1 2 1 3 Bonding Atoms trying to
1. 2 -1. 3 Bonding Atoms trying to attain the stable configuration of a noble (inert) gas - often referred to as the octet rule 1. 2 Ionic Bonding - Electrons Transferred 1. 3 Covalent Bonding - Electrons Shared type of bond that is formed is dictated by the relative electronegativities of the elements involved

Electronegativity the attraction of an atom for electrons
1. 2 Ionic bonding Electrons Transferred Big differences in E. N. values Metals reacting with non-metals
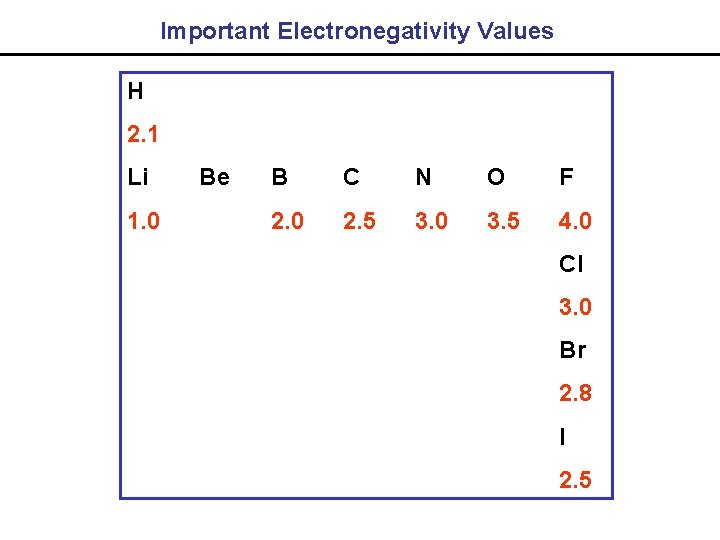
Important Electronegativity Values H 2. 1 Li 1. 0 Be B C N O F 2. 0 2. 5 3. 0 3. 5 4. 0 Cl 3. 0 Br 2. 8 I 2. 5
