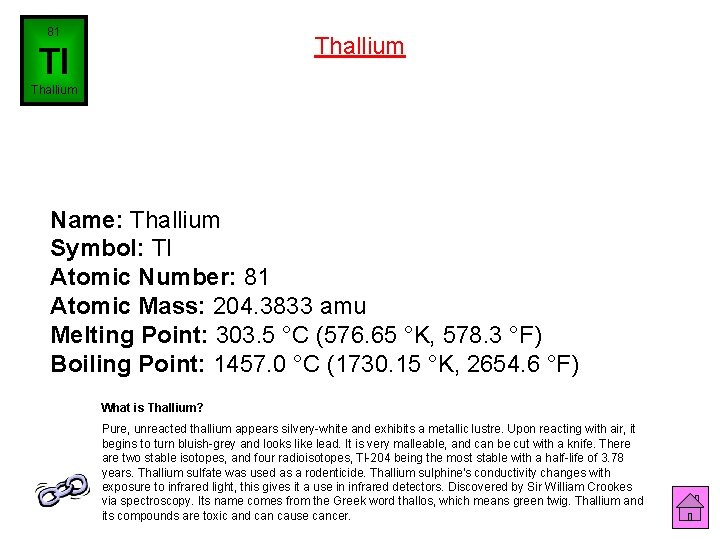
81 Thallium Tl Thallium Name: Thallium Symbol: Tl Atomic Number: 81 Atomic Mass: 204. 3833 amu Melting Point: 303. 5 °C (576. 65 °K, 578. 3 °F) Boiling Point: 1457. 0 °C (1730. 15 °K, 2654. 6 °F) What is Thallium? Pure, unreacted thallium appears silvery-white and exhibits a metallic lustre. Upon reacting with air, it begins to turn bluish-grey and looks like lead. It is very malleable, and can be cut with a knife. There are two stable isotopes, and four radioisotopes, Tl-204 being the most stable with a half-life of 3. 78 years. Thallium sulfate was used as a rodenticide. Thallium sulphine's conductivity changes with exposure to infrared light, this gives it a use in infrared detectors. Discovered by Sir William Crookes via spectroscopy. Its name comes from the Greek word thallos, which means green twig. Thallium and its compounds are toxic and can cause cancer.

82 Lead Pb Lead Name: Lead Symbol: Pb Atomic Number: 82 Atomic Mass: 207. 2 amu Melting Point: 327. 5 °C (600. 65 °K, 621. 5 °F) Boiling Point: 1740. 0 °C (2013. 15 °K, 3164. 0 °F) What is Lead? Heavy dull grey ductile metallic element, belongs to group 14. Used in building construction, lead-place accumulators, bullets and shot, and is part of solder, pewter, bearing metals, type metals and fusible alloys.

83 Bismuth Bi Bismuth Dschwen, wikipedia. org Name: Bismuth Symbol: Bi Atomic Number: 83 Atomic Mass: 208. 98038 amu Melting Point: 271. 3 °C (544. 45 °K, 520. 33997 °F) Boiling Point: 1560. 0 °C (1833. 15 °K, 2840. 0 °F) What is Bismuth? White crystalline metal with a pink tinge, belongs to group 15. Most diamagnetic of all metals and has the lowest thermal conductivity of all the elements except mercury. Lead-free bismuth compounds are used in cosmetics and medical procedures. Burns in the air and produces a blue flame. In 1753, C. G. Junine first demonstrated that it was different from lead.

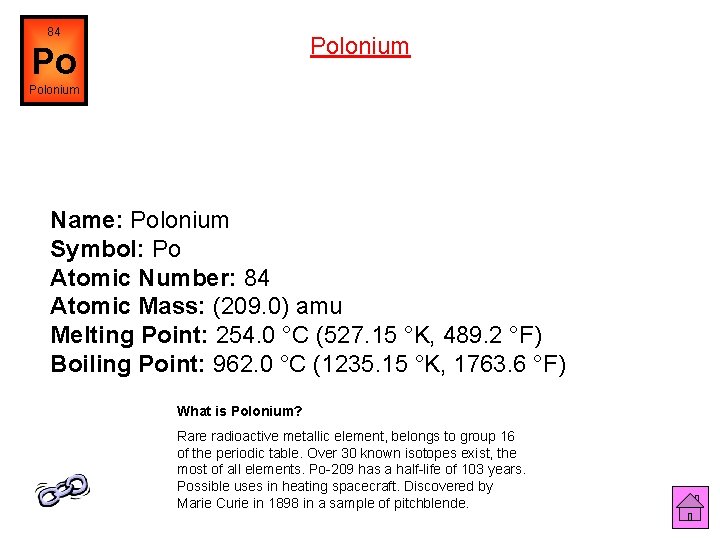
84 Polonium Po Polonium Name: Polonium Symbol: Po Atomic Number: 84 Atomic Mass: (209. 0) amu Melting Point: 254. 0 °C (527. 15 °K, 489. 2 °F) Boiling Point: 962. 0 °C (1235. 15 °K, 1763. 6 °F) What is Polonium? Rare radioactive metallic element, belongs to group 16 of the periodic table. Over 30 known isotopes exist, the most of all elements. Po-209 has a half-life of 103 years. Possible uses in heating spacecraft. Discovered by Marie Curie in 1898 in a sample of pitchblende.

