1 2 Columns vertical groups of elements on
- Slides: 3

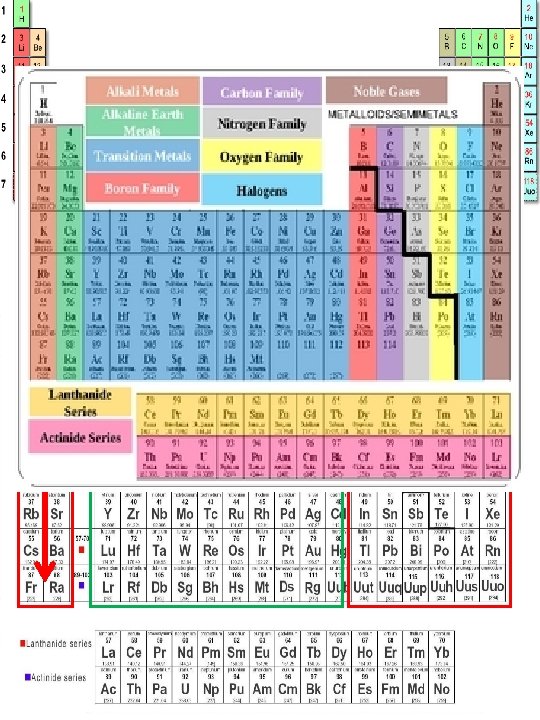
1 2 Columns – vertical groups of elements on the Periodic Table. 3 • There are 18 columns of elements 4 • Columns of elements are called groups or families 5 § 8 main group or “A” Families (1 A – 8 A) § 10 secondary group or “B” Families 6 7 • Elements in a particular group or family are related to Organization of the Periodic Table 1. each other by their similar (not identical) chemical and/or physical properties. 1 A 2 A 3 A 4 A 5 A 6 A 7 A 8 A 2. Periods – horizontal groups of elements on the Periodic Table • There are 7 periods on the Periodic Table • Elements in the same period are not alike in physical/chemical properties • The first element in a period is always an extremely reactive solid • The last element in a period is a non-reactive gas • Period # = the total number of energy levels (n) • Every element in a period has the same number of energy levels

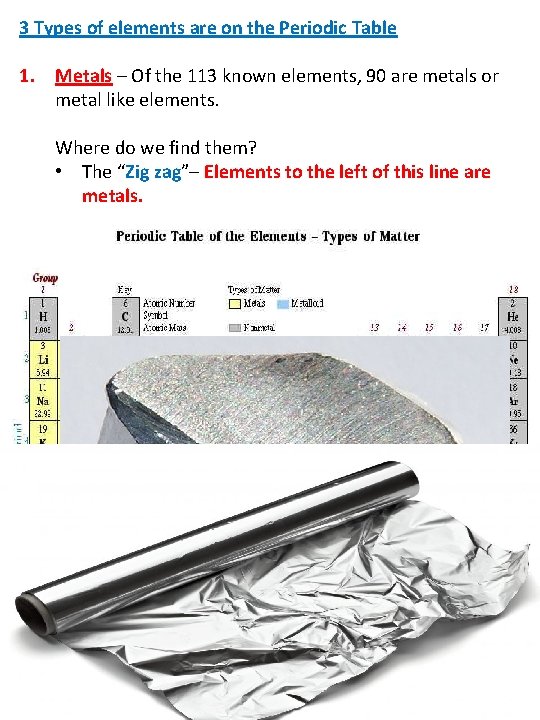
3 Types of elements are on the Periodic Table 1. Metals – Of the 113 known elements, 90 are metals or metal like elements. Where do we find them? • The “Zig zag”– Elements to the left of this line are metals. Physical Properties of Metals • Metals have luster (shiny) • Conduct heat and electricity • Most are solids (few liquids) • Have high melting points • Most metals are ductile (can be drawn into thin wires) • Most metals are malleable (can be hammered into thin sheets) Chemical Properties of Metals • Metals like to lose electrons when reacting with nonmetal elements

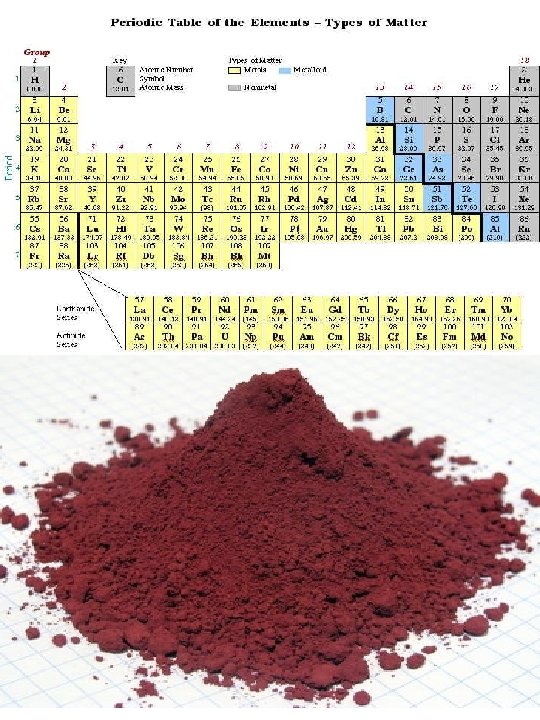
2. Nonmetals – Elements located to the right of the zig zag line Physical Properties of Nonmetals • Nonmetals are dull (not shiny) • Poor conductors • Are not ductile or malleable • Can be a solid, liquid, or gas Chemical Properties of Nonmetals • Elements in the 18 th group are non reactive • Other Nonmetals like to gain electrons from metals 3. Metalloids – Elements located on either side of the zig zag • Metalloids have properties of both metals and nonmetals • All metalloids are solids • They can be shiny or dull • They conduct heat and electricity better than nonmetals but not as well as metals • They are ductile and malleable