09012022 Measurements Lesson 1 Prefixes Scientific Notation and












- Slides: 17

09/01/2022 Measurements Lesson 1: Prefixes, Scientific Notation and Significant Figures Page 7 of Higher Physics: An Overview

What are we learning today? �What is meant by the SI system of units. �How to use prefixes and scientific notation. �The rules for using significant figures.

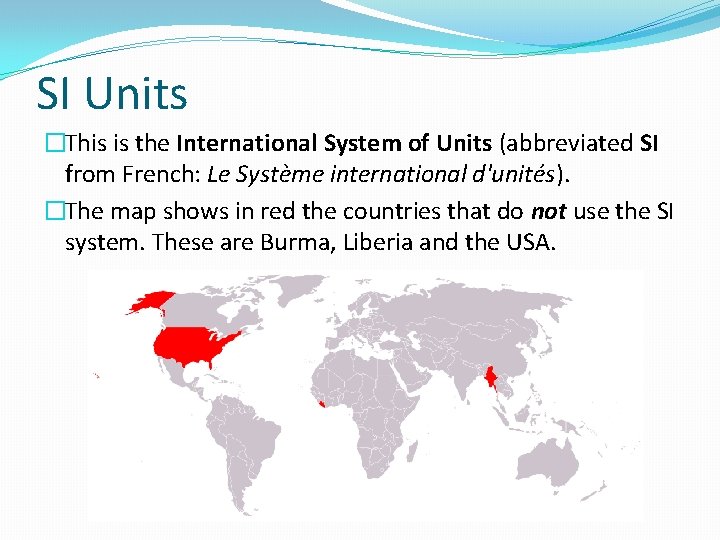
SI Units �This is the International System of Units (abbreviated SI from French: Le Système international d'unités). �The map shows in red the countries that do not use the SI system. These are Burma, Liberia and the USA.

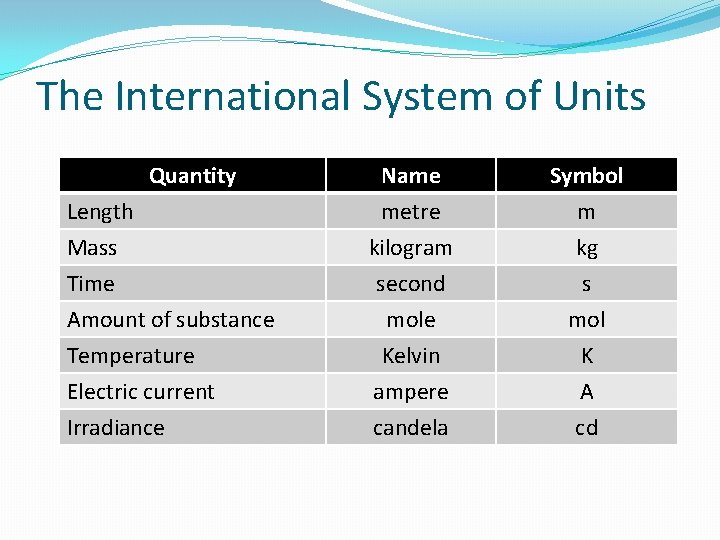
The International System of Units Quantity Length Mass Time Name metre kilogram second Symbol m kg s Amount of substance Temperature Electric current Irradiance mole Kelvin ampere candela mol K A cd

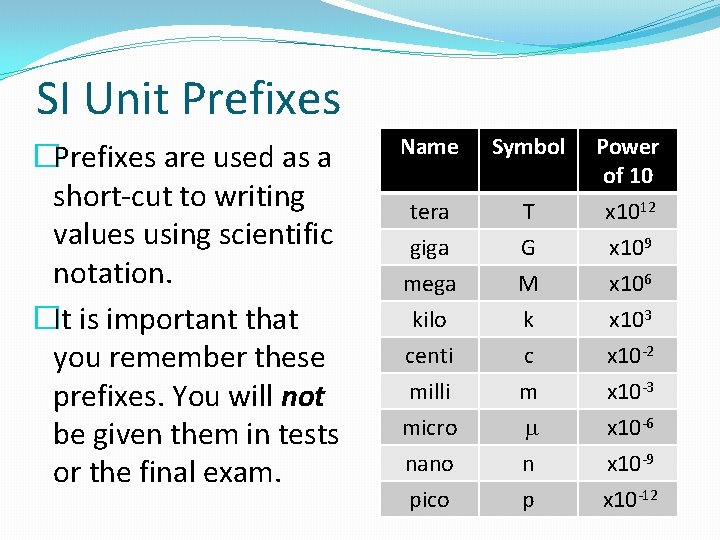
SI Unit Prefixes �Prefixes are used as a short-cut to writing values using scientific notation. �It is important that you remember these prefixes. You will not be given them in tests or the final exam. Name Symbol Power of 10 tera giga T G x 1012 x 109 mega kilo centi milli micro nano pico M k c m m n p x 106 x 103 x 10 -2 x 10 -3 x 10 -6 x 10 -9 x 10 -12

Scientific Notation �Measuring physical phenomena in the real world we encounter numbers of large magnitude. For example, the distance to quasar 3 C 273 is 2. 443 Gly which is 231127344000000000 m. �The time taken for a photon of light to cross the nucleus of an atom is 0. 000000000001 s.