85 Astatine At Astatine Name: Astatine Symbol: At Atomic Number: 85 Atomic Mass: (210. 0) amu Melting Point: 302. 0 °C (575. 15 °K, 575. 6 °F) Boiling Point: 337. 0 °C (610. 15 °K, 638. 6 °F) What is Astatine? Radioactive halogen element. Occurs naturally from uranium and thorium decay. At least 20 known isotopes. At-210, the most stable, has a half-life of 8. 3 hours. Synthesized by nuclear bombardment in 1940 by D. R. Corson, K. R. Mac. Kenzie and E. Segre at the University of California.

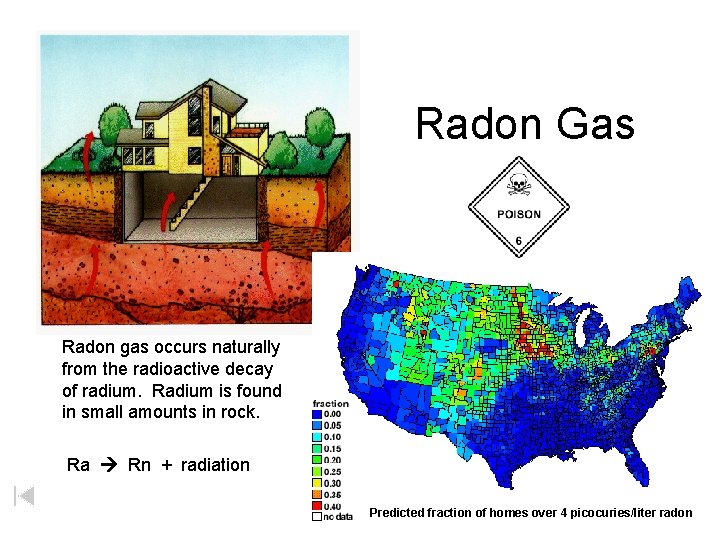
86 Radon Rn Radon Name: Radon Symbol: Rn Atomic Number: 86 Atomic Mass: (222. 0) amu Melting Point: -71. 0 °C (202. 15 °K, -95. 8 °F) Boiling Point: -61. 8 °C (211. 35 °K, -79. 24 °F) Link What is Radon? Colorless radioactive gaseous element, belongs to the noble gases. Of the twenty known isotopes, the most stable is Rn-222 with a half-life of 3. 8 days. Formed by the radioactive decay of Radium-226. Radon itself decays into polonium. Used in radiotherapy. As a noble gas, it is effectively inert, though radon fluoride has been synthesized. First isolated in 1908 by Ramsey and Gray.

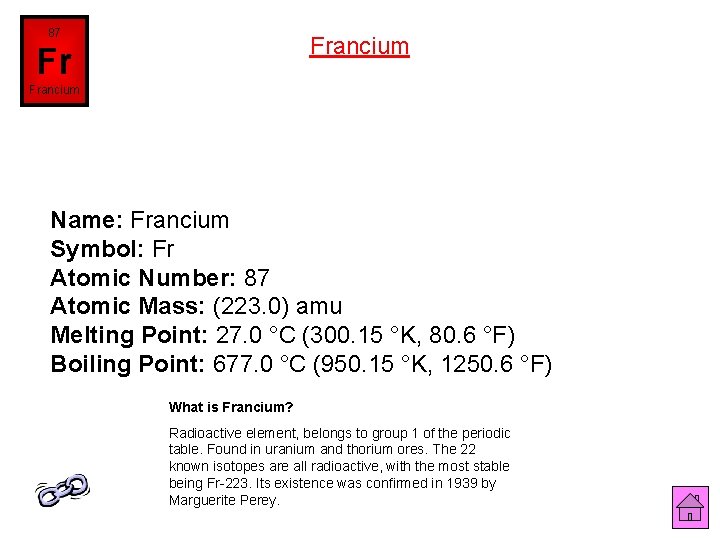
87 Francium Fr Francium Name: Francium Symbol: Fr Atomic Number: 87 Atomic Mass: (223. 0) amu Melting Point: 27. 0 °C (300. 15 °K, 80. 6 °F) Boiling Point: 677. 0 °C (950. 15 °K, 1250. 6 °F) What is Francium? Radioactive element, belongs to group 1 of the periodic table. Found in uranium and thorium ores. The 22 known isotopes are all radioactive, with the most stable being Fr-223. Its existence was confirmed in 1939 by Marguerite Perey.

