03 Colloidal Dispersions Definition the particle size of


















- Slides: 36

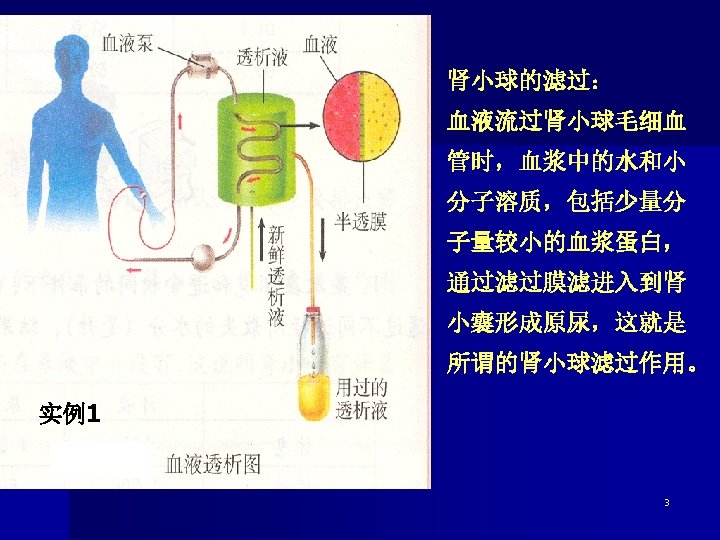
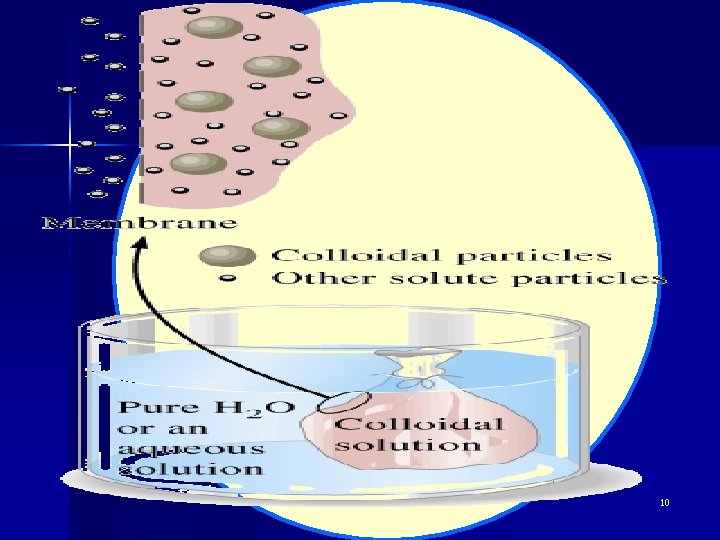
03 Colloidal Dispersions Definition: the particle size of the dispersed phase 1 nm~ 100 nm. 制备方法: 物理、化学方法 l Dialysis 渗析 The separation of ions from colloids by diffusion through a semipermeable membrane is called dialysis. 1

2



1. Properties of Colloidal Dispersions Tyndall Effect (丁达尔效应) Brownian Movement (布朗运动) Electrophoresis (电泳) Coagulation of Colloidal Dispersions(聚沉) 5

l Tyndall Effect When a light beam enter through a colloidal dispersion, the beam becomes visible. This phenomenon, known as the Tyndall effect. It is due to the fact that small particles scatter light in all directions. 6

7


l Brownian Movement Random motion of colloidal particles in a dispersing medium is called Brownian Movement. 9

10

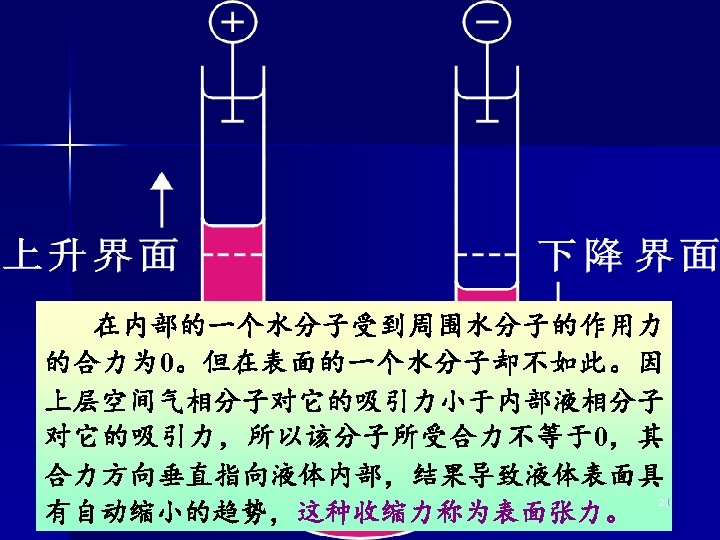
l Electrophoresis 电泳 带电荷的溶质或粒子在电场 中向着与其本身所带电荷相 Level rising Level lowing 反的电极移动的现象。 Almost all colloidal particles have an electrical chargeeither positive or negative. 11