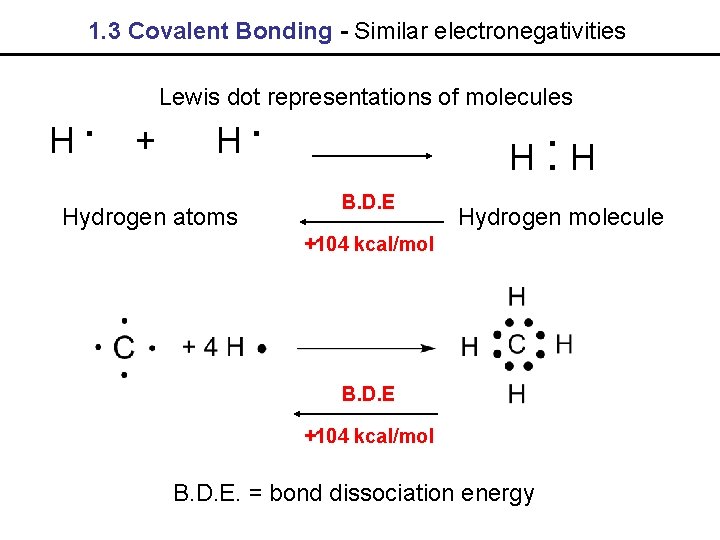
1. 3 Covalent Bonding - Similar electronegativities . H Lewis dot representations of molecules + . H Hydrogen atoms H: H B. D. E Hydrogen molecule +104 kcal/mol B. D. E. = bond dissociation energy
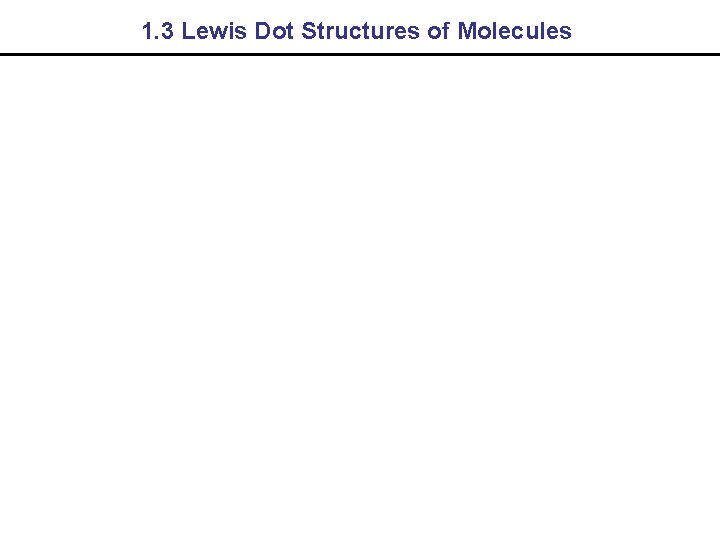
1. 3 Lewis Dot Structures of Molecules
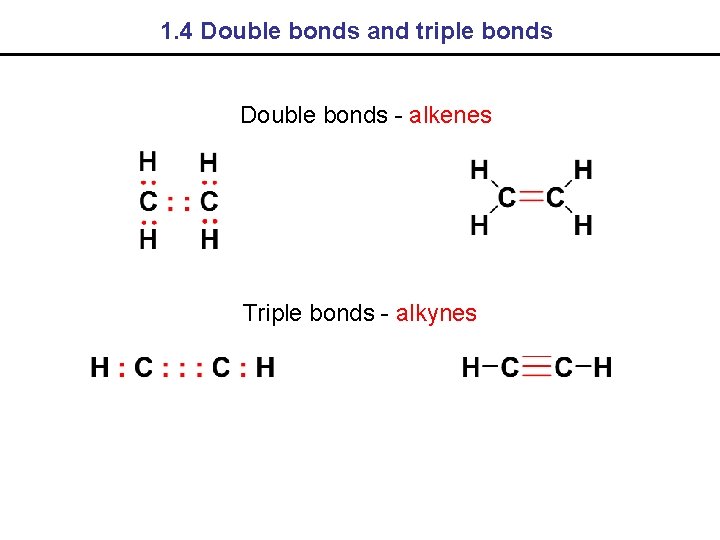
1. 4 Double bonds and triple bonds Double bonds - alkenes Triple bonds - alkynes
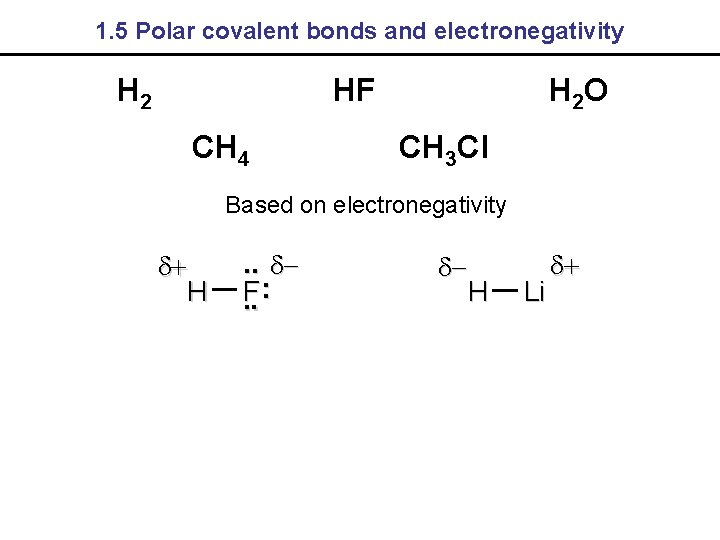
1. 5 Polar covalent bonds and electronegativity H 2 HF CH 4 H 2 O CH 3 Cl Based on electronegativity H . . F: . . H Li
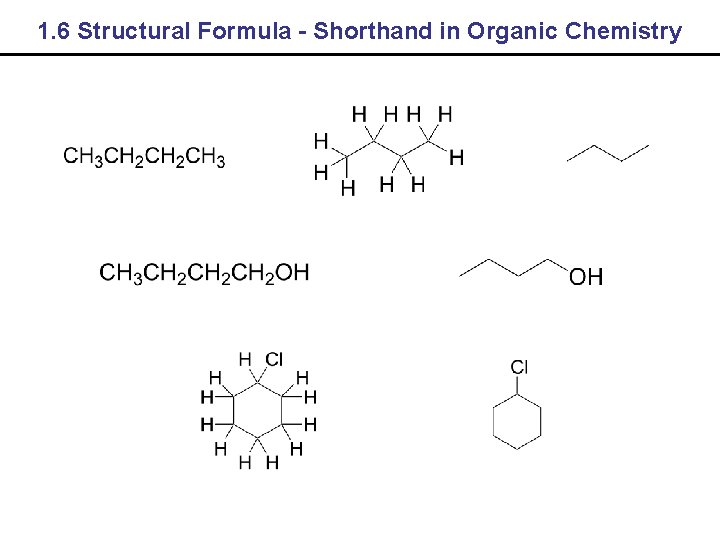