88 Radium Ra Radium Name: Radium Symbol: Ra Atomic Number: 88 Atomic Mass: (226. 0) amu Melting Point: 700. 0 °C (973. 15 °K, 1292. 0 °F) Boiling Point: 1737. 0 °C (2010. 15 °K, 3158. 6 °F) Link What is Radium? Radioactive metallic element, belongs to group 2 of the periodic table. Most stable isotope, Ra-226 has a halflife of 1602 years, which decays into radon. Isolated from pitchblende in 1898 Marie and Pierre Curie.

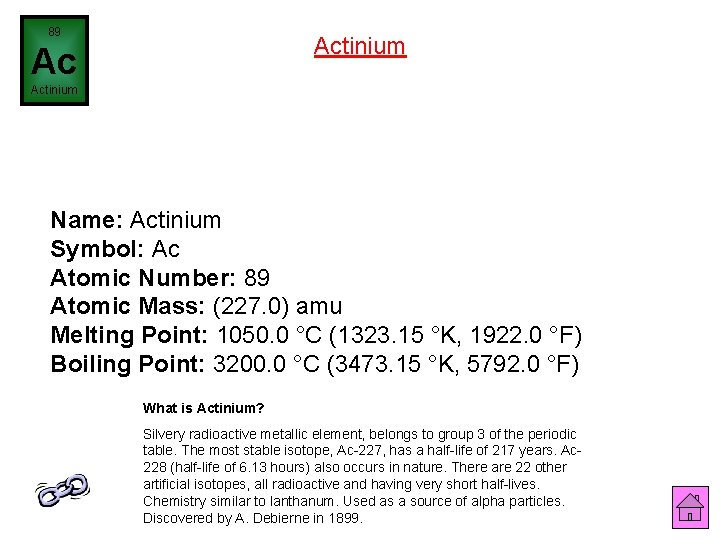
89 Actinium Ac Actinium Name: Actinium Symbol: Ac Atomic Number: 89 Atomic Mass: (227. 0) amu Melting Point: 1050. 0 °C (1323. 15 °K, 1922. 0 °F) Boiling Point: 3200. 0 °C (3473. 15 °K, 5792. 0 °F) What is Actinium? Silvery radioactive metallic element, belongs to group 3 of the periodic table. The most stable isotope, Ac-227, has a half-life of 217 years. Ac 228 (half-life of 6. 13 hours) also occurs in nature. There are 22 other artificial isotopes, all radioactive and having very short half-lives. Chemistry similar to lanthanum. Used as a source of alpha particles. Discovered by A. Debierne in 1899.

90 Thorium Th Thorium Name: Thorium Symbol: Th Atomic Number: 90 Atomic Mass: 232. 0381 amu Melting Point: 1750. 0 °C (2023. 15 °K, 3182. 0 °F) Boiling Point: 4790. 0 °C (5063. 15 °K, 8654. 0 °F) What is Thorium? Grey radioactive metallic element. Belongs to actinoids. Found in monazite sand in Brazil, India and the US. Thorium-232 has a half-life of 1. 39 x 10^10 years. Can be used as a nuclear fuel for breeder reactors. Thorium -232 captures slow neutrons and breeds uranium-233. Discovered by Jons J. Berzelius in 1829.

91 Pa Protactinium Name: Protactinium Symbol: Pa Atomic Number: 91 Atomic Mass: 231. 03587 amu Melting Point: 1600. 0 °C (1873. 15 °K, 2912. 0 °F) Boiling Point: Unknown What is Protactinium? Radioactive metallic element, belongs to the actinoids. The most stable isotope, Pa-231 has a half-life of 2. 43 x 104 years. At least 10 other radioactive isotopes are known. No practical applications are known. Discovered in 1917 by Lise Meitner and Otto Hahn.

92 Uranium U Uranium Name: Uranium Symbol: U Atomic Number: 92 Atomic Mass: 238. 0289 amu Melting Point: 1132. 0 °C (1405. 15 °K, 2069. 6 °F) Boiling Point: 3818. 0 °C (4091. 15 °K, 6904. 4 °F) What is Uranium? White radioactive metallic element belonging to the actinoids. Three natural isotopes, U-238, U-235 and U-234. Uranium-235 is used as the fuel for nuclear reactors and weapons. Discovered by Martin H. Klaproth in 1789.