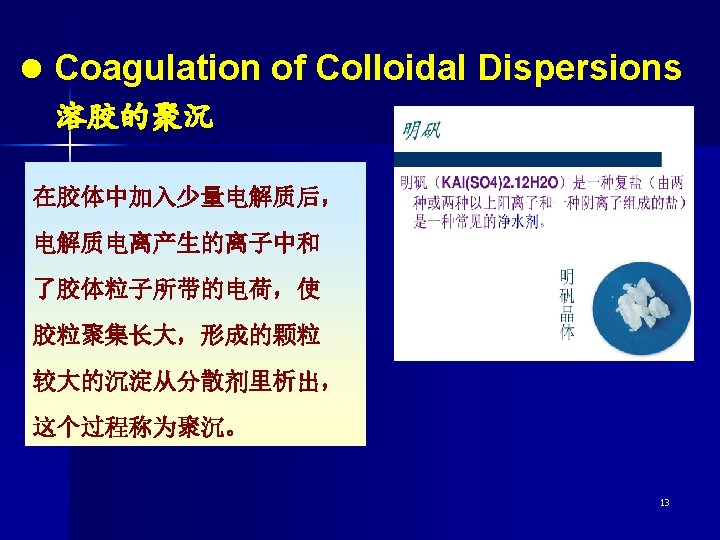
Several methods of bringing about the coagulation The most effective way of coagulating colloidal dispersions of the sol is by adding an electrolyte. n The mixing of two colloidal dispersions whose particles are oppositely charged causes both to coagulate. n n In many cases, heating is also a method of bringing about the coagulation of colloidal dispersions. 14


16

2. Surface Phenomena and Surface Tension Problem: Why, does water bead up on a newly waxed car instead of forming a sheet over it? 17

Floating a needle Walking on water 18

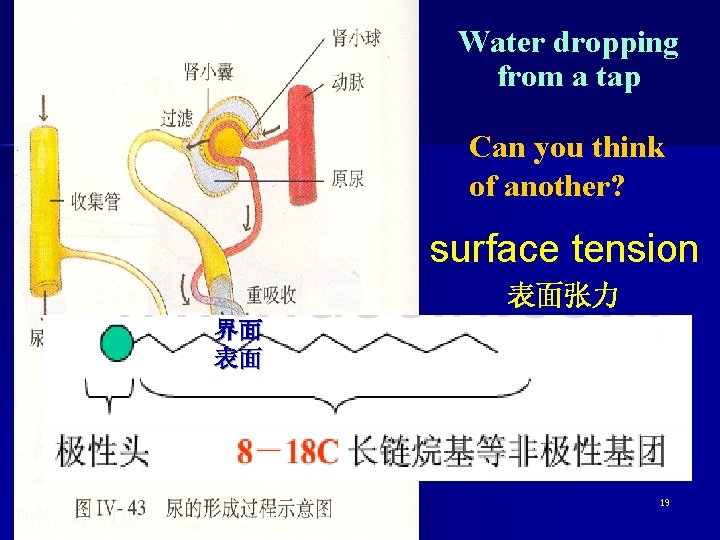
Water dropping from a tap Can you think of another? surface tension 表面张力 界面 表面 19