1. 6 Structural Formula - Shorthand in Organic Chemistry
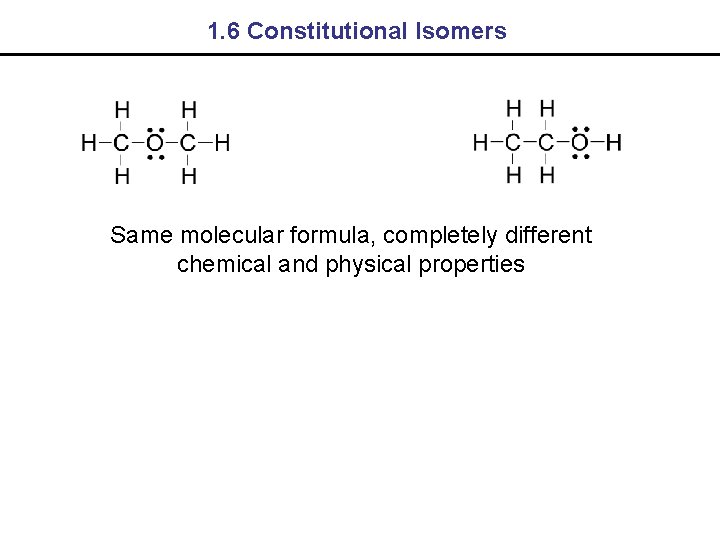
1. 6 Constitutional Isomers Same molecular formula, completely different chemical and physical properties
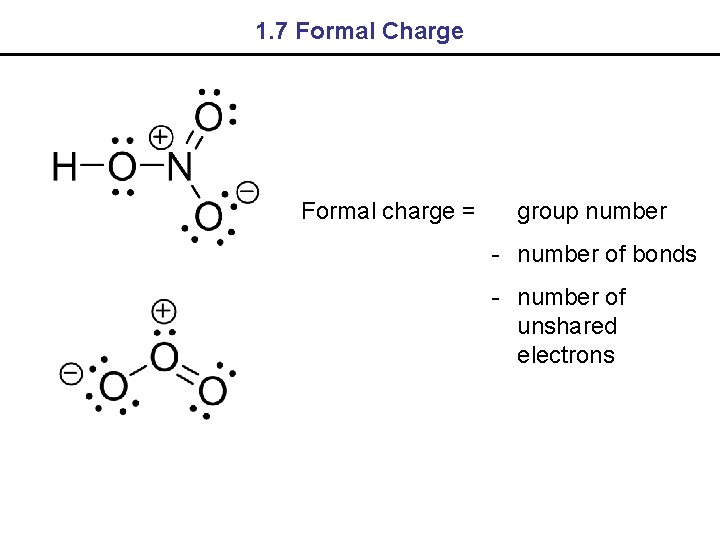
1. 7 Formal Charge Formal charge = group number - number of bonds - number of unshared electrons
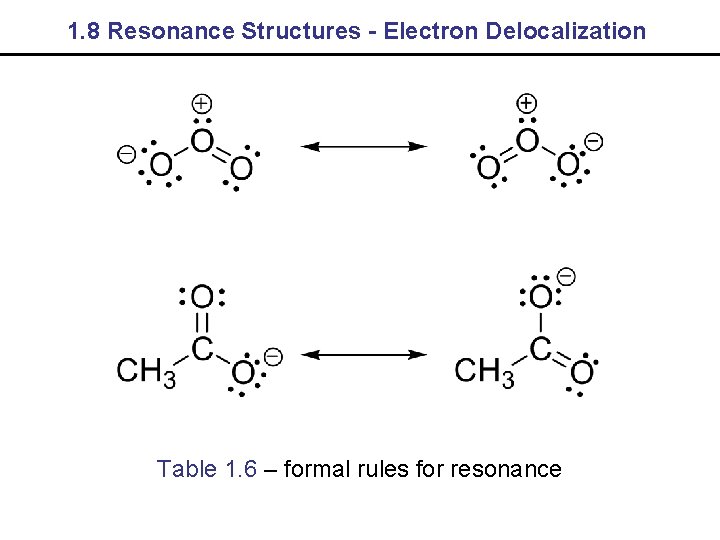
1. 8 Resonance Structures - Electron Delocalization Table 1. 6 – formal rules for resonance
1. 9 Shapes of Molecules Shapes of molecules are predicted using VSEPR theory
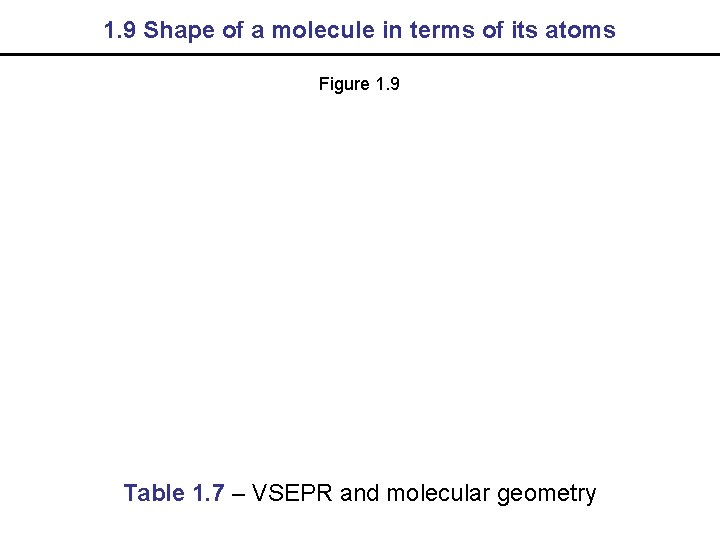
1. 9 Shape of a molecule in terms of its atoms Figure 1. 9 Table 1. 7 – VSEPR and molecular geometry