�Performing calculations with such complicated numbers is impractical. We need a 'short-hand' method for writing these numbers. �For this we use scientific notation where we write the number of zeros as the index of a power of 10. ie 105

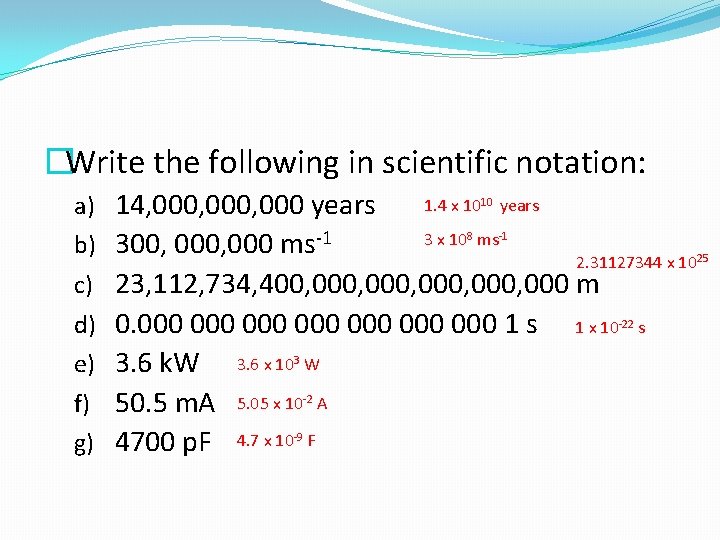
�Write the following in scientific notation: a) 14, 000, 000 years b) 300, 000 ms-1 1. 4 x 1010 years 3 x 108 ms-1 2. 31127344 x 1025 c) 23, 112, 734, 400, 000, 000 m d) 0. 000 000 1 s e) 3. 6 k. W 3. 6 x 103 W f) 50. 5 m. A 5. 05 x 10 -2 A g) 4700 p. F 4. 7 x 10 -9 F 1 x 10 -22 s

Unit 0 Tutorial 1

Significant Figures �The significant figures of a measured or calculated quantity are the meaningful digits in it. �There are rules which you should learn and follow for how to express numbers so as to properly indicate their significant figures.

The Rules: Rule 1: �Any digit that is not zero is significant. �So, 642 has three significant figures and 6. 792 has four. Rule 2: �Zeros between non zero digits are significant. �So, 4023 has four significant figures.

Rule 3: �Zeros to the left of the first non zero digit are not significant. �So, 0. 000072 has only two significant figures. �This is more easily seen if it is written as 7. 2 x 10 -5. Rule 4: �For numbers with decimal points, zeros to the right of a non zero digit are significant. �So, 6. 00 has three significant figures and 0. 050 has two significant figures.

Rule 5: �For numbers without decimal points, trailing zeros are not significant. �So, 400 has only one significant figure. �To indicate that the trailing zeros are significant a decimal point must be added. �So, 400. 0 has four significant figures.

Activity: �How many significant figures does each of the following have? 3 a) 525 3 b) 30, 600 2 c) 0. 072 5 d) 19. 000 e) 300, 000 1 5 f) 0. 26050

Calculations and Significant Figures �It is important when you carry out calculations that your answers are given to an appropriate number of significant figures. �As a general rule your answer should contain the same number of significant figures as the least accurate value in the question.

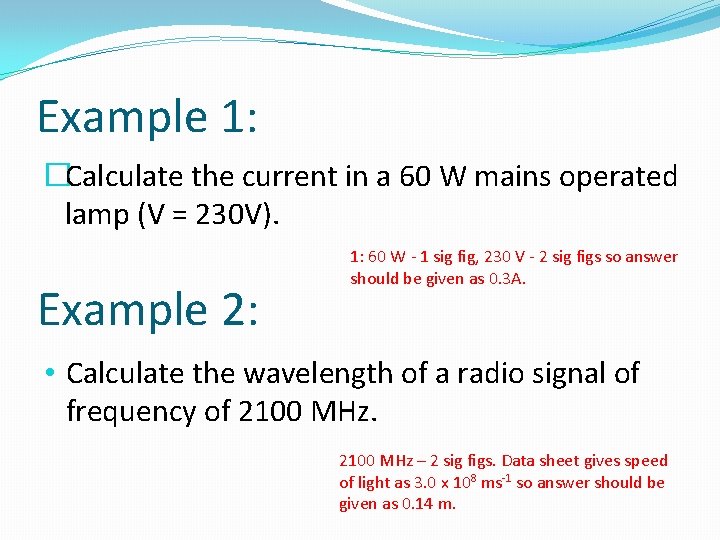
Example 1: �Calculate the current in a 60 W mains operated lamp (V = 230 V). Example 2: 1: 60 W - 1 sig fig, 230 V - 2 sig figs so answer should be given as 0. 3 A. • Calculate the wavelength of a radio signal of frequency of 2100 MHz – 2 sig figs. Data sheet gives speed of light as 3. 0 x 108 ms-1 so answer should be given as 0. 14 m.

Unit 0 Tutorial 2