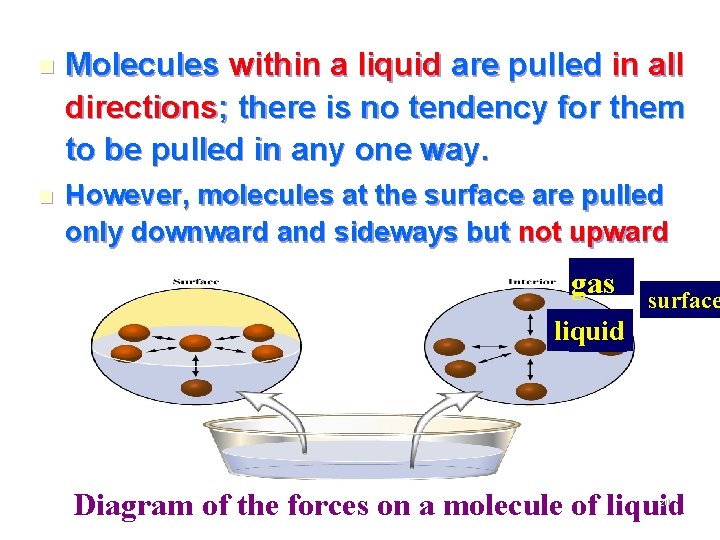
n Molecules within a liquid are pulled in all directions; there is no tendency for them to be pulled in any one way. n However, molecules at the surface are pulled only downward and sideways but not upward gas surface liquid Diagram of the forces on a molecule of liquid 20


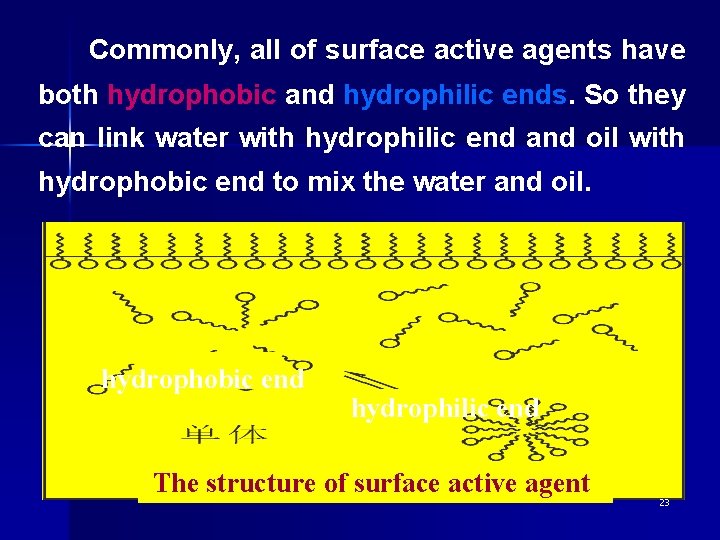
3. surface active substance (surface active agent) 表面活性剂是指能显著降低水的表面张力 的一类物质。 • The structure of surface active agent 22

Commonly, all of surface active agents have both hydrophobic and hydrophilic ends. So they can link water with hydrophilic end and oil with hydrophobic end to mix the water and oil. hydrophobic end hydrophilic end The structure of surface active agent 23

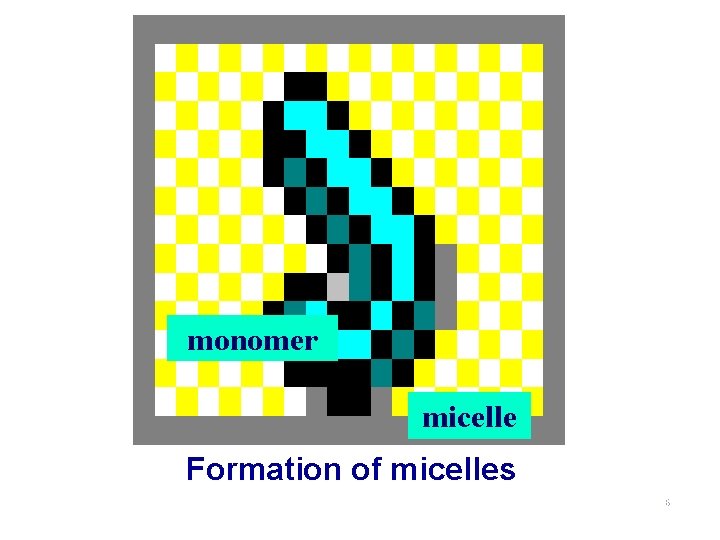
4. Association Colloid �合胶体 n micell The molecules that have both a hydrophobic end a hydrophilic end are dispersed in water, they associate or aggregate to form colloidalsized particles, or micells. n association colloid A colloid in which the dispersed phase consists of micelles is called an association colloid. 24