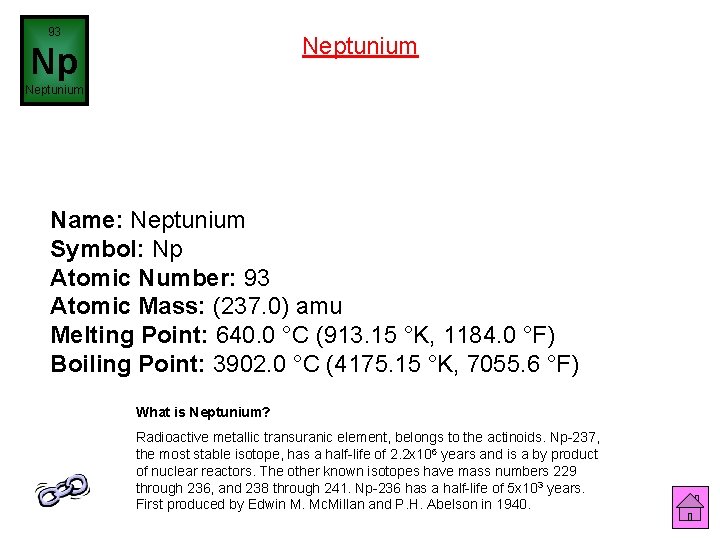
93 Neptunium Np Neptunium Name: Neptunium Symbol: Np Atomic Number: 93 Atomic Mass: (237. 0) amu Melting Point: 640. 0 °C (913. 15 °K, 1184. 0 °F) Boiling Point: 3902. 0 °C (4175. 15 °K, 7055. 6 °F) What is Neptunium? Radioactive metallic transuranic element, belongs to the actinoids. Np-237, the most stable isotope, has a half-life of 2. 2 x 106 years and is a by product of nuclear reactors. The other known isotopes have mass numbers 229 through 236, and 238 through 241. Np-236 has a half-life of 5 x 103 years. First produced by Edwin M. Mc. Millan and P. H. Abelson in 1940.

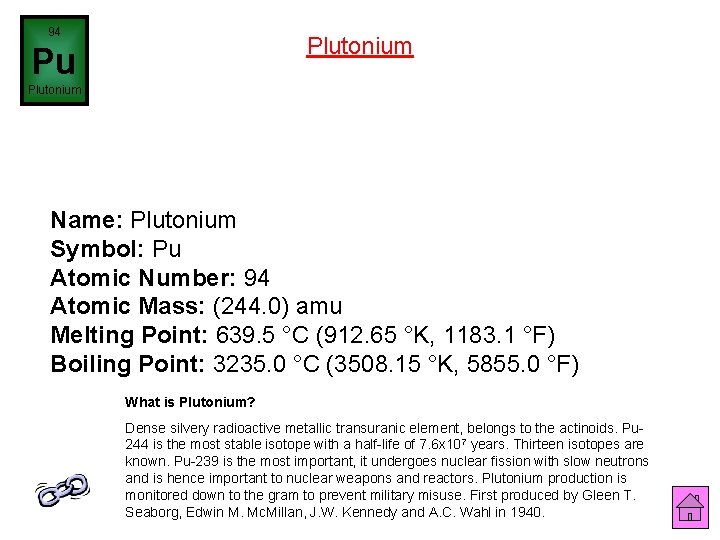
94 Plutonium Pu Plutonium Name: Plutonium Symbol: Pu Atomic Number: 94 Atomic Mass: (244. 0) amu Melting Point: 639. 5 °C (912. 65 °K, 1183. 1 °F) Boiling Point: 3235. 0 °C (3508. 15 °K, 5855. 0 °F) What is Plutonium? Dense silvery radioactive metallic transuranic element, belongs to the actinoids. Pu 244 is the most stable isotope with a half-life of 7. 6 x 107 years. Thirteen isotopes are known. Pu-239 is the most important, it undergoes nuclear fission with slow neutrons and is hence important to nuclear weapons and reactors. Plutonium production is monitored down to the gram to prevent military misuse. First produced by Gleen T. Seaborg, Edwin M. Mc. Millan, J. W. Kennedy and A. C. Wahl in 1940.

95 Am Americium Name: Americium Symbol: Am Atomic Number: 95 Atomic Mass: (243. 0) amu Melting Point: 994. 0 °C (1267. 15 °K, 1821. 2 °F) Boiling Point: 2607. 0 °C (2880. 15 °K, 4724. 6 °F) Lawrence Berkeley National Lab What is Americium? Radioactive metallic transuranic element, belongs to the actinoids. Ten known isotopes. Am-243 is the most stable isotope, with a half-life of 7. 95 x 103 years. Discovered by Glenn T. Seaborg and associates in 1945, it was obtained by bombarding uranium-238 with alpha particles.

