1 14 6 2 18 10 15 7








- Slides: 21

1 14 6 2 18 10 15 7 3 19 11 16 8 4 17 9 12 20 WHACK-A-MOLE 13 5

1 What is the most important characteristic in determining an element’s chemical properties? Students type their answers here

2 Which block contains 5 orbitals? Students type their answers here

3 Which category of elements is commonly used to make computer chips and solar cells due to their ability to conduct electricity only under certain conditions? a. metals b. metalloids c. nonmetals d. noble gases Students type their answers here

4 What is the correct electron configuration for the element molybdenum (Mo)? Students type their answers here

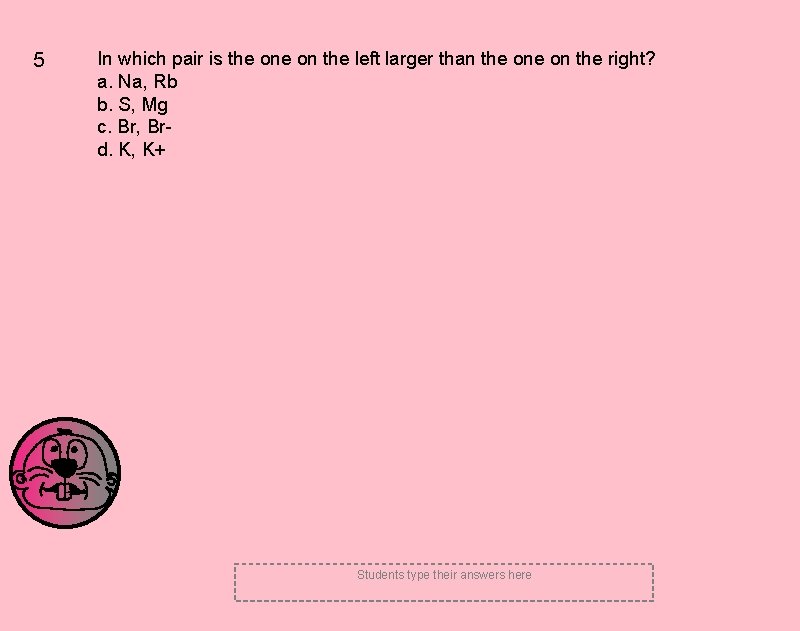
5 In which pair is the on the left larger than the on the right? a. Na, Rb b. S, Mg c. Br, Brd. K, K+ Students type their answers here

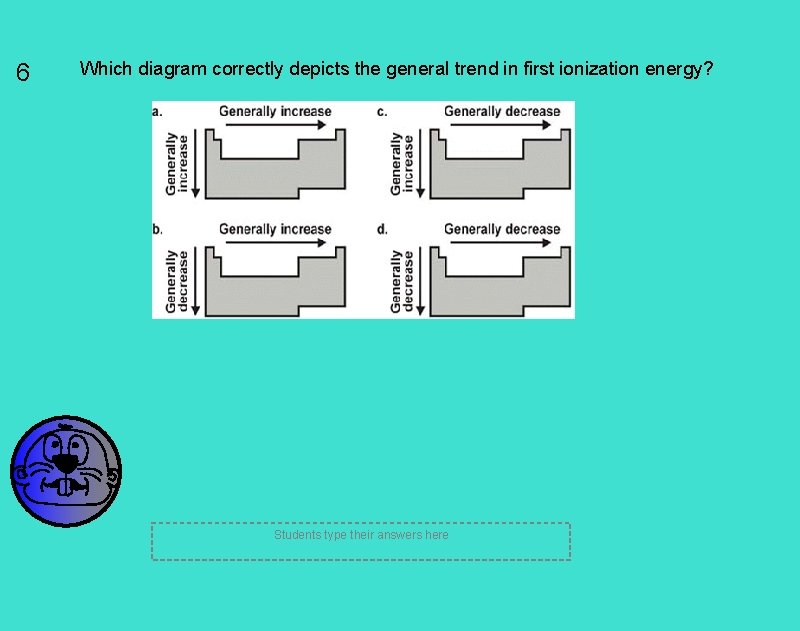
6 Which diagram correctly depicts the general trend in first ionization energy? Students type their answers here

