1 1 Periods Periodic Table of the Elements
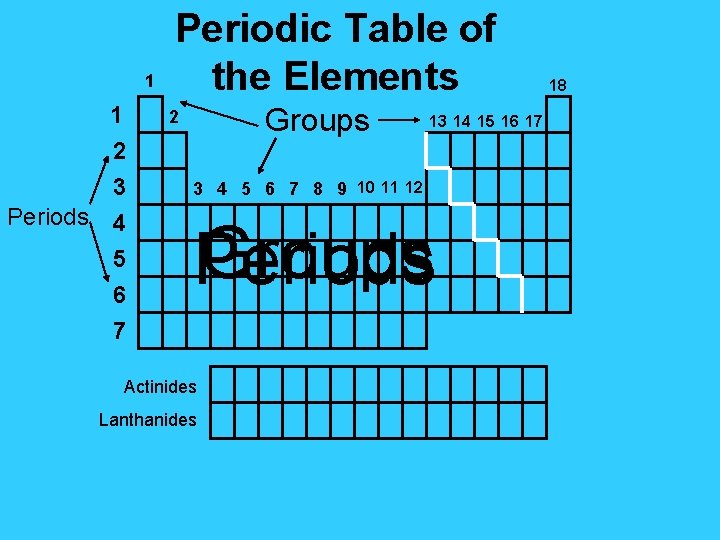
1 1 Periods Periodic Table of the Elements Groups 2 2 3 4 5 6 7 13 14 15 16 17 3 4 5 6 7 8 9 10 11 12 Groups Periods Actinides Lanthanides 18
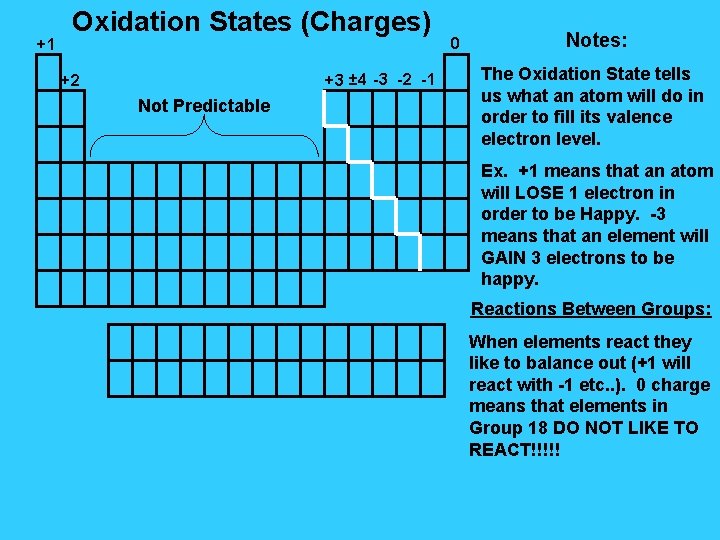
+1 Oxidation States (Charges) +3 ± 4 -3 -2 -1 +2 Not Predictable 0 Notes: The Oxidation State tells us what an atom will do in order to fill its valence electron level. Ex. +1 means that an atom will LOSE 1 electron in order to be Happy. -3 means that an element will GAIN 3 electrons to be happy. Reactions Between Groups: When elements react they like to balance out (+1 will react with -1 etc. . ). 0 charge means that elements in Group 18 DO NOT LIKE TO REACT!!!!!