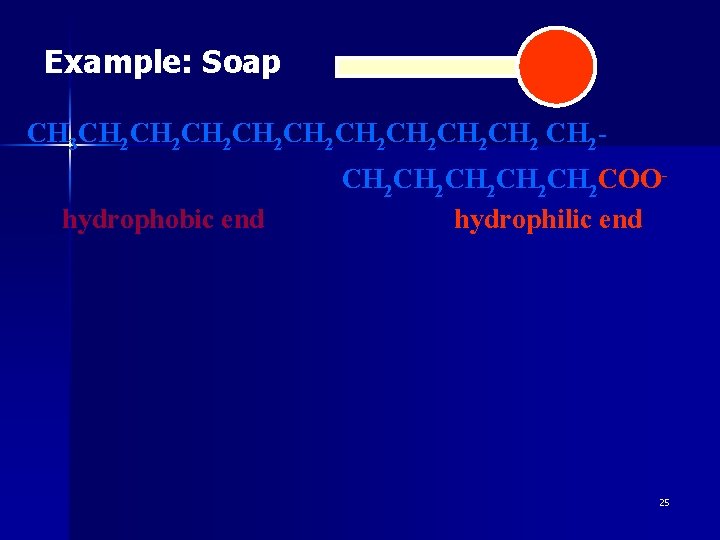
Example: Soap CH 3 CH 2 CH 2 CH 2 CH 2 hydrophobic end CH 2 CH 2 CH 2 COOhydrophilic end 25

monomer micelle Formation of micelles 26

27

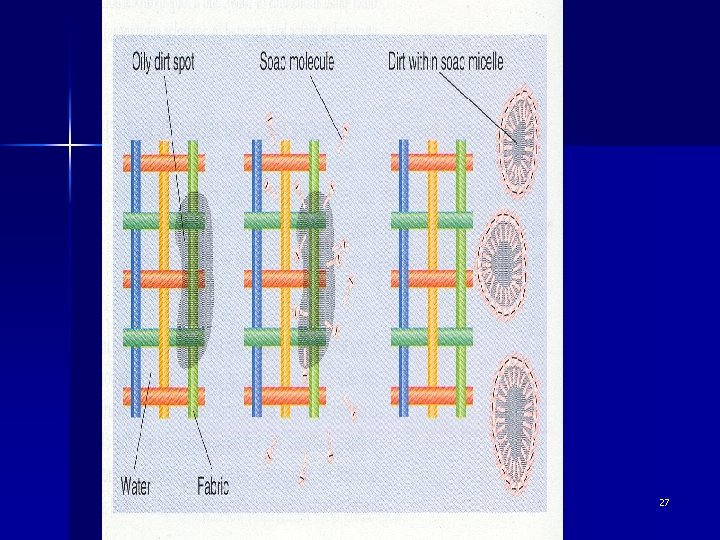
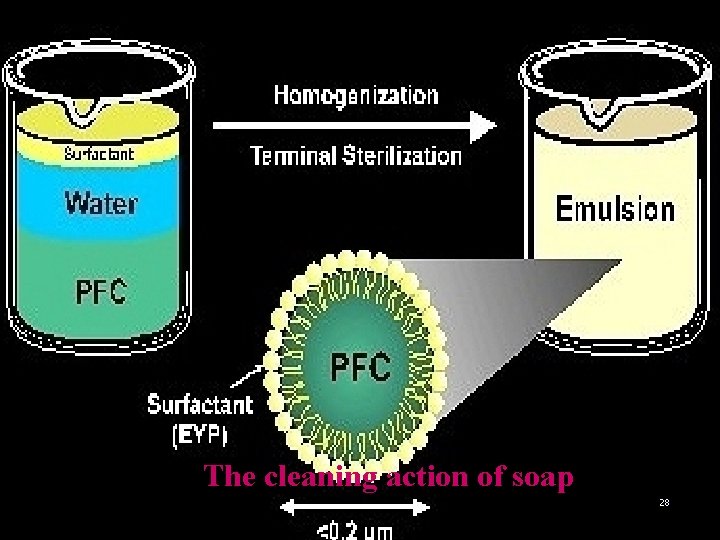
The cleaning action of soap 28