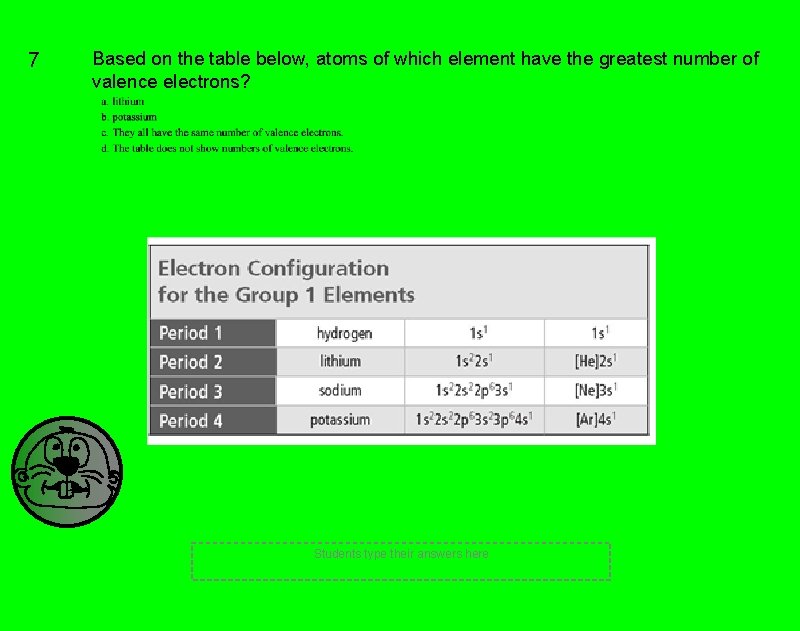
7 Based on the table below, atoms of which element have the greatest number of valence electrons? Students type their answers here

8 What is defined as the energy required to remove an electron from an atom of an element in the gaseous state? Students type their answers here

9 What element has the electron configuration 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5? Students type their answers here

10 What metalloid is in the fourth period and the same group as Carbon? Students type their answers here

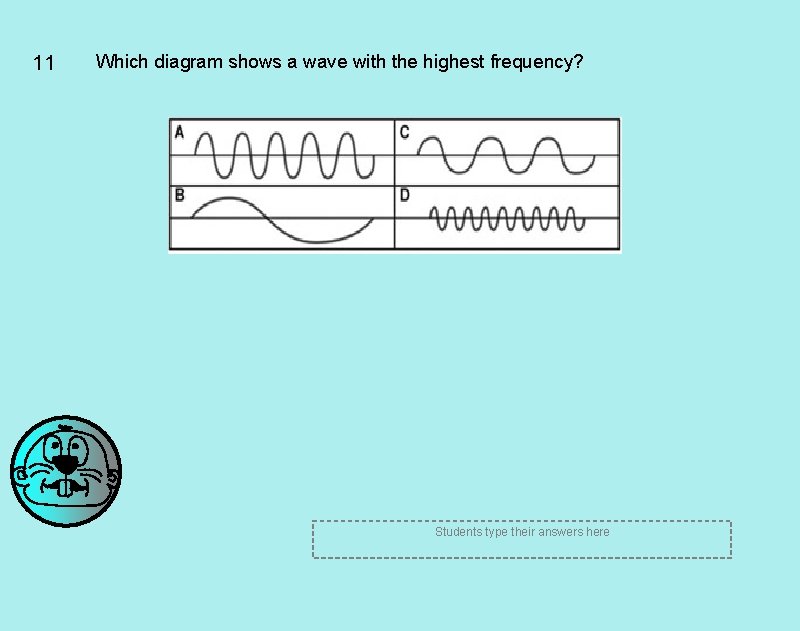
11 Which diagram shows a wave with the highest frequency? Students type their answers here

12 Use Plank’s constant, 6. 626 × 10 -34 J-s, to solve the following: What is the amount of energy carried by a photon that has a frequency of 5. 71 × 1014 Hz? Students type their answers here

13 Why do elements in the same group have similar properties? Students type their answers here

14 How many electrons maximum are present in the third energy level of an atom? Students type their answers here

15 Identify the element having the largest size and the element having the highest electronegativity from the list below: Fe Co Ni Ba Students type their answers here

16 What is the energy of a photon if it has a frequency of 4. 57 × 1025 s– 1

17 Write the electron configuration of zinc. Students type their answers here

18 Write the electron configuration of strontium.

19 Write the electron configuration of krypton.

20 What is the difference between the Bohr and quantum mechanical model of the atom? Students type their answers here