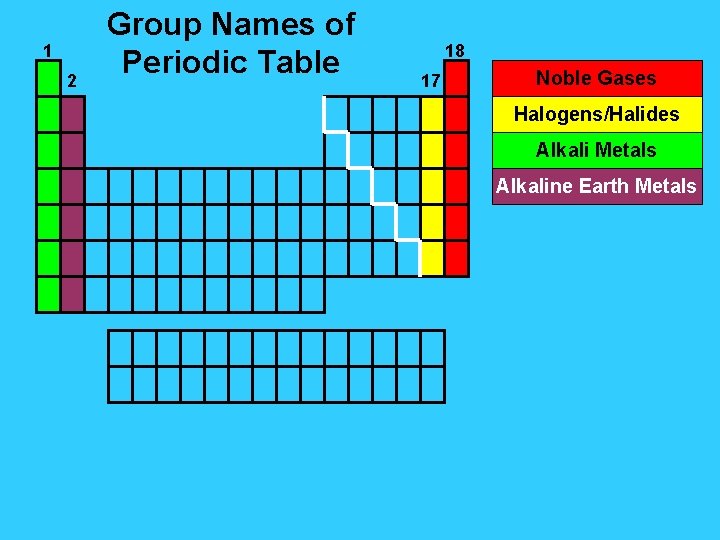
1 2 Group Names of Periodic Table 18 17 Noble Gases Halogens/Halides Alkali Metals Alkaline Earth Metals
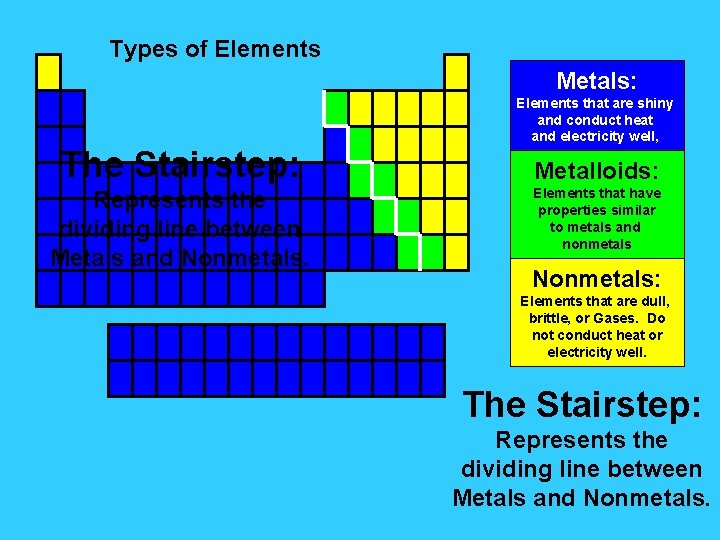
Types of Elements Metals: The Stairstep: Represents the dividing line between Metals and Nonmetals. Elements that are shiny and conduct heat and electricity well, Metalloids: Elements that have properties similar to metals and nonmetals Nonmetals: Elements that are dull, brittle, or Gases. Do not conduct heat or electricity well. The Stairstep: Represents the dividing line between Metals and Nonmetals.
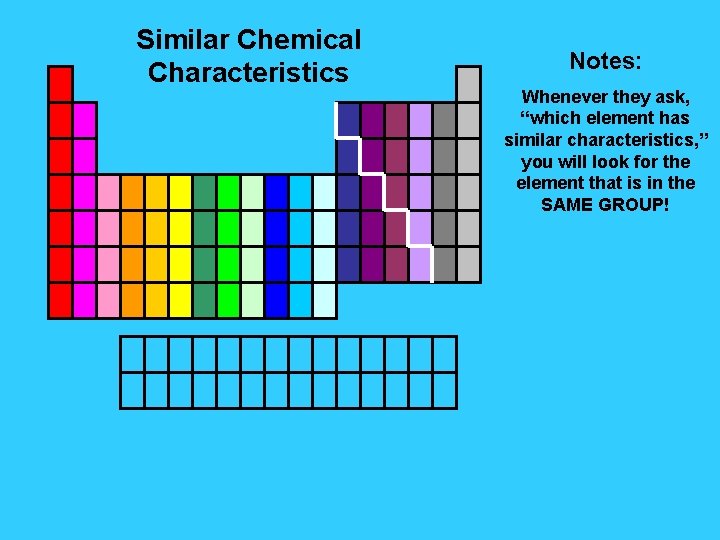
Similar Chemical Characteristics Notes: Whenever they ask, “which element has similar characteristics, ” you will look for the element that is in the SAME GROUP!