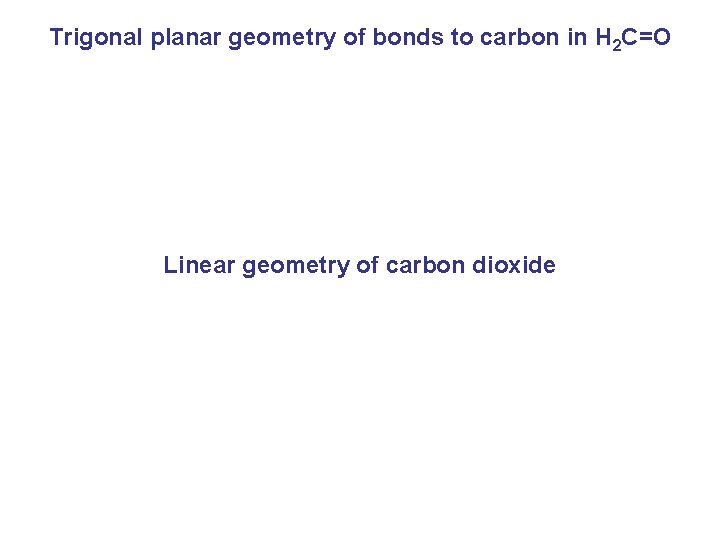
Trigonal planar geometry of bonds to carbon in H 2 C=O Linear geometry of carbon dioxide
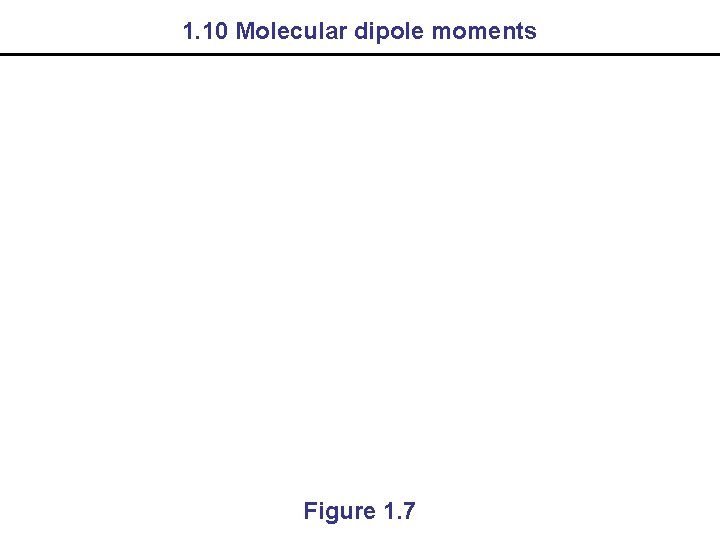
1. 10 Molecular dipole moments Figure 1. 7
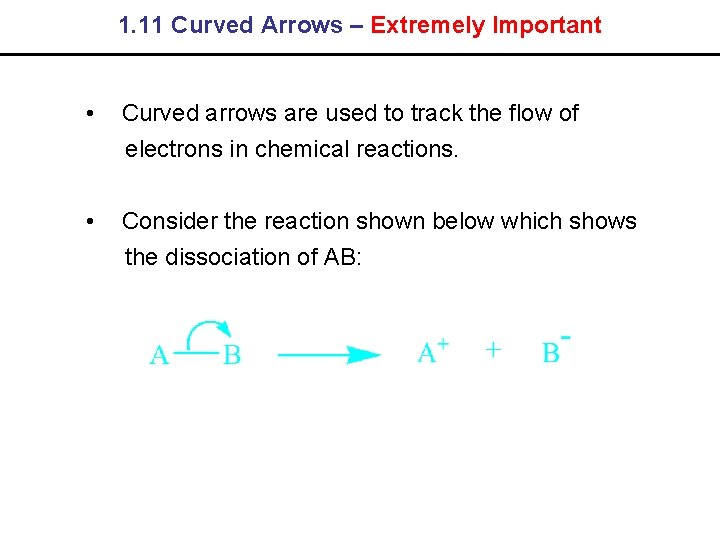
1. 11 Curved Arrows – Extremely Important • Curved arrows are used to track the flow of electrons in chemical reactions. • Consider the reaction shown below which shows the dissociation of AB:
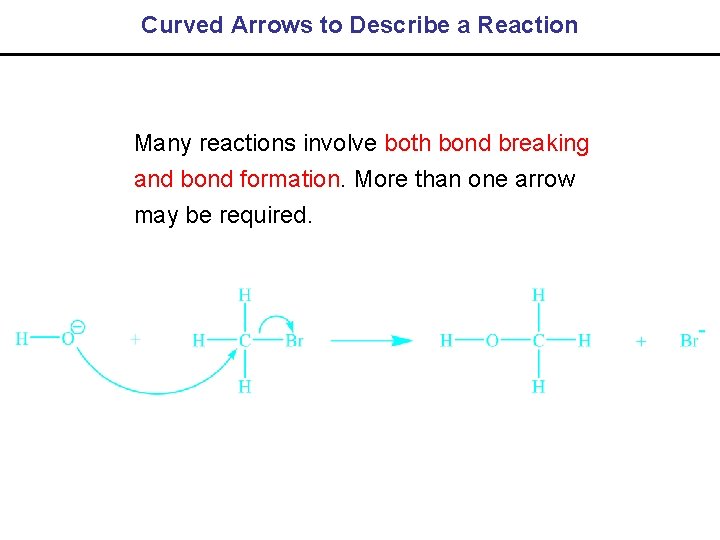
Curved Arrows to Describe a Reaction Many reactions involve both bond breaking and bond formation. More than one arrow may be required.

