1 2 Acids and Bases Overview Properties and

1. 2 Acids and Bases

Overview ● Properties and examples of ○ Acids, bases, neutral solutions ● p. H ○ ○ The p. H scale Measuring p. H ● Neutralization reactions ● Lab ● Acid rain ○ Causes, impacts, and treatment

First. . . Brainstorm with your table partner: ● Some properties of acids, bases, and neutral solutions ● Examples of each solution ● How they interact with one another, the environment, etc.

Properties of Acids ● Dissolve in water ● Has more hydrogen ions (H+) than hydroxide ions (OH-) present ● Taste sour, and can cause a stinging feeling Fun fact: the liquid in your stomach is actually very acidic!

Properties of Bases ● Soluble in water ● Has more hydroxide ions (OH-) than hydrogen ions (H+) present ● Feel slippery and taste bitter Fun fact: bases do not react with metals! This is what makes them such effective drain cleaners

Neutral Solutions ● Neither acidic nor basic
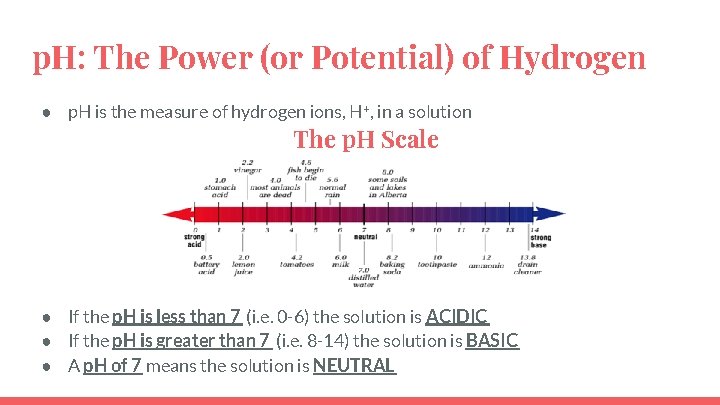
p. H: The Power (or Potential) of Hydrogen ● p. H is the measure of hydrogen ions, H+, in a solution The p. H Scale ● If the p. H is less than 7 (i. e. 0 -6) the solution is ACIDIC ● If the p. H is greater than 7 (i. e. 8 -14) the solution is BASIC ● A p. H of 7 means the solution is NEUTRAL

Strong vs. Weak Acids and Bases ● Strong acids have a p. H <2 ● Strong bases have a p. H >12 Rule: ○ ○ There is a 10 -fold difference between the numbers on the p. H scale Meaning: ■ A p. H of 2 will be 10 times more acidic than a p. H of 3 ■ A p. H of 9 will be 10 times more basic than a p. H of 8
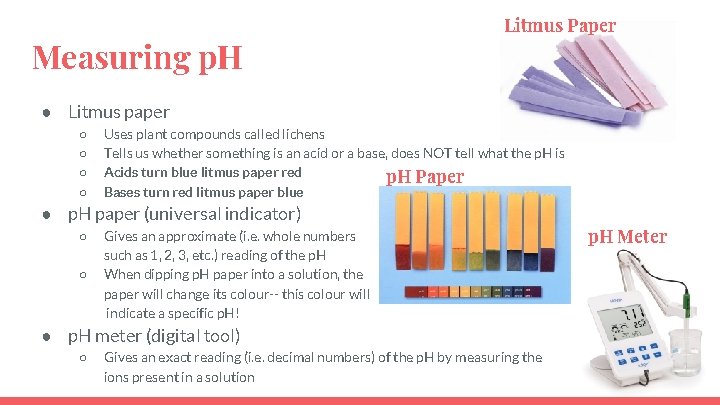
Measuring p. H Litmus Paper ● Litmus paper ○ ○ Uses plant compounds called lichens Tells us whether something is an acid or a base, does NOT tell what the p. H is Acids turn blue litmus paper red p. H Paper Bases turn red litmus paper blue ● p. H paper (universal indicator) ○ ○ Gives an approximate (i. e. whole numbers such as 1, 2, 3, etc. ) reading of the p. H When dipping p. H paper into a solution, the paper will change its colour-- this colour will indicate a specific p. H! ● p. H meter (digital tool) ○ Gives an exact reading (i. e. decimal numbers) of the p. H by measuring the ions present in a solution p. H Meter
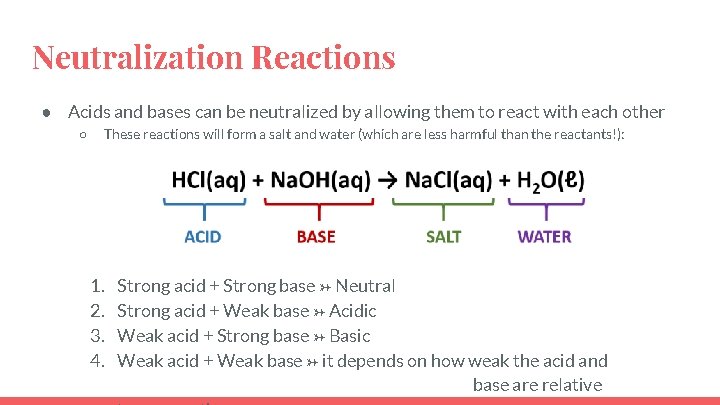
Neutralization Reactions ● Acids and bases can be neutralized by allowing them to react with each other ○ These reactions will form a salt and water (which are less harmful than the reactants!): 1. 2. 3. 4. Strong acid + Strong base → Neutral Strong acid + Weak base → Acidic Weak acid + Strong base → Basic Weak acid + Weak base → it depends on how weak the acid and base are relative

Lab Time!

Video

Wrapping Up: Acid Rain ● Normal p. H of rainwater is around 5. 4 ○ Slightly acidic because of CO 2 which reacts with water to form carbonic acid ● Acid rain usually has a p. H of around 3 -4 ○ ○ Industrial factories, coal-fired power plants, and vehicle emissions give off sulfur dioxide (SO 2) and nitrogen oxides (NOx) Water molecules in the air will react with these gases to produce sulfuric and nitric acids ● These may all contribute to acid rain
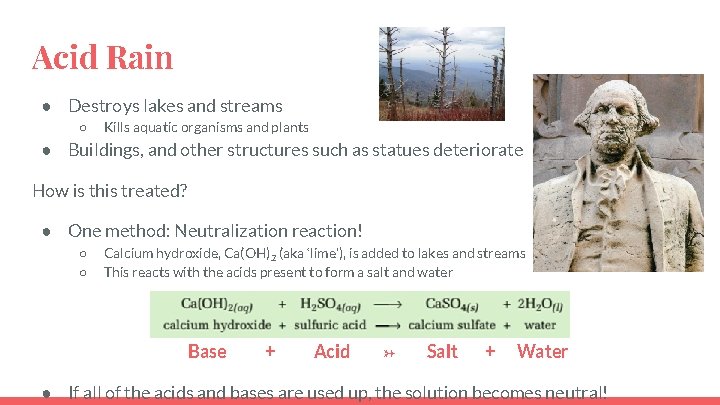
Acid Rain ● Destroys lakes and streams ○ Kills aquatic organisms and plants ● Buildings, and other structures such as statues deteriorate How is this treated? ● One method: Neutralization reaction! ○ ○ Calcium hydroxide, Ca(OH)2 (aka ‘lime’), is added to lakes and streams This reacts with the acids present to form a salt and water Base + Acid → Salt + Water ● If all of the acids and bases are used up, the solution becomes neutral!
- Slides: 14