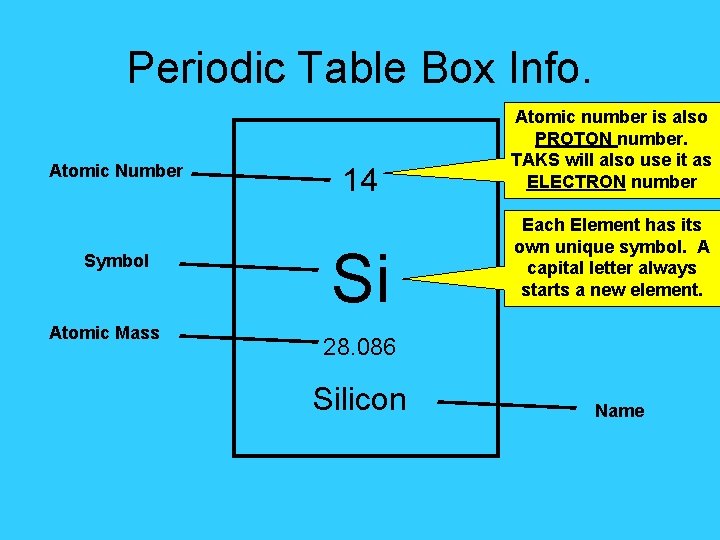
Periodic Table Box Info. Atomic Number Symbol Atomic Mass 14 Si Atomic number is also PROTON number. TAKS will also use it as ELECTRON number Each Element has its own unique symbol. A capital letter always starts a new element. 28. 086 Silicon Name
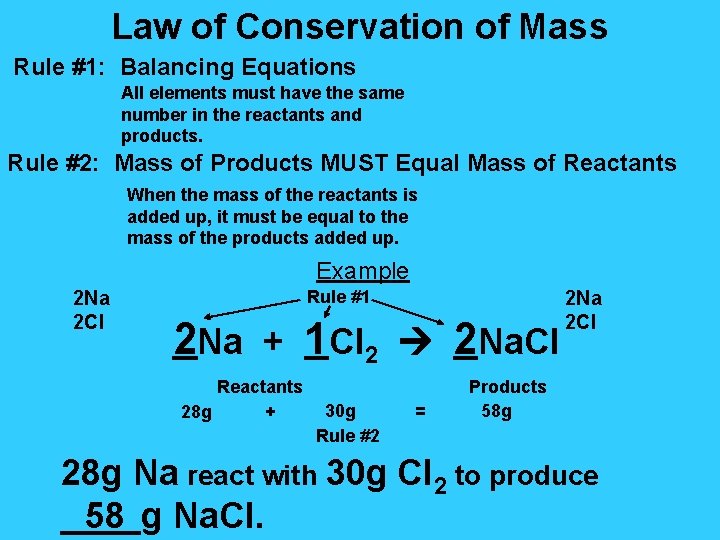
Law of Conservation of Mass Rule #1: Balancing Equations All elements must have the same number in the reactants and products. Rule #2: Mass of Products MUST Equal Mass of Reactants When the mass of the reactants