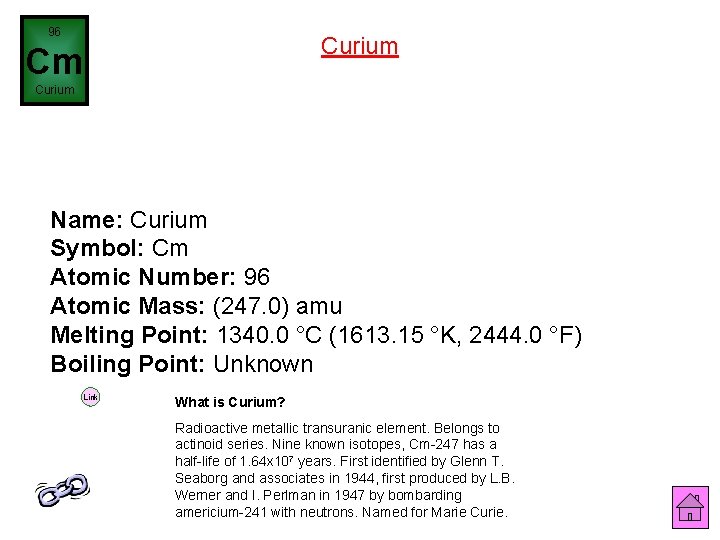
96 Curium Cm Curium Name: Curium Symbol: Cm Atomic Number: 96 Atomic Mass: (247. 0) amu Melting Point: 1340. 0 °C (1613. 15 °K, 2444. 0 °F) Boiling Point: Unknown Link What is Curium? Radioactive metallic transuranic element. Belongs to actinoid series. Nine known isotopes, Cm-247 has a half-life of 1. 64 x 107 years. First identified by Glenn T. Seaborg and associates in 1944, first produced by L. B. Werner and I. Perlman in 1947 by bombarding americium-241 with neutrons. Named for Marie Curie.

97 Bk Berkelium Name: Berkelium Symbol: Bk Atomic Number: 97 Atomic Mass: (247. 0) amu Melting Point: Unknown Boiling Point: Unknown What is Berkelium? Radioactive metallic transuranic element. Belongs to actinoid series. Eight known isotopes, the most common Bk-247, has a half-life of 1. 4 x 103 years. First produced by Glenn T. Seaborg and associates in 1949 by bombarding americium-241 with alpha particles.

98 Californium Cf Californium Name: Californium Symbol: Cf Atomic Number: 98 Atomic Mass: (251. 0) amu Melting Point: Unknown Boiling Point: Unknown What is Californium? Radioactive metallic transuranic element. Belongs to actinoid series. Cf 251 has a half life of about 700 years. Nine isotopes are known. Cf-252 is an intense neutron source, which makes it an intense neutron source and gives it a use in neutron activation analysis and a possible use as a radiation source in medicine. First produced by Glenn T. Seaborg and associates in 1950.

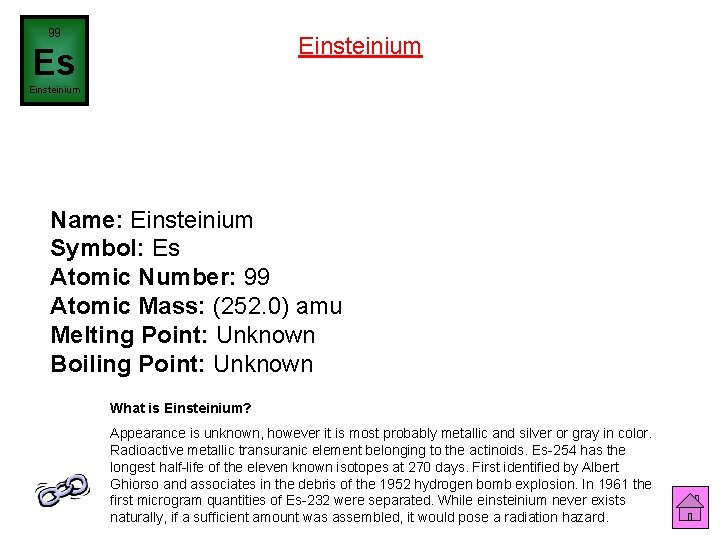
99 Einsteinium Es Einsteinium Name: Einsteinium Symbol: Es Atomic Number: 99 Atomic Mass: (252. 0) amu Melting Point: Unknown Boiling Point: Unknown What is Einsteinium? Appearance is unknown, however it is most probably metallic and silver or gray in color. Radioactive metallic transuranic element belonging to the actinoids. Es-254 has the longest half-life of the eleven known isotopes at 270 days. First identified by Albert Ghiorso and associates in the debris of the 1952 hydrogen bomb explosion. In 1961 the first microgram quantities of Es-232 were separated. While einsteinium never exists naturally, if a sufficient amount was assembled, it would pose a radiation hazard.

