1 1 Atomic Structure Objectives 1 2 Essential

1. 1 Atomic Structure Objectives 1; 2

Essential Questions What is an atom? n What is atomic # and how do you determine it? n How is atomic # to the identify an element? n What is mass # and how do you determine it? n What is the difference between atomic mass and mass number? n What is an isotope? n How do you determine the number of protons, neutrons, and electrons for an isotope? n
Elements and Atoms n An element is the smallest particle of matter that retains its chemical identity We use symbols for the names of elements n 1 or 2 letters, the first letter always is Capital and the second is lowercase n n An atom is the smallest particle of an element that retains its identity in a chemical reaction.

Parts of the Atom There are three subatomic particles that make up atoms: proton, neutron, and electron n Protons are positively charged particles found in the nucleus n Neutrons are neutral particles found in the nucleus n Electrons are negative particles found in orbitals around the nucleus n
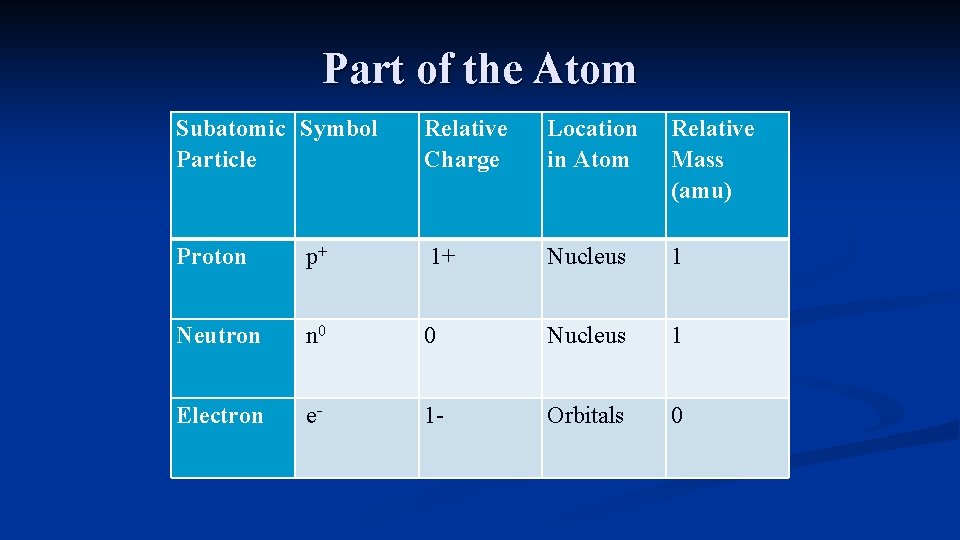
Part of the Atom Subatomic Symbol Particle Relative Charge Location in Atom Relative Mass (amu) Proton p+ 1+ Nucleus 1 Neutron n 0 0 Nucleus 1 Electron e- 1 - Orbitals 0

Atomic Number Atomic number is the number of protons. n How do you find the atomic number? n n n How do we use atomic number? n n Periodic table Identifies the type of element because each element has its own unique atomic number The atomic number of a neutral atom is equal to number of electrons

Mass Number Mass number is the total number of protons and neutrons in an atom (mass number = # of protons + # of neutrons) n Mass number can be used to find the number of neutrons n n # of neutrons = Mass number - # of protons
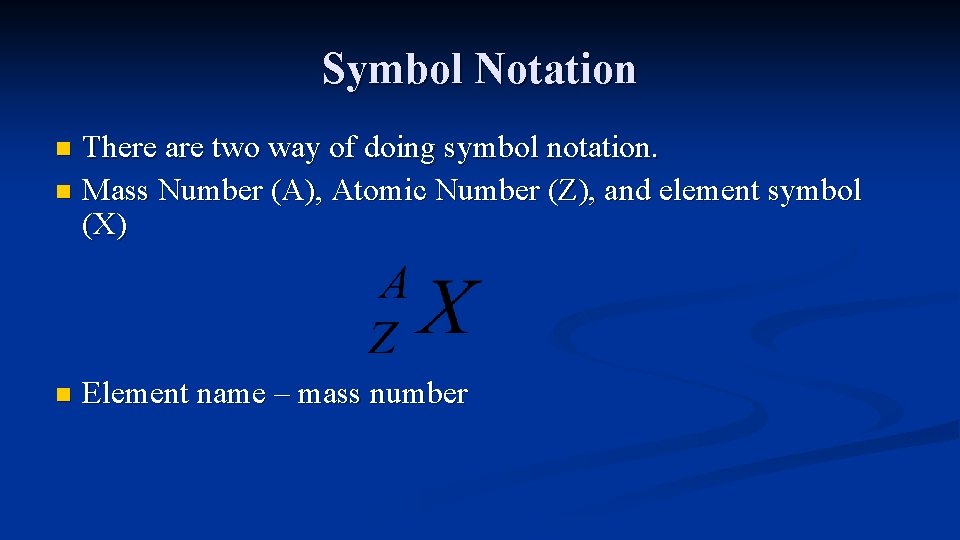
Symbol Notation There are two way of doing symbol notation. n Mass Number (A), Atomic Number (Z), and element symbol (X) n n Element name – mass number
Isotopes n Isotopes are atoms of the same element that have different mass numbers n Carbon – 14 and carbon – 12 The protons and electrons (if neutral) will be the same for isotopes of the same element n The neutrons and mass number will be different for isotopes of the same element n