is added up, it must be equal to the mass of the products added up. Example 2 Na 2 Cl Rule #1 2 Na + 1 Cl 2 2 Na. Cl Reactants 28 g + 30 g Rule #2 = 2 Na 2 Cl Products 58 g 28 g Na react with 30 g Cl 2 to produce 58 Na. Cl. ____g
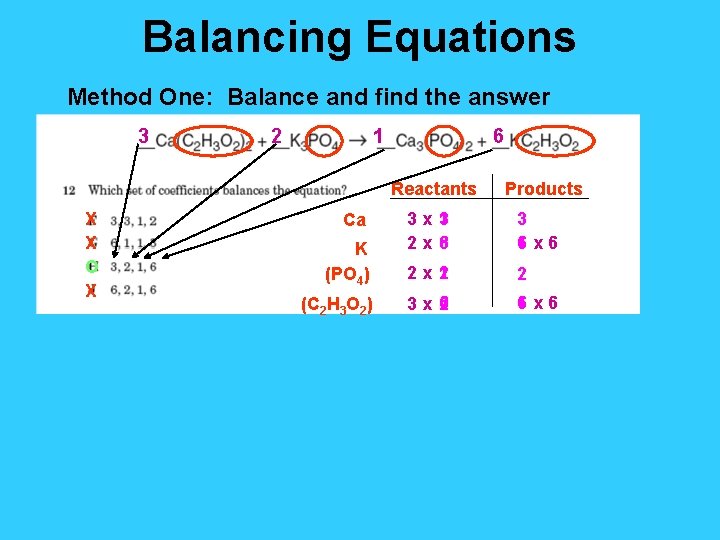
Balancing Equations Method One: Balance and find the answer 3 2 1 6 Reactants X X O X Ca K (PO 4) (C 2 H 3 O 2) Products 3 x 1 3 2 x 6 3 3 6 x 6 1 2 x 2 1 2 6 3 x 2 6 x 6 1
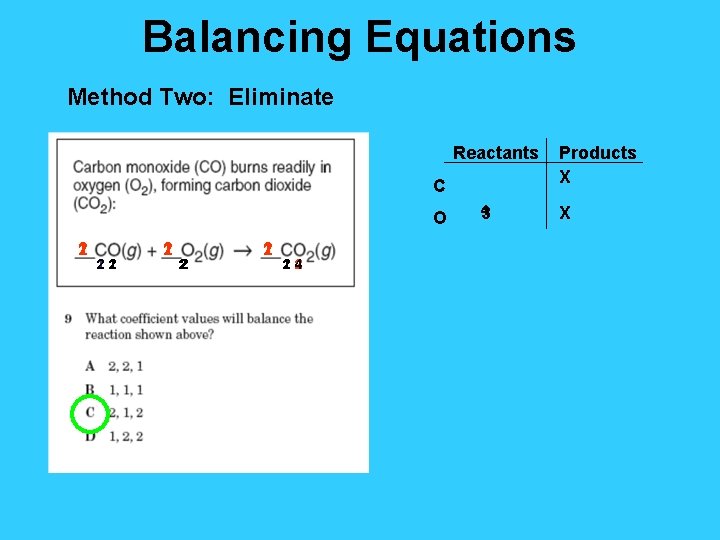
Balancing Equations Method Two: Eliminate Reactants C O 1 2 12 2 1 22 1 2 14 2 2 4 3 Products X X