100 Fermium Fm Fermium Name: Fermium Symbol: Fm Atomic Number: 100 Atomic Mass: (257. 0) amu Melting Point: Unknown Boiling Point: Unknown What is Fermium? Radioactive metallic transuranic element, belongs to the actinoids. Ten known isotopes, most stable is Fm 257 with a half-life of 10 days. First identified by Albert Ghiorso and associates in the debris of the first hydrogen-bomb explosion in 1952.

101 Mendelevium Md Mendelevium Name: Mendelevium Symbol: Md Atomic Number: 101 Atomic Mass: (258. 0) amu Melting Point: Unknown Boiling Point: Unknown What is Mendelevium? Radioactive metallic transuranic element. Belongs to the actinoid series. Only known isotope, Md-256 has a half-life of 1. 3 hours. First identified by Glenn T. Seaborg, Albert Ghiorso and associates in 1955. Alternative name unnilunium has been proposed. Named after the 'inventor' of the periodic table, Dmitri Mendeleev.

102 Nobelium No Nobelium Name: Nobelium Symbol: No Atomic Number: 102 Atomic Mass: (259. 0) amu Melting Point: Unknown Boiling Point: Unknown Link What is Nobelium? Radioactive metallic transuranic element, belongs to the actinoids. Seven known isotopes exist, the most stable being No-254 with a half-life of 255 seconds. First identified with certainty by Albert Ghiorso and Glenn T. Seaborg in 1966. Unnilbium has been proposed as an alternative name.

103 Lawrencium Lr Lawrencium Name: Lawrencium Symbol: Lr Atomic Number: 103 Atomic Mass: (262. 0) amu Melting Point: Unknown Boiling Point: Unknown What is Lawrencium? Appearance unknown, however it is most likely silvery-white or grey and metallic. Lawrencium is a synthetic rare-earth metal. There are eight known radioisotopes, the most stable being Lr-262 with a half-life of 3. 6 hours. Due to the short half-life of lawrencium, and its radioactivity, there are no known uses for it. Identified by Albert Ghiorso in 1961 at Berkeley. It was produced by bombarding californium with boron ions. The name is temporary IUPAC nomenclature, the origin of the name comes from Ernest O. Lawrence, the inventor of the cyclotron. If sufficient amounts of lawrencium were produced, it would pose a radiation hazard.

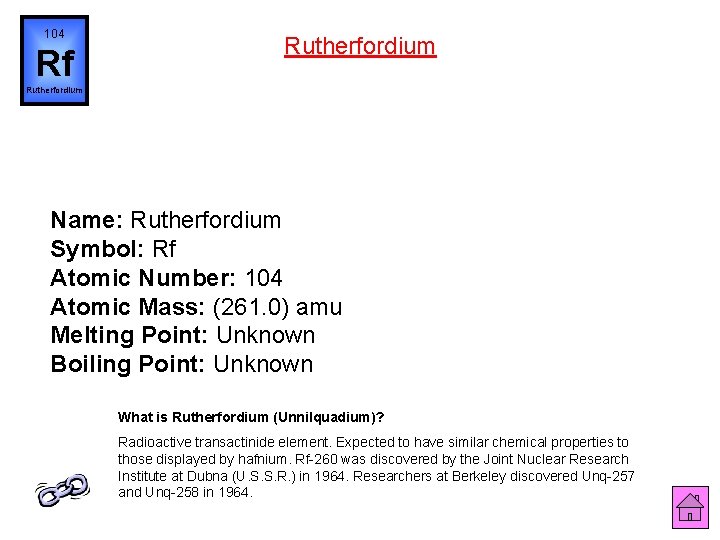
104 Rf Rutherfordium Name: Rutherfordium Symbol: Rf Atomic Number: 104 Atomic Mass: (261. 0) amu Melting Point: Unknown Boiling Point: Unknown What is Rutherfordium (Unnilquadium)? Radioactive transactinide element. Expected to have similar chemical properties to those displayed by hafnium. Rf-260 was discovered by the Joint Nuclear Research Institute at Dubna (U. S. S. R. ) in 1964. Researchers at Berkeley discovered Unq-257 and Unq-258 in 1964.

105 Db Dubnium Name: Dubnium Symbol: Db Atomic Number: 105 Atomic Mass: (262. 0) amu Melting Point: Unknown Boiling Point: Unknown What is Dubnium (Unnilpentium)? Radioactive transactinide element. Half-life of 1. 6 s. Discovered in 1970 by Berkeley researchers. So far, seven isotopes have been discovered.