Isotopes (cont) Element Proton Neutron Electron 1 26 30 26 2 25 30 25 3 27 32 26 4 26 29 25 n Which of the above would be isotopes of the same element? 1 and 4 n Which would have the same mass number? 2 and 4
Atomic Mass The atomic mass is an average mass based off of the abundance and mass of the different isotopes and is found on the periodic table. n amu – atomic mass unit n When the atomic mass is rounded to the nearest whole number that is the most common isotope of the element n n Tc atomic mass is 97. 9 so a mass number of 98 is the most common isotope

Protons, Neutrons, and Electrons Determine the number of p, n, and en Cobalt – 58 n Barium – 138 n 204 Tl n 227 Ac n In (round the mass to the most common isotope) n Yttrium n
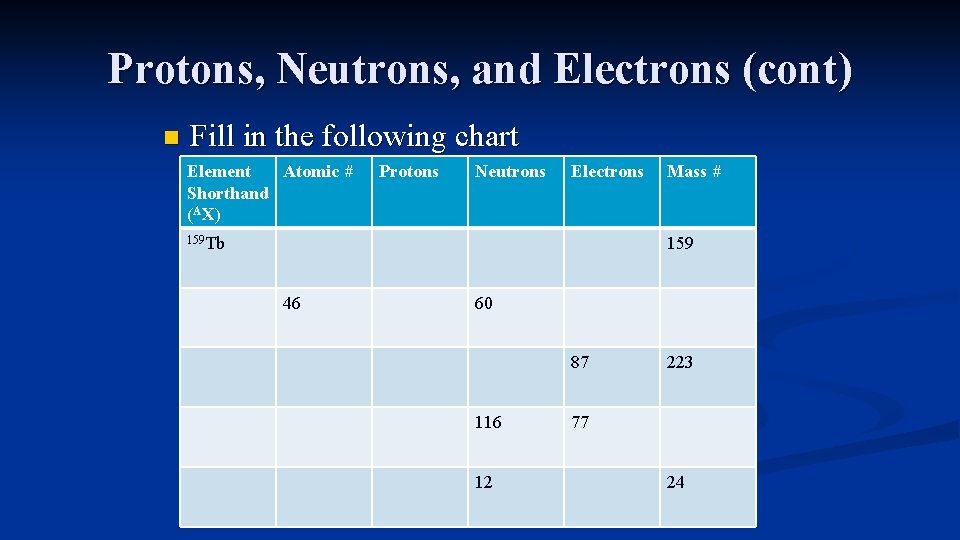
Protons, Neutrons, and Electrons (cont) n Fill in the following chart Element Atomic # Shorthand (AX) Protons Neutrons Electrons 159 Tb Mass # 159 46 60 87 116 12 223 77 24
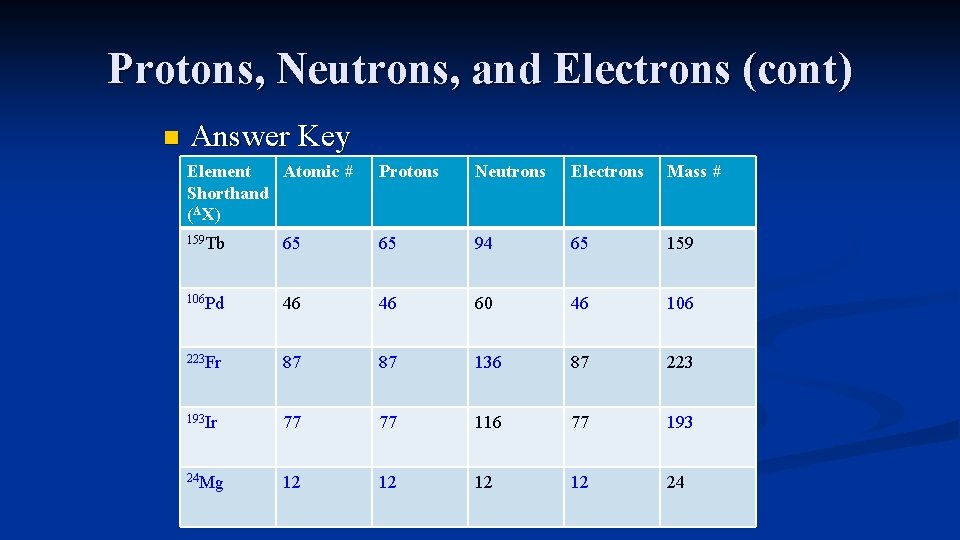