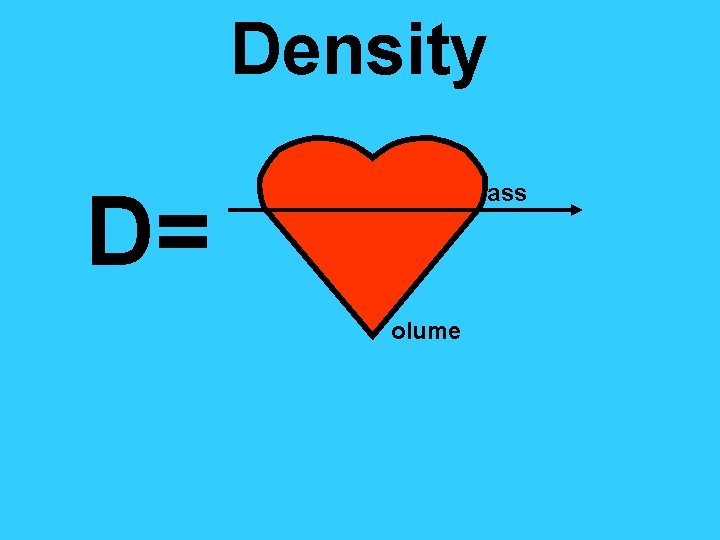
Density D= ass olume
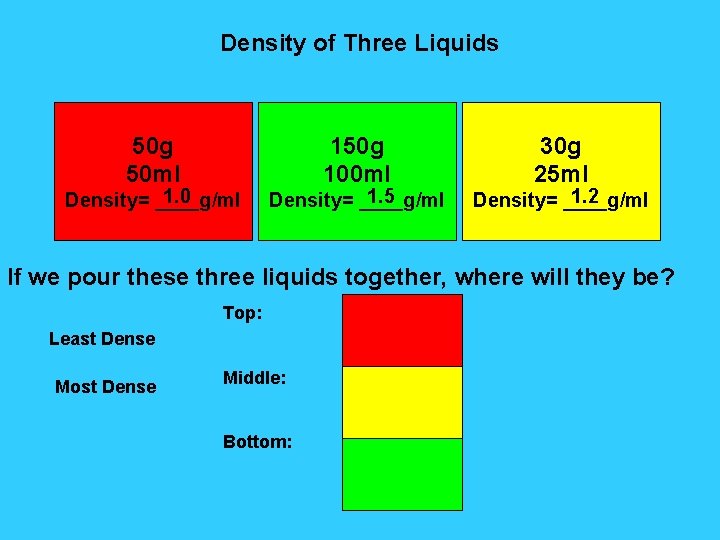
Density of Three Liquids 50 g 50 ml 1. 0 Density= ____g/ml 150 g 100 ml 1. 5 Density= ____g/ml 30 g 25 ml 1. 2 Density= ____g/ml If we pour these three liquids together, where will they be? Top: Least Dense Most Dense Middle: Bottom:
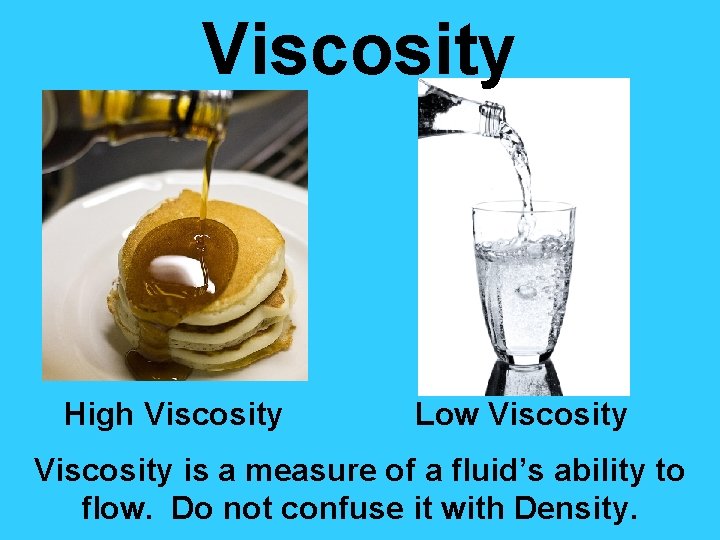
Viscosity High Viscosity Low Viscosity is a measure of a fluid’s ability to flow. Do not confuse it with Density.