1. 12 Acids and Bases - Definitions Arrhenius An acid ionizes in water to give protons. A base ionizes in water to give hydroxide ions. Brønsted-Lowry An acid is a proton donor. A base is a proton acceptor. Lewis An acid is an electron pair acceptor. A base is an electron pair donor.
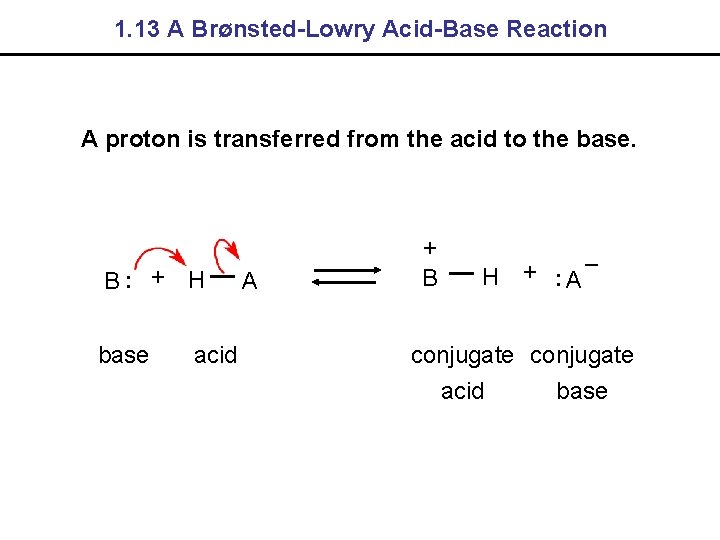
1. 13 A Brønsted-Lowry Acid-Base Reaction A proton is transferred from the acid to the base. B. . + H base acid A + B H +. . A – conjugate acid base
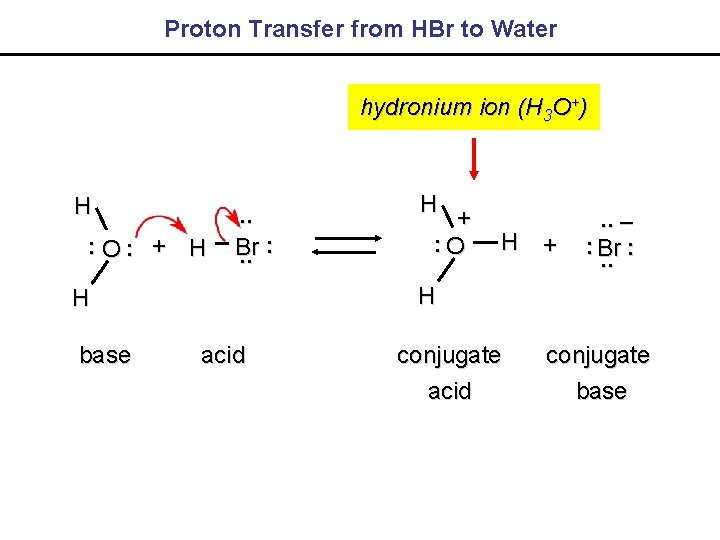
Proton Transfer from HBr to Water hydronium ion (H 3 O+) H. . O. . + H . . Br. . H + . . –. . Br. . H H base H +. . O acid conjugate base
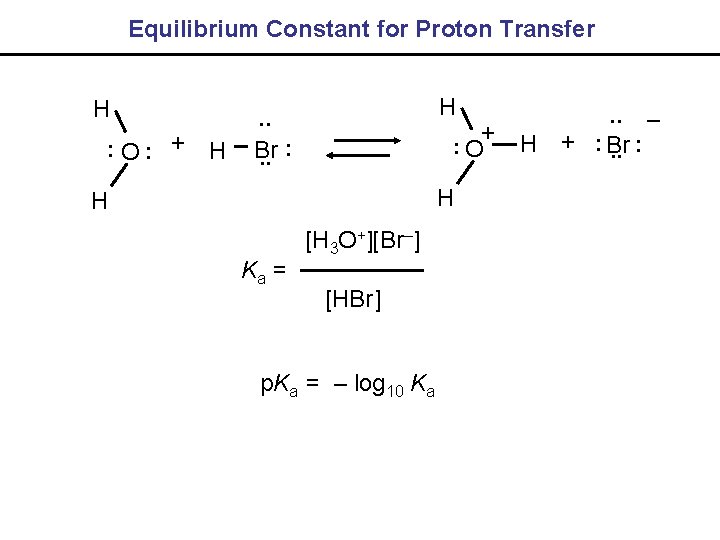
Equilibrium Constant for Proton Transfer H. . O. . + H H. . O+ . . Br. . H H Ka = [H 3 O+][Br–] [HBr] p. Ka = – log 10 Ka . . –. . H +. . Br. .