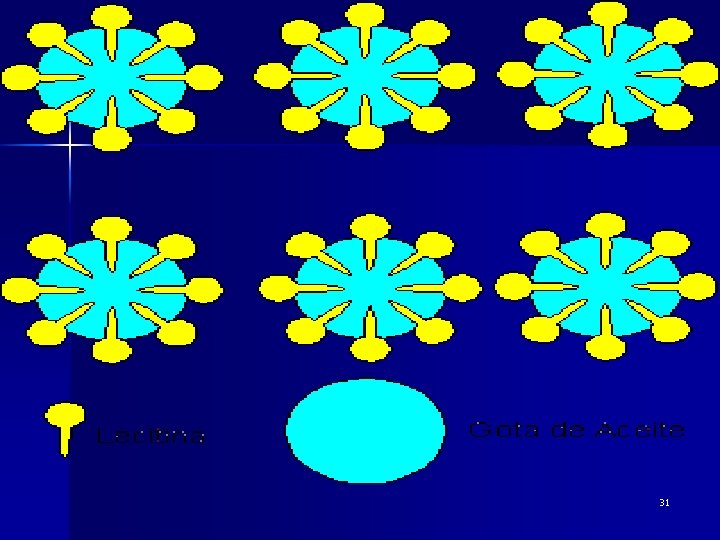
l Emulsions 乳状液 The definition of an emulsion is a stable mixture of two or more immiscible liquids where one liquid (in the form of droplets or globules) is dispersed in the other. 一种液体以液珠形式分散在与它不相混溶的另一种液体中 而形成的分散体系。 Emulsifying agent 乳化� A substance that coats the particles of the dispersed phase and prevents coagulation of colloidal particles; is called also an emulsifier. 30

31

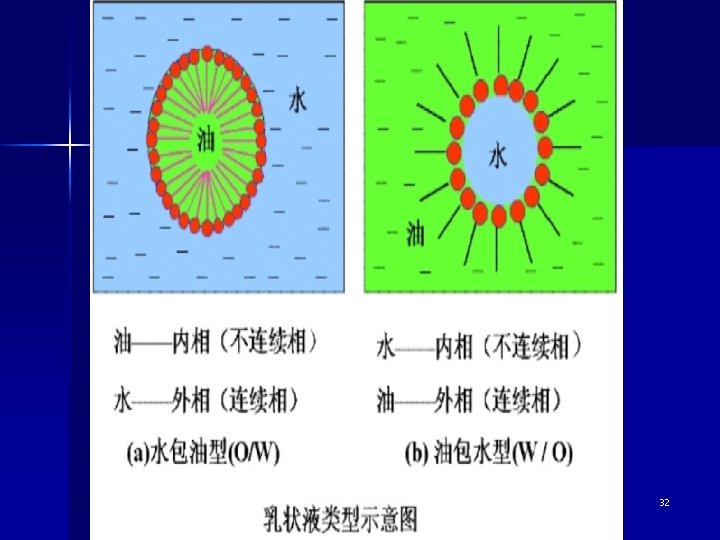
32

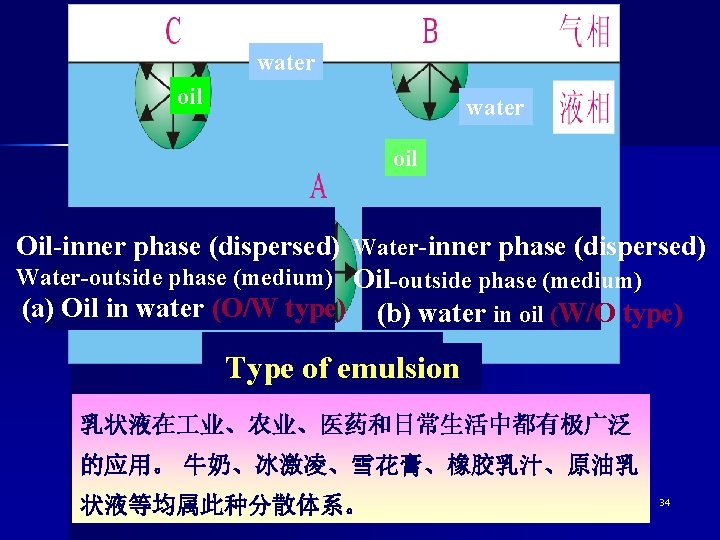
Types of emulsion Emulsions of oil in water (O/W) In which the dispersed phase is the oil while water is the medium. Emulsions of water in oil (W/O) In which the dispersed phase is the water while oil is the medium. 33

water oil Oil-inner phase (dispersed) Water-outside phase (medium) Oil-outside phase (medium) (a) Oil in water (O/W type) (b) water in oil (W/O type) Type of emulsion 乳状液在 业、农业、医药和日常生活中都有极广泛 的应用。 牛奶、冰激凌、雪花膏、橡胶乳汁、原油乳 状液等均属此种分散体系。 34