Physical vs. Chemical Change Physical Change – A change that does not result in a new chemical. Chemical Change – A change that does result in a new chemical. What type of change is this? Chemical Physical
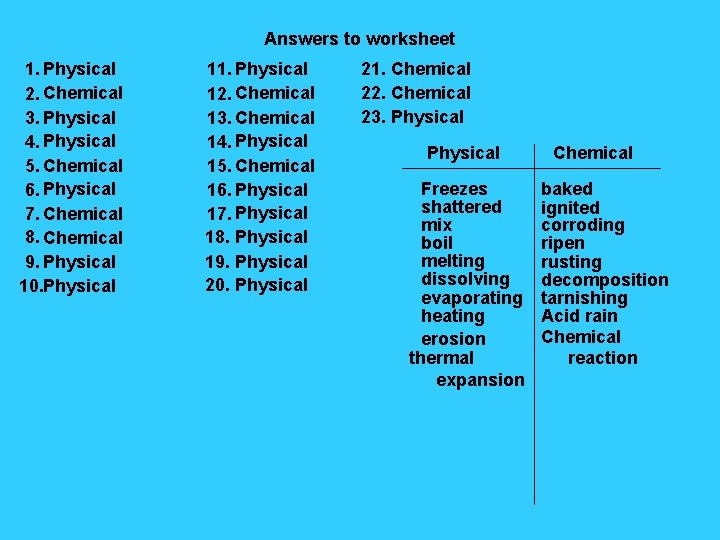
Answers to worksheet 1. Physical 2. Chemical 3. Physical 4. Physical 5. Chemical 6. Physical 7. Chemical 8. Chemical 9. Physical 10. Physical 11. Physical 12. Chemical 13. Chemical 14. Physical 15. Chemical 16. Physical 17. Physical 18. Physical 19. Physical 20. Physical 21. Chemical 22. Chemical 23. Physical Freezes shattered mix boil melting dissolving evaporating heating erosion thermal expansion Chemical baked ignited corroding ripen rusting decomposition tarnishing Acid rain Chemical reaction
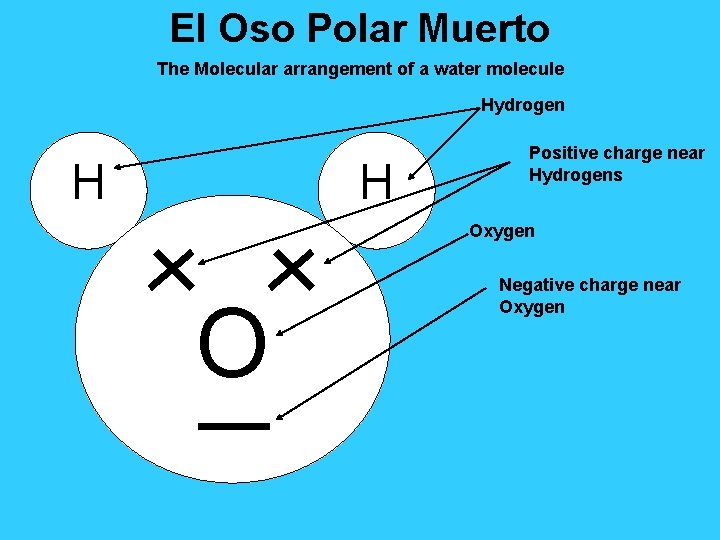
El Oso Polar Muerto The Molecular arrangement of a water molecule Hydrogen H H Positive charge near Hydrogens Oxygen O Negative charge near Oxygen

Water is special… 1. 00 1. The density of water is _____g/ml decreases 2. When water freezes, its density _________. This allows aquatic life Survive to _________ in a frozen lake. Molecular Arrangement 3. Water is unique because of its ________________ Polar 4. Water can dissolve many substances because it is ____________ slowly 5. Water has a very high specific heat. Water will heat up ______ and cool slowly down ______ compared to other substances.

Solubility is the ability of a substance to dissolve. Three factors affect solubility. 1. Surface area- The more surface area a solute has the faster it will dissolve. Sugar cube dissolves slowly while powdered sugar will dissolve quickly. 2. Agitation- Solids: The more you agitate (stir or shake) a solution, the quicker the solute will dissolve. Gases: More shake, less dissolve. 3. Temperature- Solids: The higher the temperature of solvent, the more the solute will dissolve. Gases: More temperature, less dissolve.