![Acids and Bases: Arrow Pushing Ka = [H 3 O+][Br–] [HBr] ~ 106 for Acids and Bases: Arrow Pushing Ka = [H 3 O+][Br–] [HBr] ~ 106 for](http://slidetodoc.com/presentation_image_h2/b91ff3095772a8894878a4f1ce3fb2ee/image-24.jpg)
Acids and Bases: Arrow Pushing Ka = [H 3 O+][Br–] [HBr] ~ 106 for HBr, p. Ka = - 5. 8


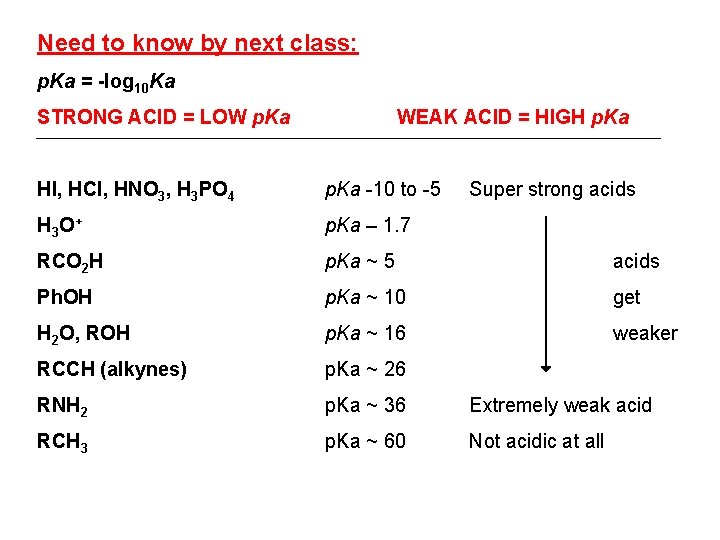
Need to know by next class: p. Ka = -log 10 Ka STRONG ACID = LOW p. Ka WEAK ACID = HIGH p. Ka HI, HCl, HNO 3, H 3 PO 4 p. Ka -10 to -5 Super strong acids H 3 O + p. Ka – 1. 7 RCO 2 H p. Ka ~ 5 acids Ph. OH p. Ka ~ 10 get H 2 O, ROH p. Ka ~ 16 weaker RCCH (alkynes) p. Ka ~ 26 RNH 2 p. Ka ~ 36 Extremely weak acid RCH 3 p. Ka ~ 60 Not acidic at all
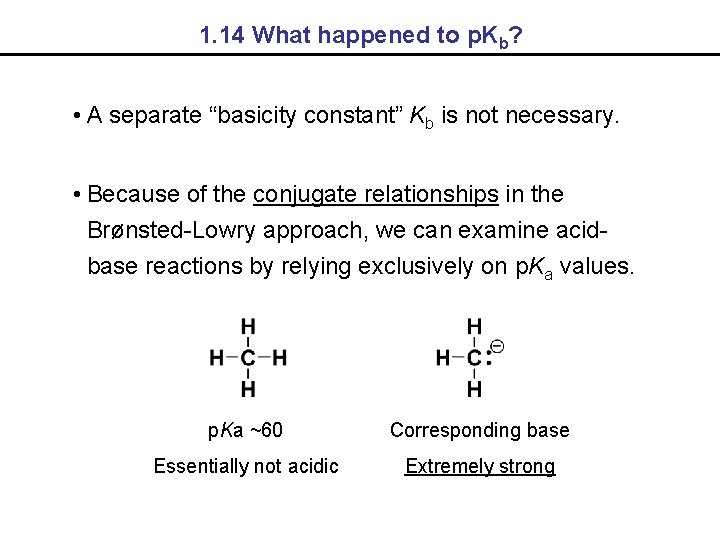