106 Sg Seaborgium Name: Seaborgium Symbol: Sg Atomic Number: 106 Atomic Mass: (263. 0) amu Melting Point: Unknown Boiling Point: Unknown Link What is Seaborgium (Unnilhexium)? Half-life of 0. 9 +/- 0. 2 s. Discovered by the Joint Institute for Nuclear Research at Dubna (U. S. S. R. ) in June of 1974. Its existence was confirmed by the Lawrence Berkeley Laboratory and Livermore National Laboratory in September of 1974.

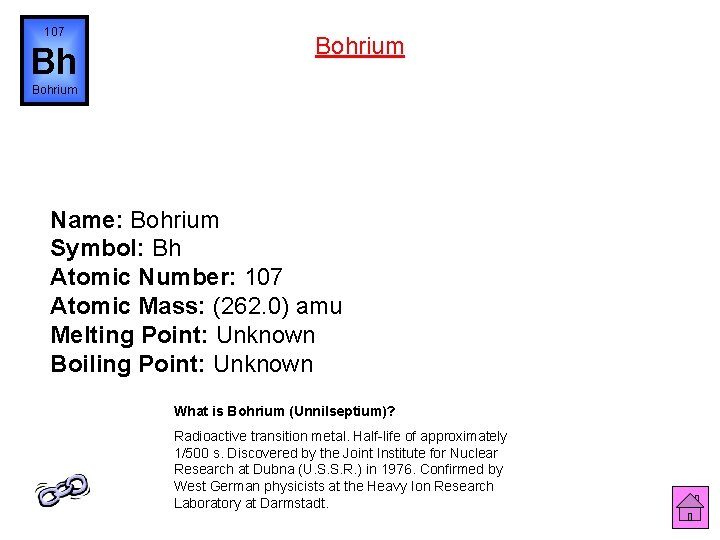
107 Bh Bohrium Name: Bohrium Symbol: Bh Atomic Number: 107 Atomic Mass: (262. 0) amu Melting Point: Unknown Boiling Point: Unknown What is Bohrium (Unnilseptium)? Radioactive transition metal. Half-life of approximately 1/500 s. Discovered by the Joint Institute for Nuclear Research at Dubna (U. S. S. R. ) in 1976. Confirmed by West German physicists at the Heavy Ion Research Laboratory at Darmstadt.

108 Hs Hassium Name: Hassium Symbol: Hs Atomic Number: 108 Atomic Mass: (265. 0) amu Melting Point: Unknown Boiling Point: Unknown

109 Mt Meitnerium Name: Meitnerium Symbol: Mt Atomic Number: 109 Atomic Mass: (266. 0) amu Melting Point: Unknown Boiling Point: Unknown

Einsteinium (Es) Albert Einstein – Relativity – E = mc 2 – Offered Presidency of Israel – Element 99 – Photoelectric effect • Solar calculator

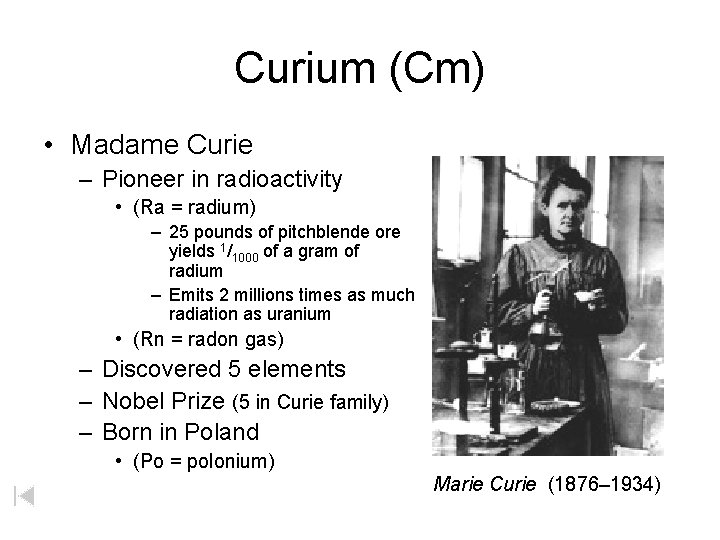
Curium (Cm) • Madame Curie – Pioneer in radioactivity • (Ra = radium) – 25 pounds of pitchblende ore yields 1/1000 of a gram of radium – Emits 2 millions times as much radiation as uranium • (Rn = radon gas) – Discovered 5 elements – Nobel Prize (5 in Curie family) – Born in Poland • (Po = polonium) Marie Curie (1876– 1934)