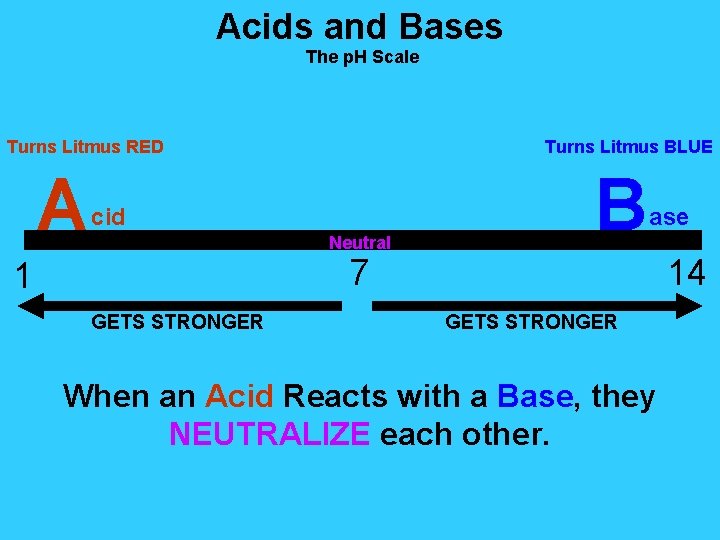
Acids and Bases The p. H Scale Turns Litmus RED A Turns Litmus BLUE cid Neutral B ase 7 1 GETS STRONGER 14 GETS STRONGER When an Acid Reacts with a Base, they NEUTRALIZE each other.
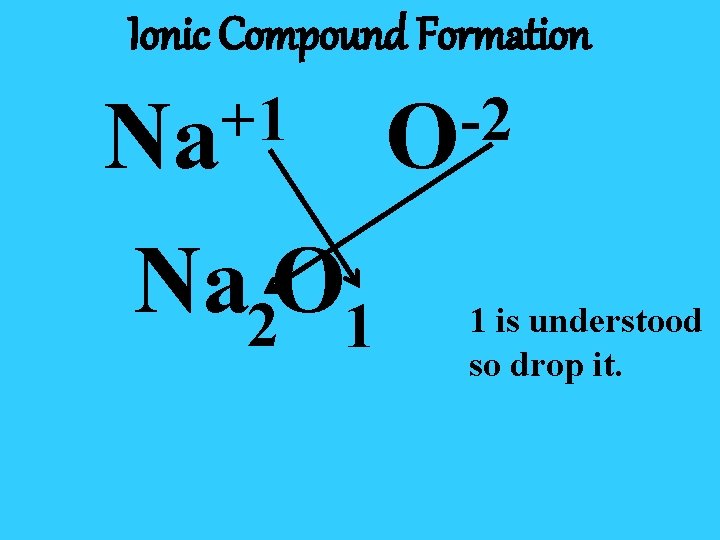
Ionic Compound Formation +1 Na Na 2 O 1 -2 O 1 is understood so drop it.
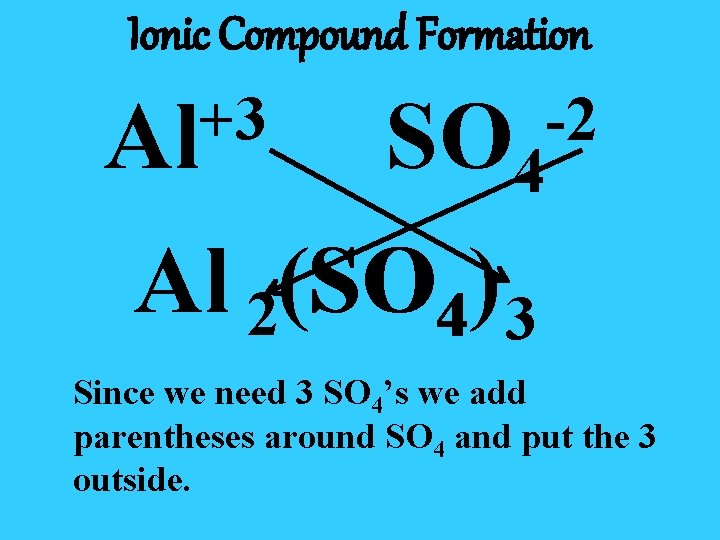
Ionic Compound Formation +3 Al -2 SO 4 Al 2(SO SO 4) 3 Since we need 3 SO 4’s we add parentheses around SO 4 and put the 3 outside.
- Slides: 20