1. 14 What happened to p. Kb? • A separate “basicity constant” Kb is not necessary. • Because of the conjugate relationships in the Brønsted-Lowry approach, we can examine acidbase reactions by relying exclusively on p. Ka values. p. Ka ~60 Corresponding base Essentially not acidic Extremely strong
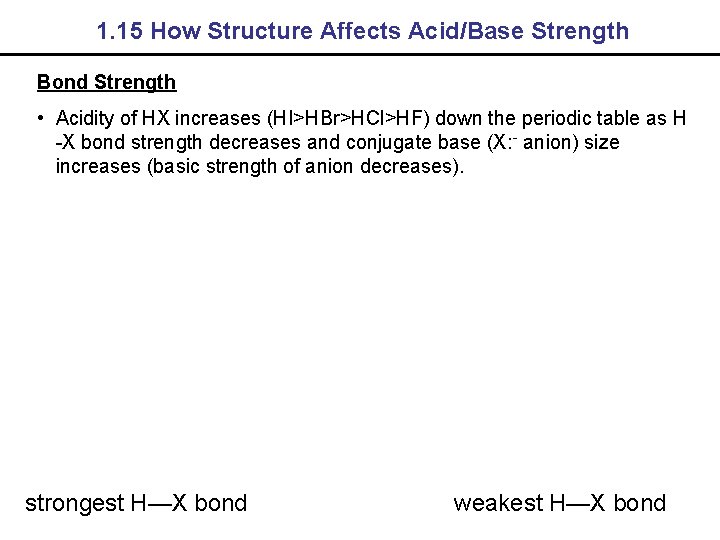
1. 15 How Structure Affects Acid/Base Strength Bond Strength • Acidity of HX increases (HI>HBr>HCl>HF) down the periodic table as H -X bond strength decreases and conjugate base (X: - anion) size increases (basic strength of anion decreases). strongest H—X bond weakest H—X bond
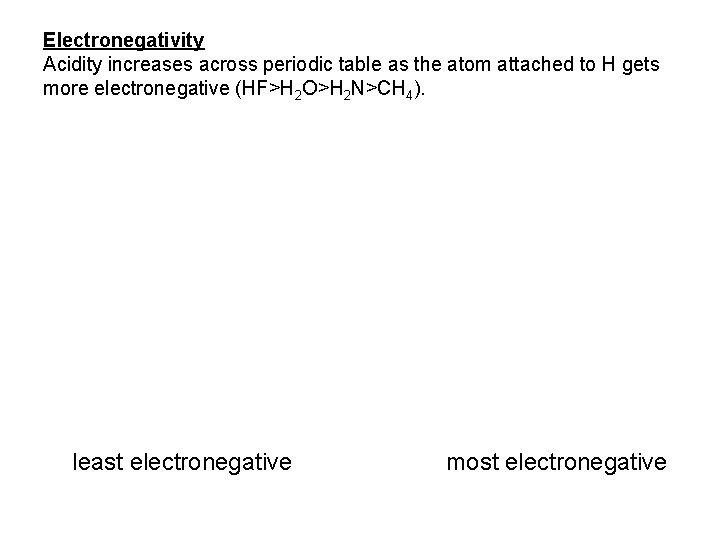
Electronegativity Acidity increases across periodic table as the atom attached to H gets more electronegative (HF>H 2 O>H 2 N>CH 4). least electronegative most electronegative
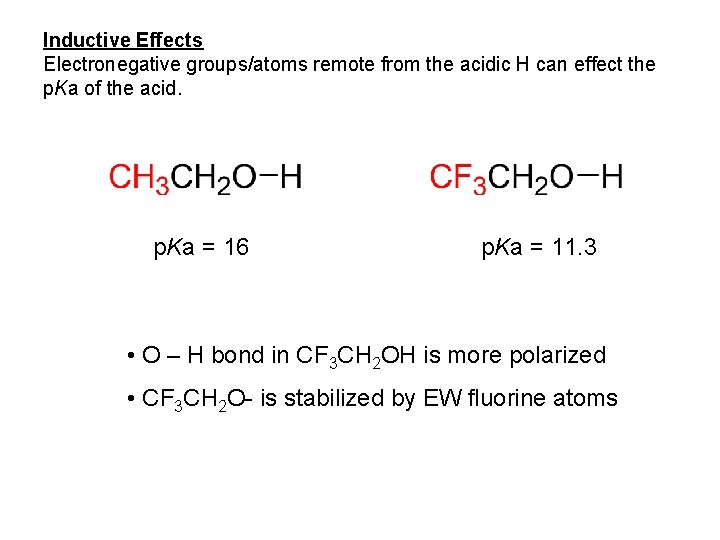