Protons, Neutrons, and Electrons (cont) n Answer Key Element Atomic # Shorthand (AX) Protons Neutrons Electrons Mass # 159 Tb 65 65 94 65 159 106 Pd 46 46 60 46 106 223 Fr 87 87 136 87 223 193 Ir 77 77 116 77 193 24 Mg 12 12 24
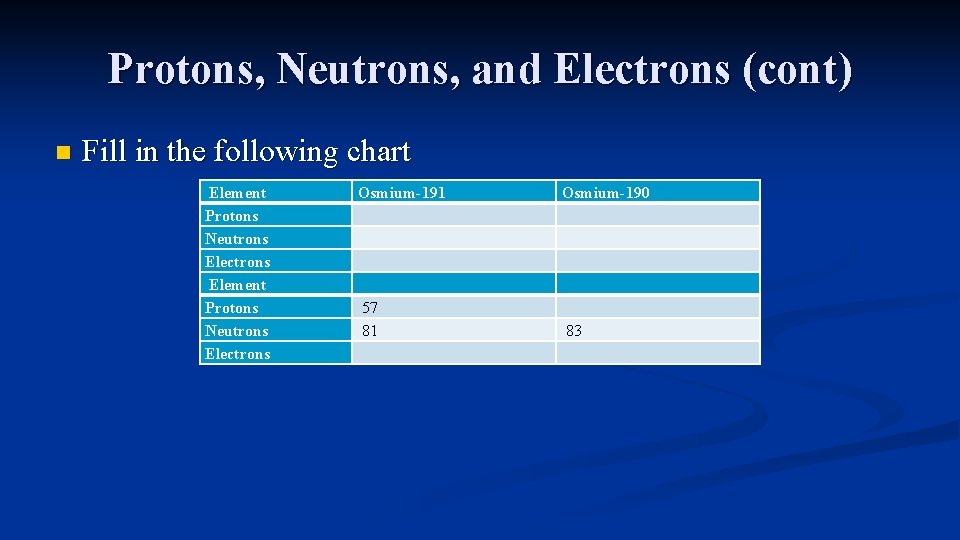
Protons, Neutrons, and Electrons (cont) n Fill in the following chart Element Protons Neutrons Electrons Osmium-191 Osmium-190 57 81 83
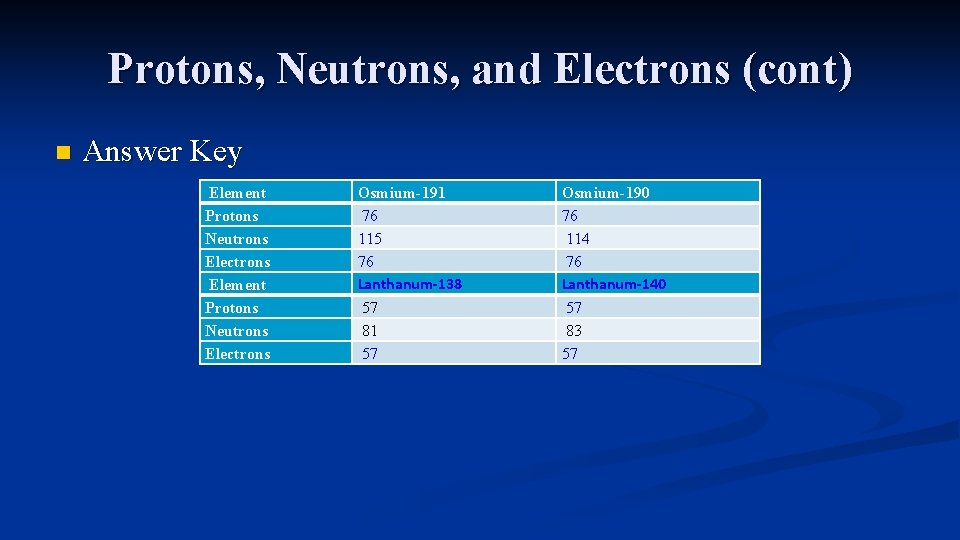
Protons, Neutrons, and Electrons (cont) n Answer Key Element Protons Neutrons Electrons Osmium-191 76 115 76 Lanthanum-138 57 81 57 Osmium-190 76 114 76 Lanthanum-140 57 83 57

Essential Questions What is an atom? n What is atomic # and how do you determine it? n How is atomic # to the identify an element? n What is mass # and how do you determine it? n What is the difference between atomic mass and mass number? n What is an isotope? n How do you determine the number of protons, neutrons, and electrons for an isotope? n

1. 1 Tracked Assignment n 1. 1 Tracked Assignment Worksheet
- Slides: 18