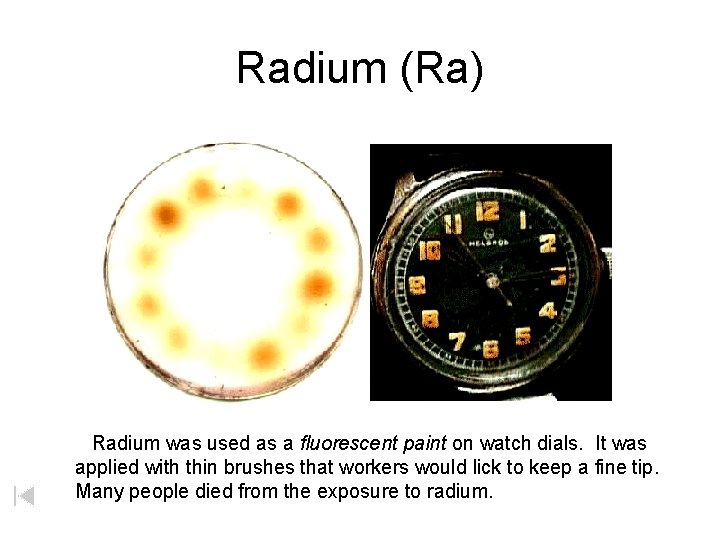
Radium (Ra) Radium was used as a fluorescent paint on watch dials. It was applied with thin brushes that workers would lick to keep a fine tip. Many people died from the exposure to radium.

Radon Gas Radon gas occurs naturally from the radioactive decay of radium. Radium is found in small amounts in rock. Ra Rn + radiation Predicted fraction of homes over 4 picocuries/liter radon

Nobelium (No) Element 102 Inventor: dynamite (TNT) blasting gelatin Trinitrotoluene Nobel Prize Alfred Nobel “Merchant of Death”

Seaborgium (Sg) Glenn Seaborg – Separated f-block from rest of periodic table – Worked on Manhattan Project (Atomic bomb) – Classified until after WW II – Element 106 • Only living person to have an element named for them

Silicon vs. Silicone • Silicon (Si) element • Silicone (…Si – O – Si…) polymer – Sealant (caulk) prevents leaks – Breast augmentation No cause-and-effect relationship exists between breast enlargement and breast cancer. Only one researcher found a causal link.

12 Magnesium Atomic Mass 24 amu melting point = silver gray metal used in flash bulbs, bombs, and flares 8 th most abundant element (2. 2% of Earth’s crust) lack of Mg produces same biological effect as alcoholism (delirium tremens) Mg 24. 305

Potassium Metal in Water Newmark, CHEMISTRY, 1993, page 25

7 1 C H 6 1 S Ir O N Mn < N e 16 77 8 7 25 H H He 1 2 3 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Na Mg 11 4 K 19 5 7 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 37 6 12 38 Cs Ba 55 56 Fr Ra 87 88 * W 25 43 26 44 Re Os 75 76 27 28 29 47 30 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 34 Sn Sb Te 45 79 48 31 52 Po At Rn 84 85 86 Rf Db Sg Bh Hs Mt 104 105 106 107 108 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 59 60 Ac Th Pa U 89 58 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103

Elements Database Printable Periodic Table Elements listed Alphabetically Actinium Aluminum Americium Antimony Argon Arsenic Astatine Barium Berkelium Beryllium Bismuth Boron Bromine Cadmium Cesium Calcium Californium Carbon Cerium Chlorine Chromium Cobalt Copper Curium Dysprosium Einsteinium Erbium Europium Fermium Fluorine Francium Gadolinium Gallium Germanium Gold Hafnium Helium Holmium Hydrogen Indium Iodine Iridium Iron Krypton Lanthanum Lawrencium Lead Lithium Lutetium Magnesium Manganese Meitnerium Mendelevium Mercury Molybdenum Neodymium Neon Neptunium Nickel Niobium Nitrogen Nobelium Osmium Oxygen Palladium Phosphorus Platinum Plutonium Polonium Potassium Praseodymium Promethium Protactinium Radon Rhenium Rhodium Rubidium Ruthenium Samarium Scandium Selenium Silicon Silver Sodium Strontium Sulfur Tantalum Technetium Tellurium Terbium Thallium Thorium Thulium Tin Titanium Tungsten Unnilhexium Unniloctium Unnilpentium Unnilquadium Unnilseptium Uranium Vanadium Xenon Ytterbium Yttrium Zinc Zirconium Get free Chemistry and Physics images for your school projects and/or research work. Feel free to use the periodic table images below in your school projects and/or research work.

Periodic Table of the Elements Images from: http: //www. chemsoc. org/viselements/pages/pertable_j. ht m Periodic Table of the Elements Data from: http: //www. chemicalelements. com/ http: //www. elementsdatabase. com/ http: //www. periodictable. com Written by: Bill Byles - bylesb@internet 4 classrooms. com & Jeff Christopherson – unit 5. org/chemistry