Inductive Effects Electronegative groups/atoms remote from the acidic H can effect the p. Ka of the acid. p. Ka = 16 p. Ka = 11. 3 • O – H bond in CF 3 CH 2 OH is more polarized • CF 3 CH 2 O- is stabilized by EW fluorine atoms
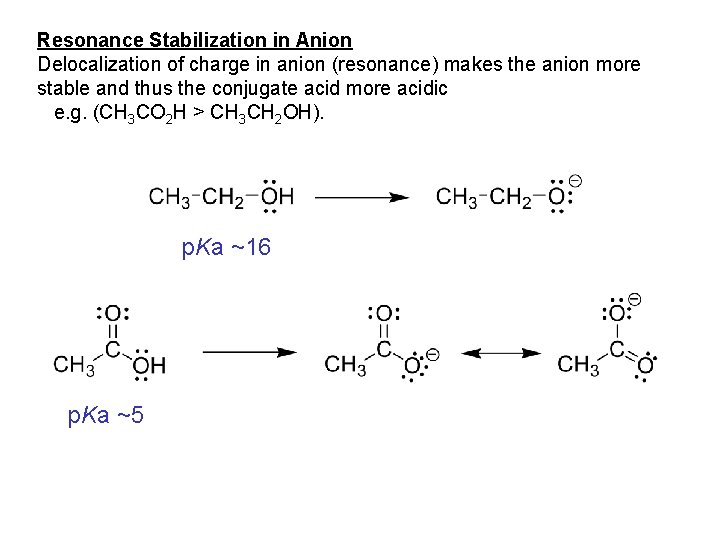
Resonance Stabilization in Anion Delocalization of charge in anion (resonance) makes the anion more stable and thus the conjugate acid more acidic e. g. (CH 3 CO 2 H > CH 3 CH 2 OH). p. Ka ~16 p. Ka ~5
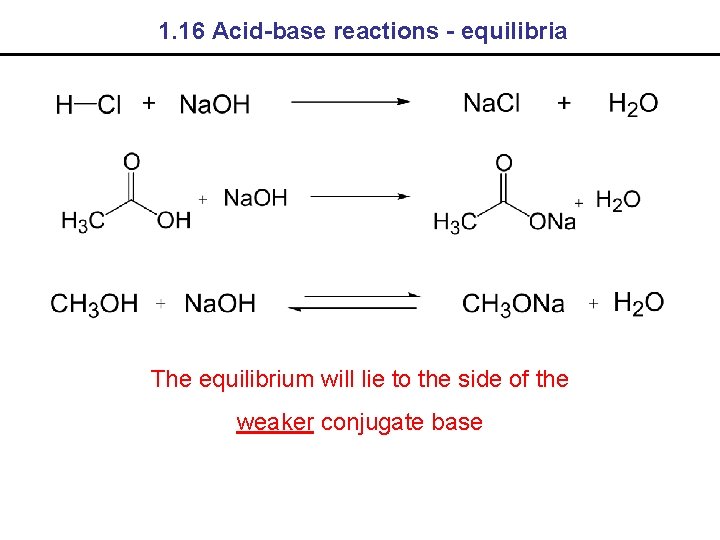
1. 16 Acid-base reactions - equilibria The equilibrium will lie to the side of the weaker conjugate base
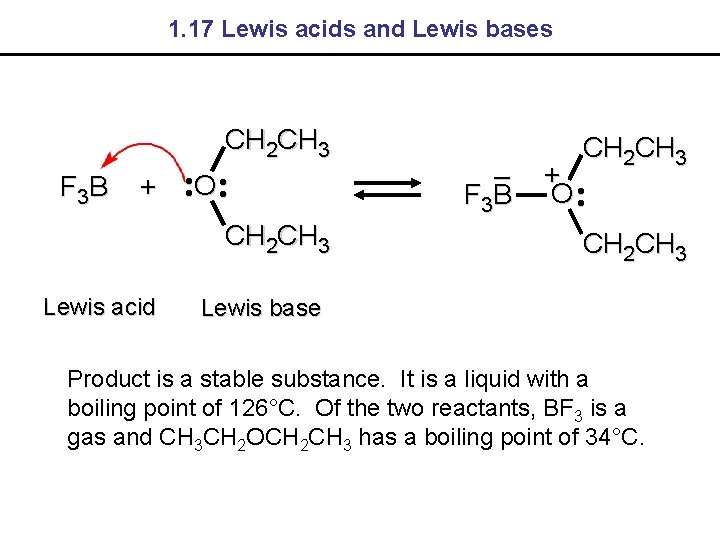
1. 17 Lewis acids and Lewis bases CH 2 CH 3 F 3 B + • • O • • CH 2 CH 3 Lewis acid – F 3 B CH 2 CH 3 + O • • CH 2 CH 3 Lewis base Product is a stable substance. It is a liquid with a boiling point of 126°C. Of the two reactants, BF 3 is a gas and CH 3 CH 2 OCH 2 CH 3 has a boiling point of 34°C.
- Slides: 34